Abstract
Objectives
The primary aim was to demonstrate that moxonidine, given in an experimental sustained release (SR) formulation, had no clinically relevant central nervous system (CNS) effects after 4 weeks of treatment. A clinically relevant CNS effect was predefined as more than 45° s−1 reduction in saccadic peak velocity (SPV), corresponding to the effects of one night's sleep deprivation.
Methods
In a randomized, double-blind fashion, 35 patients with mild to moderate essential hypertension received placebo run-in medication for 2 weeks, followed by 4 weeks’ moxonidine sustained release (1.5 mg o.d.) or placebo. On the first day and 1 and 4 weeks following the start of treatment, blood pressure was measured and CNS effects were assessed using SPV, visual analogue scales and EEG.
Results
On day 1 there was a significant, but not clinically relevant, reduction in the time-corrected area under the effect curve (AUEC) for SPV in the moxonidine group compared with placebo [difference of 38° s−1; 95% confidence interval (CI) 23, 52]. This difference was no longer significant after one (9° s−1; 95% CI −17, 35) and 4 weeks (6.9° s−1; 95% CI −16, 30). Visual analogue scales for alertness showed similar results. A decrease in EEG α- and β-power and an increase in δ-power were only found on day 1 of moxonidine treatment. The AUEC for systolic/diastolic blood pressure relative to placebo was 23 (95% CI 17, 29)/13 (9, 16) mmHg lower on day 1 and remained reduced by 20 (11, 30)/12 (6, 17) and 15 (6, 25)/9 (3, 15) mmHg after 1 and 4 weeks’ moxonidine treatment.
Conclusions
Four weeks’ treatment with an experimental SR formulation resulted in tolerance to CNS effects (equivalence to placebo) while blood pressure-lowering effects remained adequate. The tolerance to CNS effects was already observed after 1 week of treatment.
Keywords: hypertensive patients, centrally acting antihypertensive, saccadic eye movements, tolerance
Introduction
Moxonidine is a centrally acting antihypertensive agent, which binds selectively and with high affinity to the imidazoline-I1 receptor in the ventrolateral medulla of the brainstem and the kidney [1]. This receptor is thought to play an important role in central blood pressure regulation. Stimulation of I1 receptors induces sympatho-inhibition in the periphery, reflected by a lowering of blood pressure, heart rate, and plasma noradrenaline levels [2]. Moxonidine also binds to the α2-receptor in the medulla oblongata, albeit to a lesser extent than to the imidazoline-I1 receptor [2]. Stimulation of the α2-receptor in the nucleus coeruleus appears to induce the side-effects of centrally acting antihypertensive drugs, such as sedation and dry mouth [2]. The agonistic activity of moxonidine on α2-receptors is low in comparison with other first-generation centrally acting antihypertensive agents like clonidine and methyldopa [2, 3]. The improved tolerability of moxonidine compared with these drugs appears to be related to this difference in receptor activity [4–6].
The sedative properties of many drugs like benzodiazepines [7, 8] or partial α2 agonists like clonidine and rilmenidine [9] have been evaluated previously with measurements of saccadic eye movements and visual analogue scales (VAS). Sedation is typically accompanied by a drop in saccadic peak velocity (SPV), as well as a decrease in subjective alertness as measured with VAS scores. During prolonged treatment with drugs like benzodiazepines, development of tolerance to the sedative effects has been shown [8]. So far, tolerance development to central nervous system (CNS) drug effects has not been studied for imidazoline I1 agonists. This tolerance development may improve the clinical acceptability during prolonged treatment with centrally acting antihypertensive drugs.
In this study, the CNS effects of moxonidine were investigated using an experimental sustained release (SR) formulation during prolonged treatment of patients with mild to moderate hypertension. The aim was to demonstrate that moxonidine had no clinically relevant effect on the SPV after 4 weeks of treatment, compared with placebo. A clinically relevant effect on the SPV was predefined as an effect which is found after 24 h of sleep deprivation [10]. Secondary objectives were to assess the subjective effects (visual analogue scales of Bond and Lader), effects on two-lead pharmaco-EEG (fast Fourier analysis), blood pressure and heart rate. In addition, the relationship between moxonidine plasma concentration and its effects on SPV, visual analogue scales for alertness and blood pressure were modelled.
