Abstract
Objectives
The pharmacokinetic profile of antibiotics at the site of anti-infective action is one of the most important determinants of drug response, since it correlates with antimicrobial effect. Up to now, only limited information on the lung tissue pharmacokinetics of antibiotic agents has been available. The aim of this study was to measure, using a new microdialysis-based approach, antibiotic penetration into the extracellular space fluid of pneumonic human lung parenchyma.
Patients and methods
The lung penetration of a combination of piperacillin and tazobactam, substances with low protein binding, was determined in five patients suffering from pneumonia and metapneumonic pleural empyema. The condition was treated by decortication after lateral thoracotomy. Intra-, or post-operatively, respectively, two microdialysis probes were inserted into pneumonic lung tissue, and into healthy skeletal muscle to obtain reference values. Serum and microdialysis samples were collected at 20-min intervals for at last 8 h following i.v. administration of a single dose of 4 g piperacillin and 500 mg tazobactam.
Results
The mean free interstitial concentration profiles of piperacillin in infected lung tissue and serum showed a maximal tissue concentration (Cmax) of 176.0 ± 105.0 mg l−1 and 326.0 ± 60.6 mg l−1, respectively. The mean AUC (area under the curve) for infected lung tissue was 288.0 ± 167.0 mg.h l−1 and for serum 470.0 ± 142.0 mg.h l−1. There was a statistically significant difference between AUC (lung) and AUC (serum) (P = 0.018) as well as between AUC (lung) and AUC (muscle) (P = 0.043). The intrapulmonary concentrations of piperacillin and tazobactam exceeded the minimum inhibitory concentrations (MIC) for most relevant bacteria for 4–6 h. The procedure was well tolerated by all patients and no adverse events or microdialysis-associated side-effects were observed.
Conclusion
This microdialysis technique enabled continuous tissue pharmacokinetic measurement of free, unbound anti-infective agents in the lung tissue of patients with pneumonia. The present data corroborate the use of piperacillin and tazobactam in the treatment of lung infections caused by extracellular bacteria and demonstrate the distribution of piperacillin and tazobactam in the interstitial space of pneumonic lung tissue.
Keywords: human lung, in vivo, microdialysis, pharmacokinetics, piperacillin, target site, tazobactam
Introduction
In spite of advances in the development of antibiotics, infections of the lower respiratory tract (RTI) and their associated complications, such as metapneumonic empyema, are still a major clinical problem [1]. The choice of antibiotic treatment is based on the likely susceptibility pattern of a suspected microorganism and on the pharmacokinetic properties of the antibiotic itself.
Recent studies indicated that target site drug concentrations may be substantially lower than serum concentrations in various organs, including the lung [2]. High in vitro minimum inhibitory concentrations (MIC) against bacteria [2] and suboptimal target site concentrations may cause therapeutic failure and may also trigger bacterial resistance [3].
A variety of sophisticated methods have been developed to quantify the penetration of antibiotics into lung tissue [4–15]. Major disadvantages of these methods are the inability to (i) discriminate between the free and microbiologically active drug, and the bound drug, which is inactive, and (ii) determine drug concentration in the interstitial space of lung tissue. Microdialysis overcomes these limitations and allows the determination of free drug at the site of antibacterial action.
Based on these considerations we studied by in vivo microdialysis, the pharmacokinetic properties of piperacillin and tazobactam (Tazonam“, Wyeth Lederle, Vienna, Austria) in the interstitial lung tissue in patients with pneumonia.
Methods
The local ethics committee (in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the European Commission) at the Medical Faculty of the University of Graz approved the study. All patients were given a detailed description of the study, and their written informed consent was obtained.
Patients
From June 2000 to April 2001, five patients (four males, one female) were included in the study (mean age 51.3 years, range 35–70 years; mean bodyweight 72.3 kg, range 55–86 kg; mean body mass index 23; range 18–26).
All patients suffered from sepsis due to pneumonia and metapneumonic pleural empyema, necessitating decortication following thoracotomy [16–18].
Microdialysis
Microdialysis is based on sampling of analytes from the extracellular space by means of a semipermeable membrane at the tip of a microdialysis probe [19, 20]. Once the probe, perfused with a perfusion solution at a constant low flow rate, is implanted into the tissue, substances present in the extracellular fluid at concentration (Ctissue) will be filtered by diffusion out of the extracellular fluid into the probe, resulting in a concentration (Cdialysate) in the perfusion medium.
