Abstract
Aims
Cough, one of the main symptoms of bronchial asthma, is a chronic airway inflammatory disease with functionally damaged bronchial epithelium. Recently, we established an animal model with cough hypersensitivity after antigen challenge and clearly showed the protective effect of carbocysteine in this model. This study was designed to investigate the clinical effect of carbocysteine for cough sensitivity in patients with bronchial asthma.
Methods
The effects of the two orally active mucoregulatory drugs, carbocysteine and ambroxol hydrochloride, on cough response to inhaled capsaicin were examined in 14 patients with stable asthma. Capsaicin cough threshold, defined as the lowest concentration of capsaicin eliciting five or more coughs, was measured as an index of airway cough sensitivity.
Results
Geometric mean values of the cough threshold at run-in (baseline) and after 4 weeks’ treatment of placebo, 1500 mg day−1 of carbocysteine and 45 mg day−1 of ambroxol hydrochloride were 12.8 µM (95% confidence interval [CI] 5.5, 29.6), 11.0 µM (95% CI 4.4, 27.5), 21.0 µM (95% CI 8.8, 50.2) and 11.6 µM (95% CI 5.8, 23.3), respectively. The cough threshold for carbocysteine was significantly greater than those of ambroxol hydrochloride (P = 0.047) and placebo (P = 0.047), respectively.
Conclusions
These findings indicate that carbocysteine administration may be a novel therapeutic option for asthmatic patients, especially with cough variant asthma.
Keywords: airway cough sensitivity, bronchial asthma, capsaicin, carbocysteine, neutral endopeptidase
Introduction
Chronic cough is one of the most common symptoms of respiratory disease, which can interfere with social activities and adversely affect the quality of patients’ lives [1]. It is well known that chronic nonproductive cough is a main symptom in bronchial asthma. A previous study revealed that patients with persistent cough had three times the risk of developing chronic wheeze compared with normal subjects [2]. Thus, it is important to investigate the treatment and the mechanism for persistent cough, but the mechanism by which the cough reflex may be altered in humans remains unclear. Cough is considered to be a result of stimulation of airway sensory nerve endings by inhaled particles or irritants [1]. Capsaicin, which is the active ingredient of red pepper, is a commonly used cough-receptor stimulant and has been presumed to produce cough mainly by stimulating C-fibre endings [3–5].
Recently, it has been postulated that bronchial asthma is a chronic airway inflammatory disease with functional damage as well as destruction and desquamation of bronchial epithelium [6], which may lead to bronchial hyper-responsiveness [7]. Therefore, successful treatment of this condition should decrease the cough sensitivity to an inhaled tussigen. After mucosal injury induced by inflammatory diseases, infiltrating cells and activated resident cells release a wide spectrum of secretagogues that promote mucus secretion by direct stimulation of secretary cells or by mucus gland hyperplasia [8, 9].
Although carbocysteine (molecular formula C5H9NO4S, molecular mass 179.19; Kyorin Pharmaceutical Co. Ltd, Tokyo, Japan) is a well-known mucoactive and mucoregulatory drug, in current clinical use for the therapy of patients suffering from mucus hypersecretion, several studies indicate that carbocysteine does not have a direct mucolytic activity [10, 11]. Colombo and colleagues [10] indicated that carbocysteine is able to increase the chloride transport in the airway epithelium, contributing to its mucoregulatory action. Daffonchio and coworkers [11] also demonstrated that carbocysteine can ameliorate impairment of mucociliary clearance induced by human neutrophil elastase, possibly through a direct inhibition of the mucus discharge mediated by elastase. Together with these findings, carbocysteine possesses properties additional to direct mucus regulation which might contribute to its clinical efficacy. Recently, we succeeded in developing an animal model for increased cough sensitivity in allergic bronchitis and clearly showed the antitussive effect of carbocysteine on cough hypersensitivity with restoration of depressed neural endopeptidase (NEP) activity in tracheal tissue [12, 13].
This study was designed to investigate the effect of carbocysteine on cough sensitivity to inhaled capsaicin in asthmatic patients in its clinical use.
Subjects and methods
Subjects
A total of 14 patients with bronchial asthma (five males and nine females) with a mean age of 45.4 ± 4.5 (± SEM) (range 19–72) years participated in this study. All patients were lifetime nonsmokers or ex-smokers with no history of viral infection for at least 4 weeks prior to the study. Characteristics of individual patients are shown in Table 1. Informed consent was obtained from all subjects. This study was approved by the ethics committee of our hospital.
Table 1.