Methods
The study was conducted according to the principles of the Declaration of Helsinki and in accordance with the Guideline for Good Clinical Practice. The study protocol was approved by the Committee on Medical Ethics of Leiden University Medical Centre. All patients were included after giving written informed consent.
This was a randomized, parallel study in which patients received placebo run-in medication for 2 weeks in a single-blind fashion, followed by a double-blind 4-week treatment with moxonidine using an experimental SR formulation of 1.5 mg o.d. or placebo. After screening and approval by their own physician, eligible patients entered a variable washout period of up to 8 weeks, in which their own antihypertensive treatment was withdrawn. As soon as a diastolic blood pressure of 90–104 mmHg had been reached and the patient was off antihypertensive medication for at least 2 weeks, the patient entered the placebo run-in period, during which the patients were acquainted with the experimental methods and conditions during a short training session. Patients with a diastolic blood pressure of 90–104 mmHg without previous antihypertensive treatment could directly enter the single-blind placebo run-in period. Patients whose blood pressure increased above 120 mmHg after withdrawal of their antihypertensive medication were excluded from further participation. At the end of the run-in period eligible patients were randomized to one of the study treatments: moxonidine or placebo. Patients were studied on the first day of allocated randomized treatment, and at 1 and 4 weeks following the start of treatment. After the final study day, medication was tapered off to 0.5 mg o.d. for 3 days. The patients were instructed to take their study medication regularly at breakfast with liquids. Compliance was checked by tablet counting. Sufficient compliance was assumed when the study drug intake was within 80–120% of the planned intake. The moxonidine SR dose was based on the maximum dose intended to be developed, to be able to investigate the course of effects of the highest dose likely to be used in clinical practice.
On the study days, the patients arrived at the facility in the morning in a fasted state. Inclusion/exclusion criteria were checked, if applicable a pregnancy test (CARDS O.S.®H.C.G.-Urine test kits; Pacific Biotech, Inc., San Diego, CA, USA) was performed and the urine was checked for use of illicit or sedative drugs (amphetamines, cocaine, morphine, tetrahydrocannabinol, barbiturates and benzodiazepines using Abuscreen ONTRAK® Rapid Assays for Drug abuse; Roche Diagnostic Systems, Mijdrecht, the Netherlands). In addition, an alcohol breath test was performed with a Lion Alcol Meter (TAXA meter, Amsterdam, the Netherlands). After cannulation of a forearm vein and attachment of scalp, periorbital and ECG electrodes, patients received a light standardized breakfast, and were allowed a 30-min adaptation period in quiet surroundings. Randomized trial medication was administered (t = 0 h) after a baseline blood sample (drug-assay) had been drawn, and after two (t = −0.5 and 0 h) baseline measurements of heart rate, blood pressure, saccadic eye movements, pharmaco-EEG and VAS. These measurements were repeated every half hour for the first hours, and every hour from 3 to 10 h.
Measurements
The blood pressure measurements were made using an automated oscillometric blood pressure monitor (MPV1072; Nihon Kohden, Tokyo, Japan), which displays an average value for two sequential (duplicate) measurements at each time point. All measurements were made after the patient had been in a semirecumbent position for at least 5 min.
Recording of eye movements was performed in a quiet room with ambient illumination. Recording and analysis of saccadic eye movements was conducted with a microcomputer-based system for sampling and analysis of eye movements (customized CED software; Cambridge Electronic Design, Cambridge, UK) as described previously [7, 10]. The equipment used for stimulus display, signal collection and amplification was from Nihon Kohden. Disposable silver–silver chloride electrodes (Medicotest N-OO-S, Olstykke, Denmark) were applied on the forehead and beside the lateral canthi of both eyes of the patient for registration of the electro-oculographic signals. Skin resistance was reduced to < 5 kΩ before application of the electrodes. Head movements were restrained using a fixed head support. The target consisted of an array of light-emitting diodes on a bar, fixed at 50 cm in front of the head support. Saccadic eye movements were recorded for stimulus amplitudes of ± 15° to either side. Fifteen saccades were recorded for each stimulus amplitude with interstimulus intervals varying randomly between 3 and 6 s. Average values of latency (= reaction time), SPV and inaccuracy of all artefact-free saccades were used as parameters. Saccadic inaccuracy was calculated as the absolute value of the difference between the stimulus angle and the corresponding saccade, expressed as a percentage of the stimulus angle.