By fractionated collection of samples, pharmacokinetic parameters from specified tissue can be determined. For most analytes, the equilibrium between extracellular tissue fluid and the perfusion medium will be incomplete, resulting in Ctissue being greater than Cdialysate. The factor interrelating these concentrations is termed recovery, which was determined using the retrodialysis method [2, 19–21].
Drugs
The combination of piperacillin and tazobactam (Tazonam© 4.5 g; Wyeth-Lederle, Vienna, Austria) was used. Both are low-molecular-weight antibiotics with no significant protein binding. The combination is well-established in the treatment of pneumonia [22–25].
Experimental design
Under steady-state pharmacokinetic conditions for the drugs, two microdialysis probes were implanted into pneumonic tissue and one additional probe was inserted into healthy skeletal muscle at the end of the operation.
Microdialysis was started about 30 min after the insertion of the probes when patients were on the intensive care unit. The calibration procedure was followed by a 20-min washout period. Thereafter, piperacillin (4 g) and tazobactam (500 mg) were administered intravenously over 20 min.
Sampling of dialysates and venous blood was performed at 20-min intervals for a period of 8 h. Blood samples were collected in plastic tubes, immediately centrifuged at 1600 g for 5 min and frozen at −70°C until analysis.
Drug analysis
Piperacillin and tazobactam were assayed by using a previously published and validated high-performance liquid chromatography (HPLC) method with a limit of determination of 1 mg l−1[26]. Serum samples were analysed by diluting 0.2 ml serum with an equal volume of phosphate buffer (50 mm pH 6.0) and precipitating the proteins with 0.8 ml acetonitrile. After centrifugation (10 min, 15 800 g) the clear supernatant was decanted and mixed with 2.0 ml dichloromethane. After further centrifugation (10 min, 3000 g), 100 µl of the upper aqueous phase were transferred to an autosampler vial and 20 µl were injected onto the HPLC. Microdialysate samples were injected directly without preparation. The chromatographic system consisted of a Wisp 717+ autosampler with a refrigerated sample compartment set to 4°C, two pumps model 510, a model 2487 absorbance detector (set to 220 nm) and a Millenium 3.2 data system (Waters, Milford, MA, USA). A Lichrospher 100 (Merck AG, Darmstadt, Germany; 5 µm particle size; 125 × 4 mm) was used as the analytical column. A linear calibration curve was obtained in the range 1–500 mg l−1 for piperacillin and 1–100 mg l−1 for tazobactam. Control samples with 10 mg l−1 and 100 mg l−1 of both analytes were prepared and assayed together with the patients’ samples. Between-day variability ranged from 9% to 15% (n = 15 for serum and n = 24 for dialysate).
Data analysis
Pharmacokinetic calculations were performed using the commercial software Kinetica 3.0 [27] based on a one-compartment model [28]. Demographic and pharmacokinetic parameters were analysed by descriptive methods (mean and s.d.). Comparisons were made using Wilcoxon's matched pairs signed-rank test.
Results
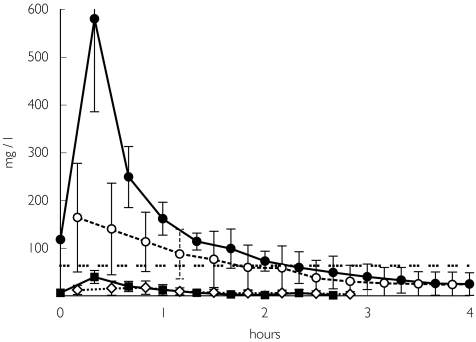
The free interstitial concentration–time profiles of piperacillin and of tazobactam at tissue steady state in infected lung are shown in Figure 1 and the corresponding pharmacokinetic parameters are summarized in Table 1.
Figure 1.
Concentration vs time profile for piperacillin, at tissue steady state, in infected lung tissue, serum and healthy skeletal muscle after i.v. administration of 4 g piperacillin over 20 min. The minimum inhibitory concentration (MIC) against Pseudomonas aeruginosa is 64 mg l−1. •, Piperacillin in serum; ○, piperacillin in lung; ▪, tazobactam in serum; ◊, tazobactam in lung; … …, MIC against P. aeruginosa.
Table 1.