Clinical characteristics of patients.
| Patient number | Age (yr) | Sex | Height (cm) | Type | Severity | Total IgE in serum (IU/ml) | Specific IgE in serum | Complication of allergic disease | PC20-FEVt (mg/ml)* | BDP (µg/day) | Treatment Theophylline (mg/day) | Clenbuterol (µg/day) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | F | 160 | Ext | Moderate | 204 | HD, Mite | AR | 2.50 | 400 | 400 | 40 |
| 2 | 33 | M | 176 | Ext | Moderate | 39 | HD, Ceder | AR | 1.25 | 400 | 200 | 0 |
| 3 | 72 | M | 165 | Ext | Mild | 325 | Mite, Ceder | AR | 2.50 | 0 | 600 | 30 |
| 4 | 68 | M | 138 | Ext | Mild | 137 | Ceder | – | 0.16 | 0 | 300 | 0 |
| 5 | 66 | F | 146 | Int | Moderate | 2 | – | – | 0.63 | 400 | 400 | 0 |
| 6 | 19 | F | 183 | Ext | Moderate | 86 | HD, Cedex | – | 2.5 | 400 | 600 | 30 |
| 7 | 32 | M | 150 | Ext | Moderate | 689 | HD | – | 0.31 | 800 | 600 | 30 |
| 8 | 65 | F | 143 | Int | Mild | 2 | – | – | 2.50 | 0 | 400 | 40 |
| 9 | 52 | M | 167 | Int | Mild | 3 | – | – | 0.63 | 0 | 400 | 40 |
| 10 | 36 | F | 157 | Ext | Mild | 143 | HD | AR, UR | 1.25 | 0 | 200 | 0 |
| 11 | 53 | F | 160 | Ext | Mild | 357 | HD, Ceder | AR | 2.50 | 200 | 400 | 40 |
| 12 | 42 | F | 152 | Ext | Mild | 432 | HD, Ceder | AR | 1.25 | 0 | 400 | 40 |
| 13 | 30 | F | 163 | Ext | Moderate | 42 | Mite, Ceder | – | 2.50 | 400 | 0 | 0 |
| 14 | 34 | F | 160 | Ext | Mild | 155 | HD | AR | 0.31 | 200 | 400 | 0 |
Ext, extrinsic; Int, intrinsic; HD, house dust; AR allergic rhinitis; UR, urticaria; BDP, beelomerhasone diproprionate inlialation.
PC20-FEV1 shows concentration of inhaled merhaclvoline causing a 20% fall in FEV1.
All patients used inhaled β2-agonists (salbutamal or procaterol) on demand.
Each asthmatic patient satisfied the American Thoracic Society definition of asthma, with symptoms of episodic wheezing, cough, and shortness of breath responding to bronchodilators, and reversible airflow obstruction documented on at least one previous pulmonary function study [14]. Reversibility was defined as> 12% increase in the forced expiratory volume in 1 s (FEV1) following a bronchodilator inhalation. All patients had bronchial hyper-responsiveness as shown in Table 1.
This study was carried out when symptoms were mild and stable, while patients were taking oral theophylline; Nikken Chemical Co., Tokyo, Japan, oral (short-acting clenbuterol; Teijin Ltd, Osaka, Japan) and/or aerosol β2-agonists (short-acting procaterol; Otsuka Pharmaceutical Co. Ltd, Tokyo, Japan), and/or inhaled steroids (beclomethasone dipropionate; Kline KK, Tokyo, Japan) (Table 1). They had not received systemic corticosteroids or visited an emergent room for at least 8 weeks.
Assessment of cough receptor sensitivity to inhaled capsaicin
Cough receptor sensitivity was measured by capsaicin provocation test [15]. Capsaicin (30.5 mg) was dissolved in Tween 80 (1 ml) and ethanol (1 ml) and then dissolved in physiological saline (8 ml) to make a stock solution of 1 × 10−2 M, which was stored at −20°C. This solution was diluted with physiological saline to make solutions starting at a concentration of 0.49 µM and increasing it by doubling concentrations up to 1000 µM. Each subject inhaled a control solution of physiological saline followed by progressively increasing concentrations of the capsaicin solution. Solutions were inhaled for 15 s every 60 s, by tidal mouth-breathing, wearing a noseclip from a Bennett twin nebulizer (3012–60cc; Puritan-Bennett Co., Carlsbad, CA, USA). Increasing concentrations were inhaled until five or more coughs were elicited. The nebulizer output was 0.21 ml min−1. The number of capsaicin-induced coughs was counted by a blinded medical technician in our pulmonary function laboratory. The cough threshold was defined as the lowest concentration of capsaicin that elicited five or more coughs.