EEG registrations were made as described previously [7], using silver–silver chloride electrodes, fixed with collodion at median frontal (Fz), median central (Cz), median parietal (Pz) and median occipital (Oz), with the same common ground electrode as for the eye movement registration (international 10/20 system). The electrode resistances were kept below 5 kΩ. EEG signals were obtained from leads Fz-Cz and Pz-Oz. The signals were amplified by use of a Nihon Kohden AB-621G bioelectric amplifier with a time constant of 0.3 s and a low pass filter at 100 Hz. For the fast Fourier analysis, data collection and analysis were performed using customized CED software (Cambridge Electronics Design). Per session, eight consecutive blocks of 8 s were recorded. The signal was AD-converted using a CED 1401 laboratory interface (Cambridge Electronics Design) and electronically stored for subsequent analysis. Data blocks containing artefacts were identified by visual inspection and these were excluded from analysis. Fast Fourier analysis was performed to obtain the sum of amplitudes in the delta (0.5–3.5 Hz), theta (3.5–7.5 Hz), alpha (7.5–11.5 Hz) and beta (11.5–30 Hz) frequency ranges.
Visual analogue scales were used as described by Bond and Lader [11] and Norris [12]. From the lines three factors were derived, corresponding to alertness, mood and calmness.
Blood samples for drug assay were collected using lithium heparin-containing polystyrene tubes (Vacuette; Greiner, Alphen a/d Rijn, the Netherlands) and centrifuged immediately after sampling for 10 min at 4 °C and 1500 g. Plasma was stored at −20 °C until shipment and drug analysis. Drug concentrations were measured using HPLC/MS with atmospheric pressure chemical ionization at Oneida Research Services (Whitesboro, NY, USA). Validation range was 0.050–8.000 ng mL−1, accuracy and precision were < 14% and < 4%.
Calculations and statistical analysis
Data from all subjects were used in the analysis, including the dropouts during treatment with randomized trial medication. The primary statistical analysis was aimed at differences between the two study treatments at day 29 (after 4 weeks’ treatment) in overall effects on SPV. The overall effect was characterized by calculating the area under the effect curves (AUEC) over 10 h and dividing this area by the corresponding time span. An analysis of covariance was performed with the value prior to initial drug administration (on day 1) as covariate. A one-sided t-test using the mean square error from the ANCOVA was used for testing one-sided equivalence of the SPV response at day 29. Clinical equivalence was predefined to be proven, if the upper value of the 90% confidence interval of the difference was below 45° s−1. This difference is found after one night of sleep deprivation and can be considered to be a maximally acceptable sedative effect [10]. All secondary parameters were analysed using an analysis of covariance. For the estimates of the treatment differences, 95% confidence intervals (CIs) were calculated. All parameters are given as time-corrected AUECs as a representation of the average effect over the day.
SPV, VAS alertness and diastolic blood pressure concentration–effect relationships were investigated using nonlinear, mixed effect modelling as implemented in NONMEM (Version 5; NONMEM Project Group, UCSF, CA, USA). This methodology analyses all occasions simultaneously: all subjects are described using the same structural model, and mean and interindividual variability estimates are generated for the entire population [13]. Significance of parameters was assessed by comparing models with and without the specific parameter(s) using a likelihood ratio test, where a conservative P-value of 0.001 was used to compensate for the large sample normal approximation. In order to correct for the placebo response, the average placebo vs. time profile was calculated over all placebo subjects and subtracted from the individual moxonidine responses at corresponding protocol times. The relationship was described using a simple linear model. In order to relate concentrations to effects, linear interpolation was used. This was implemented in NONMEM by adding, for each data point, the corresponding slope and intercept of the interpolation line for that particular segment. This makes it possible to assess and estimate the need for an effect compartment to model the possible delay between concentrations and effects. Concentration–effect parameters were estimated assuming a parameter value for day 1 and a within-subject deviation (with a fixed and random component) for days 8 and 29. Fixed and/or random components were subsequently eliminated using the likelihood ratio test. An effect compartment was implemented in all cases.