Pharmacokinetic parameters for piperacillin and for tazobactam, after intravenous administration to patients with pneumonia.
| Ratio | AUC | ||||
|---|---|---|---|---|---|
| AUCinterstitium/ AUCserum | (mg.h l−1) | t½(h) | tmax(h) | Cmax(mg l−1) | |
| Serum | |||||
| Piperacillin | 470 ± 142 | 0.943 ± 0.52 | – | 326 ± 60.6 | |
| Tazobactam | 36.2 ± 26.0 | 0.656 ± 0.456 | – | 30.7 ± 5.88 | |
| Lung | |||||
| Piperacillin | 0.63 ± 0.29 | 288 ± 167 | 1.47 ± 1.28 | 0.627 ± 0.501 | 176 ± 105 |
| Tazobactam | 1.93 ± 1.56 | 45.7 ± 44.8 | 1.2 ± 1.53 | 0.575 ± 0.121 | 20.5 ± 14.5 |
| Muscle | |||||
| Piperacillin | 0.40 ± 0.13 | 197 ± 122.0 | 1.30 ± 0.615 | 0.62 ± 0.323 | 76.1 ± 22.4 |
| Tazobactam | 0.73 ± 0.64 | 19.6 ± 21.8 | 0.713 ± 0.557 | 0.463 ± 0.343 | 11.4 ± 8.12 |
AUC, Area under the concentration–time curve; t1/2, elimination half-life; tmax, time to maximum concentration; Cmax, maximum concentration; mean values ± standard deviation (n = 5).
The AUC (area under the curve)lung tissue/AUCserum and the AUCskeletal muscle/AUCserum ratios revealed excellent penetration of piperacillin into the interstitial space of infected lung tissue. Statistical comparison of the AUC values of infected lung tissue (288.0 ± 167.0 mg.h l−1) and skeletal muscle (197.0 ± 122.0 mg.h l−1) compared with serum (470.0 ± 142.0 mg.h l−1) showed significant differences between serum and lung tissue (P = 0.018) and between serum and muscle (P = 0.043). The mean difference in the AUC of piperacillin between serum and lung was 158.8 mg.h l−1 with a 95% confidence interval (CI) of 49.6, 268.2 mg.h l−1, and between serum and skeletal muscle it was 273.6 mg.h l−1 with a 95% CI of 213.9, 333.2 mg.h l−1.
Discussion
Microdialysis is a promising in vivo technique allowing the determination of free interstitial space drug concentrations in a defined target tissue [29–31]. Accordingly, a US-FDA advisory committee recently acknowledged that microdialysis might be a potentially attractive approach for clinical studies on tissue distribution of antimicrobial drugs, since it allows the measurement of unbound drug at the site of infection [32–34].
In the present study microdialysis was successfully performed in a clinical setting for the determination of the concentration of antibiotics in infected lung tissue. At the dosage used adequate concentrations of unbound piperacillin in the interstitial space of infected lung tissue were achieved.
However, concentrations of piperacillin measured by two probes inserted into each pneumonic lung differed substantially within an individual patient, suggesting that the pneumonic lung is not a homogeneous area. Moreover, the movement of antibiotics across the various barriers into the interstitial space fluid is a dynamic process, which might be additionally influenced by the distance of the probe from a pulmonary vessel [35]. Though the AUCs of piperacillin in lung were significantly different from serum, its tissue concentrations reached the MIC threshold for many clinically relevant pathogens for a maximum interval of 4–6 h. However, the MIC value for the important Pseudomonas aeruginosa was only sustained for a much shorter period of 2–2.5 h. Since treatment failure and development of resistant microorganisms may both derive from inadequate tissue concentrations of antibiotics, the results from microdialysis studies can offer useful information for future dosage modifications.
Tazobactam is a β-lactamase inhibitor which protects piperacillin from degradation by β-lactamase enzymes. Concentrations of 0.05–0.5 mg l−1 are required for a 50% inhibition of these enzymes [36]. In the present study mean maximal concentrations of tazobactam of 20.5 ± 14.5 mg l−1 after 0.57 ± 0.21 h were measured in the lung.
Tazobactam concentrations in healthy skeletal muscle of all patients were surprisingly low, because the pectoralis muscle has an abundant blood supply. However, these findings are in accordance with results from other microdialysis studies in healthy volunteers. As an explanation, tissue-specific differences in protein binding have been suggested [37].
In conclusion, microdialysis is a useful method for measuring interstitial lung tissue concentrations of piperacillin and tazobactam in patients with pneumonia and associated metapneumonic empyema. Although our data have to be interpreted with caution because of the small number of patients studied, the results may be useful for the adjustment of dosage schedules to optimize antibiotic treatment.
Acknowledgments
Wyeth-Lederle supported this study. The fruitful discussions with Mr Erwin Spannraft and the excellent technical assistance of Ing. I. Trebuch are gratefully acknowledged.