EEG data were analysed after log transformation because of clear indications of skewness and only log EEG results were reported. All other variables were analysed untransformed.
Results
Subjects
Two patients dropped out during the placebo run-in period, one because of an adverse event (depression) and one because of frequent benzodiazepine use. Thirty-five patients were randomized into the double-blind treatment period: 16 to moxonidine SR and 19 to placebo. Demographics and baseline characteristics of the subjects who started randomized trial medication are summarized in Table 1. Two patients in the moxonidine SR group dropped out: one patient after 1 week's treatment because of adverse event (dizziness), and one patient after day 1 because of a serious adverse event (aorta dissection leading to hospitalization and death). This serious adverse event was regarded as unrelated to the study drug. In the placebo group, also two patients dropped out: one patient because of severe hypertension and one patient because of depression, both after day 1.
Table 1.
Demographic characteristics and baseline variables
| Moxonidine SR (n = 16) | Placebo (n = 19) | |
|---|---|---|
| Age (years)* | 55 (34–71) | 56 (35–78) |
| Numbers/%, per age group | ||
| < 50 | 6/38 | 3/16 |
| 50–59 | 3/19 | 9/47 |
| ≥ 60 | 7/44 | 7/37 |
| Sex (n, male/female) | 7/9 | 10/9 |
| Weight (kg)* | 78 (59–102) | 81 (61–105) |
| Height (cm)* | 172 (160–190) | 172 (158–186) |
| Body mass index (kg m−2)* | 26 (22–37) | 28 (21–38) |
| Systolic blood pressure*† | 157 (135–189) | 161 (146–178) |
| Diastolic blood pressure*† | 91 (72–106) | 91 (77–100) |
| Heart rate*† | 71 (60–86) | 72 (56–91) |
| Ethnic origin (n/%) | ||
| Caucasian | 15/94 | 19/100 |
| Negroid | 1/6 | 0/0 |
Given as means (range).
Predose value at first study day.
Adverse events
A total of 30 patients (86%) reported treatment-emergent adverse events. The most frequently reported adverse events were somnolence and dry mouth. On the first treatment day, somnolence was reported by all 16 subjects on moxonidine SR (100%) and by 10/19 patients (58%) in the placebo group, and dry mouth by 10 patients (63%) in the moxonidine group, vs. two patients (11%) on placebo. After 4 weeks of treatment, the number of moxonidine-treated patients reporting sedation had diminished to 9/16 (56%), compared with 7/19 (37%) on placebo. At that time, dry mouth was noted by 7/16 patients on moxonidine SR (44%) and 2/19 on placebo (11%).
Compliance
The median percentage of tablets taken during treatment was 100.0% (range 96.0–105.6%) in the moxonidine treatment group, and 100.0% (95.5–114.3%) in the placebo group.
Saccadic peak velocity
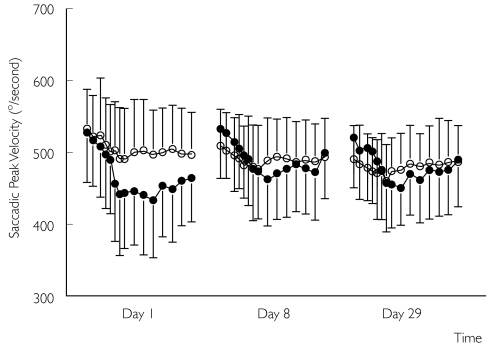
The SPV profile showed a clear treatment-related curve for all moxonidine SR treatment study days (Figure 1). On day 1 the time-corrected AUEC for peak velocity was significantly lower in the moxonidine SR group (38° s−1; 95% CI 23, 52). However, as predose moxonidine baseline values increased, the moxonidine treatment profile shifted upwards, and the difference with placebo was no longer present on day 8 (difference 9° s−1; 95% CI −17, 35) and day 29 (difference 7° s−1; 95% CI −16, 30). The upper value of the 90% CI (−12, 26) was well below 45° s−1, the predefined level of clinical significance [10]. The two treatments were therefore considered equivalent with respect to the AUEC curve for SPV after 4 weeks of treatment. No significant differences were observed for accuracy and reaction times (data not shown).
Figure 1.
Mean ± SD saccadic peak velocity profiles for the moxonidine sustained release (SR) (•) and placebo (○) treatment groups during study day 1, day 8 and day 29. Each profile represents a different study day; measurements were performed from predose (first two data points) until 10 h postdose.
Visual analogue scales
Analogously to the SPV profile, the alertness VAS showed a treatment-related curve for all moxonidine SR treatment study days (Figure 2). The time-corrected alertness AUEC was significantly lower on day 1 in the moxonidine SR group (6.7 mm; 95% CI 0.2, 13.2). As predose baseline VAS alertness increased and the profile shifted upwards, this difference disappeared on day 8 (difference of 5.4 mm; 95% CI −2.6, 13.5) and day 29 (difference of 0.9 mm; 95% CI −8.3, 10.1). No significant differences between the moxonidine SR and placebo groups were observed with respect to the mood and calmness AUECs (data not shown).
Figure 2.
Mean ± SD visual analogue scale (VAS) alertness profiles for the moxonidine sustained release (SR) (•) and placebo (○) treatment groups during study day 8 and day 29. Each profile represents a different study day; measurements were performed from predose (first two data points) until 10 h postdose.
Electroencephalogram
No clear treatment profiles were observed for EEG parameters. Significant differences between treatment groups were shown for all mid-parietal–occipital (PzOz) leads. In the moxonidine SR group on day 1, log transformed AUEC of total α- and β-power was significantly lower (difference 0.08, 95% CI 0.005, 0.16; and 0.12, 95% CI 0.01, 0.23, respectively), while total δ-power was significantly higher (difference −0.10; 95% CI −0.20, −0.01). These differences were not observed on day 8 and day 29, nor were there significant differences between study days (data not shown). The log transformed AUEC of total θ-power was higher in the moxonidine SR group on day 8 and day 29 (difference −0.16, 95% CI −0.33, −0.001; and −0.21, 95% CI −0.40, −0.03, respectively). No significant differences between treatment groups were observed for any of the mid-frontal-central (FzCz) lead EEG parameters. The log transformed AUEC of total θ-power (FzCz lead) on day 1, however, came close to a significant increase for the moxonidine SR group compared with placebo (difference −0.15; 95% CI −0.31, 0.003).
Blood pressure and heart rate
Systolic and diastolic blood pressure profiles showed clear treatment profiles on all study days (Figures 3 and 4). Mean (95% CI) systolic AUECs were significantly lower in the moxonidine SR group on all study days: 23 (17, 29), 20 (11, 30) and 15 (6, 25) mmHg difference for days 1, 8 and 29. Also the mean (95% CI) diastolic blood pressure AUECs were significantly lower in the moxonidine SR group on all study days: 13 (9, 16), 12 (6, 17) and 9 (3, 15) mmHg difference for days 1, 8 and 29. Only on day 8 was predose (trough) diastolic blood pressure significantly lower in the moxonidine SR group compared with placebo (difference 5.3 mmHg; 95% CI 0.2, 10.5). Average heart rate did not show a treatment-related response (not shown).
Figure 3.
Mean ± SD systolic blood pressure profiles for the moxonidine sustained release (SR) (•) and placebo (○) treatment groups during study day 1, day 8 and day 29. Each profile represents a different study day; measurements were performed from predose (first two data points) until 10 h postdose.
Figure 4.