References
- 1.Chesnutt MS, Prendergast TJ. Pulmonary infections. In: Tierny LW, McPhee SJ, Papadakis MA, editors. Current Medical Diagnosis and Treatment. New York: Lange Medical Publishing/McGraw-Hill; 2001. pp. 291–300. [Google Scholar]
- 2.Joukhadar C, Derendorf H, Müller M. Microdialysis, a novel tool for clinical studies of anti-infective agents. Eur J Clin Pharmacol. 2001 doi: 10.1007/s002280100301. 10.1007/s002280100301 Article in HTML, published online at: http://link.springer.de/link/service/journals/00228/contents/01/00301/ 8 May. [DOI] [PubMed]
- 3.Hyatt JM, McKinnon PS, Zimmer GS, Schentag JJ. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bergogne-Berezin E. Penetration of antibiotics into the respiratory tree. J Antimicrob Chemother. 1981;8:171–174. doi: 10.1093/jac/8.3.171. [DOI] [PubMed] [Google Scholar]
- 5.Drusano GL. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988;32:289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pennington JE. Penetration of antibiotic into respiratory secretions. Rev Infect Dis. 1981;3:67–73. doi: 10.1093/clinids/3.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Jehl F, Muller-Serieys C, de Larminat V, Monteil H, Bergogne-Berezin E. Penetration of piperacillin–tazobactam into bronchial secretions after multiple doses to intensive care patients. Antimicrobial Agents Chemother. 1994;38:2780–2784. doi: 10.1128/aac.38.12.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honeybourne D, Andrews JM, Ashby JP, Lodwick R, Wise R. Evaluation of the penetration of ciprofloxacin and amoxycillin into the bronchial mucosa. Thorax. 1988;43:715–719. doi: 10.1136/thx.43.9.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schentag JJ. Clinical significance of antibiotic tissue penetration. Clin Pharmacokinet. 1989;16(Suppl. 1):25–31. doi: 10.2165/00003088-198900161-00005. [DOI] [PubMed] [Google Scholar]
- 10.The BAL Cooperative Group Steering Committee. Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am Rev Respir Dis. 1990;141:S166–S202. doi: 10.1164/ajrccm/141.5_Pt_2.S169. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin DR, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob Agent Chemother. 1992;36:1171–1175. doi: 10.1128/aac.36.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hand WL, King-Thompson NL, Steinberg TH. Interactions of antibiotics and phagocytes. J Antimicrob Chemother. 1983;12(Suppl. C):1–11. doi: 10.1093/jac/12.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- 13.Harf R, Panteix G, Desnottes F, Diallo N, Leciereq M. Spiramycin uptake by alveolar macrophages. J Antimicrob Chemother. 1988;22(Suppl. B):135–140. doi: 10.1093/jac/22.supplement_b.135. [DOI] [PubMed] [Google Scholar]
- 14.Le Conte P, Potel G, Peltier P, et al. Lung distribution and pharmacokinetics of aerosolized tobramycin. Am Rev Respir Dis. 1993;147:1279–1282. doi: 10.1164/ajrccm/147.5.1279. [DOI] [PubMed] [Google Scholar]
- 15.Wollmer P, Pride NB, Rhodes CG, et al. Measurement of pulmonary erythromycin concentration in patients with lobar pneumonia by means of positron tomography. Lancet. 1982;2:1361–1364. doi: 10.1016/s0140-6736(82)91269-7. [DOI] [PubMed] [Google Scholar]
- 16.Ali I, Unruh H. Management of empyema thoracis. Ann Thor Surg. 1990;55:355–359. doi: 10.1016/0003-4975(90)90474-k. [DOI] [PubMed] [Google Scholar]
- 17.Renner H, Gabor S, Pinter H, Maier A, Friehs G, Smolle-Jüttner FM. Is aggressive surgery in pleural empyema justified? Eur J Cardiothorac Surg. 1998;14:117–122. doi: 10.1016/s1010-7940(98)00165-1. [DOI] [PubMed] [Google Scholar]
- 18.Maier A, Domej W, Anegg U, et al. CT or ultrasonically guided pigtail catheter drainage in multiloculated pleural empyema: a recommended procedure? Respirology. 2000;5:119–124. doi: 10.1046/j.1440-1843.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- 19.Müller M, Haag O, Burgdorff T, et al. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob Agents Chemother. 1996;40:2703–2709. doi: 10.1128/aac.40.12.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ståhle L, Arner P, Ungerstedt U. Drug distribution studies with microdialysis III. Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 1991;49:1853–1858. doi: 10.1016/0024-3205(91)90488-w. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Wong SL, Sawchuk RJ. Microdialysis calibration using retrodialysis zeronet flux: application to a study of the distribution of zidovudine to rabbit cerebrospinal fluid and thalamus. Pharm Res. 1993;10:1411–1419. doi: 10.1023/a:1018906821725. [DOI] [PubMed] [Google Scholar]