Mean ± SD diastolic blood pressure profiles for the moxonidine sustained release (SR) (•) and placebo (○) treatment groupsduring study day 1, day 8 and day 29. Each profile represents a different study day; measurements were performed from predose (first two data points) until 10 h postdose.
Pharmacokinetics
The moxonidine time profiles were clearly the result of an extended release oral formulation. Average moxonidine concentration profiles shifted upwards on day 8 and day 29 as baseline values increased (Figure 5). The area under the curve (AUC) increased 18% (95% CI 2, 37) from day 1 to day 8 and the AUC increased 14% (95% CI −1, 33) from day 1 to day 29. The maximum concentration increased 19% (95% CI 6, 34) from day 1 (2.6 ± 0.7 ng mL−1) to day 8 (3.1 ± 1.1 ng mL−1) and 20% (95% CI 6, 35%) from day 1 to day 29 (3.1 ± 0.8 ng mL−1). The time to maximum concentration (4 h 28 min on day 1 and 3 h 26 min on day 29) did not change significantly between study days.
Figure 5.
Mean ± SD moxonidine plasma concentration profiles during study day 1, day 8 and day 29. Each profile represents a different study day; measurements were performed from predose (first two data points) until 10 h postdose.
Concentration–effect modelling
Concentration–effect modelling was performed by linearly interpolating the concentration profile and by linking these concentrations to the effects. In the concentration–effect graphs the average effect (moxonidine minus placebo effect) is plotted against the corresponding average interpolated moxonidine concentration. All effects tended to lag behind relative to the plasma concentrations. This hysteresis was dealt with by assuming a hypothetical effect compartment, characterized by an equilibration half-life that describes the speed with which plasma concentrations would be equilibrated with effect compartment concentrations if the drug was to be infused at a constant rate.
Saccadic peak velocity
For SPV the best fit was given by a model including an effect compartment where the equilibration half-life (58 min; 95% CI 39, 77) and slope (−22.9° s−1 ng−1 mL; 95% CI −4, −42) were similar between study days (Figure 6). From day 1 to 8 and 29 the profile shifted upwards, leading to an increase in intercept of 36° s−1 (95% CI 30, 41) from day 1 to day 8 and an increase of 41° s−1 (95% CI 26, 55) from day 1 to day 29.
Figure 6.
Delta (placebo minus moxonidine response) saccadic peak velocity profiles vs. interpolated moxonidine concentration. The broken line represents the modelled profile for day 1 (with average original data, ○), the solid lines represent the modelled profile for days 8 and 29.
VAS alertness
For VAS alertness, the best model was analogous to SPV. The slope (−3.9 mm ng−1 mL; 95% CI −1.6, −6.2) and equilibration half-life (43 min; 95% CI 10, 76) did not differ between occasions (Figure 7). Only the intercept was different between occasions: a nonsignificant increase of 3.0 mm (95% CI −1.3, 7.3) from day 1 to day 8 and a significant increase of 6.6 mm (95% CI 3.6, 9.6) from day 1 to day 29.
Figure 7.
Delta (placebo minus moxonidine response) visual analogue scale (VAS) alertness vs. interpolated moxonidine concentration. The broken line represents the modelled profile for day 1 (with average original data, ○), the solid lines represent the modelled profile for days 8 and 29.
Diastolic blood pressure
For diastolic blood pressure, no shift of the concentration–δ diastolic blood pressure curve was observed, but the profile levelled off on day 8 and day 29 (Figure 8). The best fit was given by a model in which only the slope was significantly different between study days. Estimated equilibration half-life was 86 min (95% CI 52, 119) and intercept was −0.15 mmHg (95% CI −4.5, 4.2). The slope decreased 4.4 mmHg ng−1 mL (95% CI 2.3, 6.6) from day 1 to day 8 and 3.2 mmHg ng−1 mL (95% CI 0.3, 6.1) from day 1 to day 29.
Figure 8.
Delta (placebo minus moxonidine response) diastolic blood pressure vs. interpolated moxonidine concentration. The broken line represents the modelled profile for day 1 (with average original data, ○), the solid lines represent the modelled profile for days 8 and 29.
Discussion
This study showed that after 4 weeks of treatment, moxonidine 1.5 mg o.d. given in an experimental SR formulation was equivalent to placebo for the overall effects on SPV, an objective measure of alertness. On each study day, a treatment-related decrease in SPV relative to predose values at each study day was observed, but only on the first day of moxonidine SR treatment was the SPV AUEC significantly lower compared with placebo. As a result of an upward shift of the curve, the average effects of moxonidine SR on SPV were at placebo level after 8 and 29 days of treatment. Although its face validity is not very high, SPV is one of the most reproducible and sensitive measures of sedation [10]. No method applicable in drug research is available that shows direct relationships to clinical consequences of sedation, like reduced working performance or increased accident rates. The SPV results were corroborated by similar findings with visual analogue scales for alertness, which have a higher face validity. The subjective VAS score for alertness showed treatment-related curves, which shifted upwards during treatment. The EEG results further support these observations, as only on day 1 were significant effects consistent with CNS depression observed [14]. All these results point to the development of tolerance to the CNS effects of moxonidine 1.5 mg. It should be noted that the recommended starting dose for moxonidine is considerably lower than the dosage used in this study. The observed CNS effects for the first treatment day are therefore likely to be smaller during normal clinical use. Both the average subjective and objective CNS effects on day 1 returned to placebo levels within the first week of treatment, although treatment-related decreases in SPV and VAS were still present after 1 and 4 weeks. These observations are further clarified by concentration–effect modelling. The slope of the modelled effect remained unchanged, indicating that the response to moxonidine was similar on all study days. However, the concentration–effect curves shifted upwards during treatment, resulting in net CNS effects comparable to placebo.
The increase in baseline alertness as shown by two independent measures of alertness (SPV and VAS) indicates that after 1 and 4 weeks of treatment the patients in the moxonidine SR group became more alert in the morning before receiving their daily dose. This phenomenon has not been observed previously during treatment with moxonidine, and its causes are unclear. The CNS effects of moxonidine are thought to be mainly due to weak α2 receptor agonism, which decreases central noradrenergic activity [2]. An increase in alertness during treatment with α2 agonists could be the result of a hyperresponsiveness of central noradrenergic pathways during prolonged treatment [15]. However, such a putative central noradrenergic increase did not seem to affect blood pressure.
Systolic and diastolic blood pressure showed clear treatment effects in the moxonidine SR group, with a lowering of blood pressure to nonhypertensive levels. Despite this clear treatment effect, predose blood pressure did not differ between the treatment groups, except for a lower diastolic blood pressure in the moxonidine SR group on day 8. Thus, 24-h effective control of systolic and diastolic blood pressure was not fully maintained with a single dose of this experimental moxonidine SR formulation. However, it has to be considered that the study was not powered to detect clinically meaningful blood pressure effects at trough. Further improvement of the extended release profile could increase the trough levels of 0.7 ng mL−1 observed on day 29, and also reduce the mean maximum concentrations of 3.1 ng mL−1 that were found in this study.
Moxonidine plasma concentrations increased by 14–18% compared with day 1, but the average blood pressure remained unchanged throughout the 4-week treatment period. Modelling showed a small decrease in slopes of the concentration–effect curves, which may be interpreted as an indication of tolerance. Tolerance is often observed among agonist-stimulated, G-protein-coupled receptors, such as the I1-receptor. On the other hand, no rebound hypertension has been reported after the discontinuation of moxonidine treatment [16], arguing against such a phenomenon. Systemic counter-regulation could be a more plausible explanation, considering the strong antihypertensive effects of this moxonidine dose, as exemplified by an average maximum systolic/diastolic blood pressure reduction of 36/21 mmHg on day 1 (Figures 3 and 4). In view of the sustained antihypertensive effects, the lack of further blood pressure reduction despite increasing moxonidine concentrations may be regarded as clinically nonsignificant.
In conclusion, this study showed that after 4 weeks of treatment, moxonidine SR 1.5 mg o.d. is equivalent to placebo for average CNS effects. Treatment–effect curves over the day and modelling of the pharmacodynamic and pharmacokinetic data provided indications of an increased alertness at predose (trough level) after 1 and 4 weeks treatment.