- 22.Perry CM, Morkham A. Piperacillin/tazobactam: an updated review of its use in the treatment of bacterial infections. Drugs. 1999;57:805–843. doi: 10.2165/00003495-199957050-00017. [DOI] [PubMed] [Google Scholar]
- 23.Mouton Y, Leroy O, Beuscart C. Efficacy, safety and tolerance of parenteral piperacillin/tazobactam in the treatment of patients with lower respiratory tract infections. J Antimicrob Chemother. 1993;31(Suppl. A):87–95. doi: 10.1093/jac/31.suppl_a.87. [DOI] [PubMed] [Google Scholar]
- 24.Sanders CV., Jr Piperacillin/tazobactam in the treatment of community-acquired and nosocomial respiratory tract infections: a review. Intensive Care Med. 1994;20:21–26. doi: 10.1007/BF01745247. [DOI] [PubMed] [Google Scholar]
- 25.Smith DL. Parenteral piperacillin/tazobactam with amikacin for the treatment of severe pulmonary infections in intensive care units. Infect Dis Clin Pract. 1995;4(Suppl. 1):33–36. [Google Scholar]
- 26.Ocampo AP, Hoyt KD, Wadgaonkar N, Carver AH, Puglisi CV. Determination of tazobactam and piperacillin in human serum, serum, bile and urine by gradient elution reversed-phase high-performance liquid chromatography. J Chromatogr Biomed Applications. 1989;496:167–179. doi: 10.1016/s0378-4347(00)82563-3. [DOI] [PubMed] [Google Scholar]
- 27.Kinetica 2000. Philadelphia, PA: InnaPhase Corp; 2000. Version 3. [Google Scholar]
- 28.Stahle L. Pharmacokinetic estimations from microdialysis data. Eur J Clin Pharmacol. 1992;43:289–294. doi: 10.1007/BF02333025. [DOI] [PubMed] [Google Scholar]
- 29.Lönnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987;253:E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- 30.Müller M, Schmid R, Georgopoulos A, Buxbaum A, Wasicek C, Eichler HG. Application of microdialysis to clinical pharmacokinetics in humans. Clin Pharmacol Ther. 1995;57:371–380. doi: 10.1016/0009-9236(95)90205-8. [DOI] [PubMed] [Google Scholar]
- 31.Ryan DM. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J Antimicrob Chemother. 1993;31(Suppl. D):1–16. doi: 10.1093/jac/31.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- 32.FDA. Developing Antimicrobial Drugs—General Considerations for Clinical Trials. Guidance for Industry. Published at: http://www.fda.gov/cder/guidance/2580dft.pdf.
- 33.The European Agency for the Evaluation of Medicinal Products. Evaluation of Medicines for Human UsePoints to Consider on Pharmacokinetics and Pharmacodynamics in the Development of Antibacterial Medicinal Products. London: CPMP/EWP/2655/ 99 Committee for Proprietary Medicinal Products (CPMP). Published electronically at: http://www.emea.eu.int/pdfs/human/ewp/265599en.pdf.
- 34.Department of Health and Human Service, Food and Drug Administration. Guidance Documents on Developing Antimicrobial Drugs. Vol. 496. Washington, DC: Miller Reporting Co., Inc; 1989. pp. 167–179. Anti-infective drugs advisory committee meeting, 64th meeting. [Google Scholar]
- 35.Honeybourne D, Baldwin DR. The site concentrations of antimicrobial agents in the lung. J Antimicrob Chemother. 1992;30:249–260. doi: 10.1093/jac/30.3.249. [DOI] [PubMed] [Google Scholar]
- 36.Perry CM, Markham A. Piperacillin/Tazobactam: an updated review of its use in the treatment of bacterial infections. Drugs. 1999;57:805–843. doi: 10.2165/00003495-199957050-00017. [DOI] [PubMed] [Google Scholar]
- 37.Müller M, Rohde B, Kovar A, Georgopoulos A, Eichler HG, Derendorf H. Relationship between serum and free interstitial concentrations of Cefodizime and Cefpirome in muscle and subcutaneous adipose tissue of healthy volunteers measured by microdialysis. J Clin Pharmacol. 1997;37:1008–1113. doi: 10.1002/j.1552-4604.1997.tb04294.x. [DOI] [PubMed] [Google Scholar]