Acknowledgments
This study was sponsored by Solvay Pharmaceuticals, Weesp, the Netherlands.
References
- 1.Ernsberger P, Damon TH, Graff LM, Schafer SG, Christen MO. Moxonidine, a centrally acting antihypertensive agent, is a selective ligand for I1-imidazoline sites. J Pharmacol Exp Ther. 1993;264:172–182. [PubMed] [Google Scholar]
- 2.Van Zwieten PA. The renaissance of centrally acting antihypertensive drugs. J Hypertens. 1999;17(3):S15–S21. [PubMed] [Google Scholar]
- 3.Prichard BN, Graham BR, Owens CW. Moxonidine: a new antiadrenergic antihypertensive agent. J Hypertens. 1999;17(3):S41–S54. [PubMed] [Google Scholar]
- 4.Macphee GJ, Howie CA, Elliott HL, Reid JL. A comparison of the haemodynamic and behavioural effects of moxonidine and clonidine in normotensive subjects. Br J Clin Pharmacol. 1992;33:261–267. doi: 10.1111/j.1365-2125.1992.tb04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planitz V. Crossover comparison of moxonidine and clonidine in mild to moderate hypertension. Eur J Clin Pharmacol. 1984;27:147–152. doi: 10.1007/BF00544037. [DOI] [PubMed] [Google Scholar]
- 6.Schachter M. Moxonidine: a review of safety and tolerability after seven years of clinical experience. J Hypertens. 1999;17(3):S37–S39. [PubMed] [Google Scholar]
- 7.van Steveninck AL, Mandema JW, Tuk B, et al. A comparison of the concentration–effect relationships of midazolam for EEG-derived parameters and saccadic peak velocity. Br J Clin Pharmacol. 1993;36:109–115. doi: 10.1111/j.1365-2125.1993.tb04205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Steveninck AL, Wallnofer AE, Schoemaker RC, et al. A study of the effects of long-term use on individual sensitivity to temazepam and lorazepam in a clinical population. Br J Clin Pharmacol. 1997;44:267–275. doi: 10.1046/j.1365-2125.1997.t01-1-00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harron DW, Hasson B, Regan M, McClelland RJ, King DJ. Effects of rilmenidine and clonidine on the electroencephalogram, saccadic eye movements, and psychomotor function. J Cardiovasc Pharmacol. 1995;26(2):S48–S54. [PubMed] [Google Scholar]
- 10.van Steveninck AL, van Berckel BN, Schoemaker RC, Breimer DD, van Gerven JM, Cohen AF. The sensitivity of pharmacodynamic tests for the central nervous system effects of drugs on the effects of sleep deprivation. J Psychopharmacol. 1999;13:10–17. doi: 10.1177/026988119901300102. [DOI] [PubMed] [Google Scholar]
- 11.Bond AJ, Lader MH. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. [Google Scholar]
- 12.Norris H. The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology. 1971;10:181–191. doi: 10.1016/0028-3908(71)90039-6. [DOI] [PubMed] [Google Scholar]
- 13.Schoemaker HC, Cohen AF. Estimating impossible curves using NONMEM. Br J Clin Pharmacol. 1996;42:283–290. doi: 10.1046/j.1365-2125.1996.04231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santamaria J, Chiappa KH. The EEG of drowsiness in normal adults. J Clin Neurophysiol. 1995;4:327–382. doi: 10.1097/00004691-198710000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Geyskes GG, Boer P, Dorhout Mees EJ. Clonidine withdrawal. Mechanism and frequency of rebound hypertension. Br J Clin Pharmacol. 1979;7:55–62. doi: 10.1111/j.1365-2125.1979.tb00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster J, Koch HF. Aspects of tolerability of centrally acting antihypertensive drugs. J Cardiovasc Pharmacol. 1996;27(Suppl 3):S49–S54. doi: 10.1097/00005344-199627003-00007. [DOI] [PubMed] [Google Scholar]