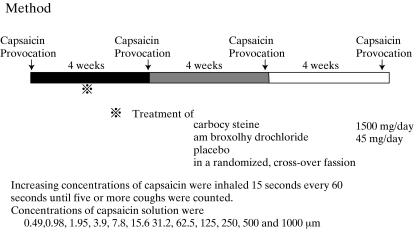
Study protocol (Figure 1)
Figure 1.
Study protocol
The medication was stopped at 21.00 h on the previous day to allow a washout time of 12 h or more before the measurement of cough threshold to inhaled capsaicin at 10.00 h on each test day to reduce the diurnal variability of cough response.
Each patient attended four times separated by 4 weeks, at the same time each day. Control measurement of capsaicin cough threshold was carried out before the first treatment in this study. Then treatment with carbocysteine, ambroxol hydrochloride (Teijin Ltd) and placebo was performed in a randomized, crossover fashion. Two carbocysteine tablets (500 mg), an ambroxol hydrochloride tablet (150 mg) and their placebo were taken orally three times a day for 28 days and at 08.00 h on the test day. FEV1 was measured on a dry wedge spirometer (Transfer Test, P.K. Morgan Ltd, London, UK) before capsaicin challenge to assess the bronchoactive effect of the treatment regimens. Serum transaminases, lactic dehydrogenase, alkaline phosphatase, γ-glutamyltranspeptidase, total bilirubin, blood urea, serum creatinine and serum uric acid were measured to assess adverse reactions caused by the test drugs.
Data analysis
Capsaicin cough threshold values were expressed as geometric means and median with 95% confidence interval (CI). Forced vital capacity (FVC) and FEV1 were shown as arithmetic mean values ± SEM The cough threshold values, the FVC values and the FEV1 values were compared between each pair of the four groups (control, carbocysteine, ambroxol hydrochloride and placebo) by the Wilcoxon signed ranks test. Data are transformed to logarithmic values for cough threshold at this test. A P-value ≤ 0.05 was taken as significant.
Results
Geometric mean values and medians of the cough threshold to inhaled capsaicin before treatment (control) and after treatment with carbocysteine, ambroxol hydrochloride and placebo are shown in Table 2. Baseline (control) cough threshold was heightened compared with our previous report because some patients had been using inhaled corticosteroid therapy [15–17]. The cough threshold after carbocysteine treatment was significantly greater than the values after placebo (P = 0.047) and ambroxol hydrochloride (P = 0.047), respectively. FVC or FEV1 values were not significantly different between control and the three treatments.
Table 2.
Pulmonary function and capsaicin cough threshold on carbocysteine, ambroxol hydrochloride and placebo treatments in patients with stable bronchial asthma.
| Control | Placebo | Carbocysteine | Ambroxol hydrochloride | |
|---|---|---|---|---|
| FVC as % pred. (%) | 98.7 ± 8.0 | 94.0 ± 8.2 | 99.2 ± 7.3 | 97.1 ± 7.5 |
| FEV1 as % pred. (%) | 88.1 ± 7.5 | 86.0 ± 6.9 | 90.8 ± 6.5 | 88.8 ± 6.9 |
| Cough threshold (κM) | ||||
| Geometric mean | 12.8 (5.5, 29.6) | 11.0 (4.4, 27.5) | 21.0 (8.8, 50.2)* | 11.6 (5.8, 23.3) |
| Mean values of difference† | ||||
| Treatment regiments vs. control | – | 0.86 (0.60, 1.23) | 1.64 (1.01, 2.66) | 0.91 (0.62, 1.32) |
| Mucoregulatory drugs vs. placebo | – | 1.90 (1.05, 3.46) | 1.05 (0.63, 1.76) | |
| Carbocysteine vs. ambroxolhydrochloride | 1.81 (1.06, 3.11) | – | ||
| Median | 7.8 (3.9, 125.0) | 7.8 (3.9, 31.2) | 15.6 (3.9, 125.0) | 7.8 (3.9, 62.5) |
| Mean values of difference‡ | ||||
| Treatment regiments vs. control | – | 0.00 (−3.90, 62.5) | 3.90 (0.00, 54.70) | 0.00 (−31.25, 3.90) |
| Mucoregulatory drugs vs. placebo | – | 3.90 (0.00, 29.30) | 0.00 (−62.50, 11.70) | |
| Carbocysteine vs. ambroxolhydrochloride | 5.85 (0.00, 64.45) | – | ||
Data are shown as standard error of the mean for FVC and FEV1, and as geometric mean value and median (95% CI) for capsaicin cough threshold, respectively.
P < 0.05 compared with placebo and ambroxol hydrochloride treatment respectively (Wilcoxon signed-ranks test data are transformed to logarithmic values for cough threshold).
If 95% CI does not include 1, showing significant difference between each group.
If 95% CI does not include 0, showing significant difference between each group.
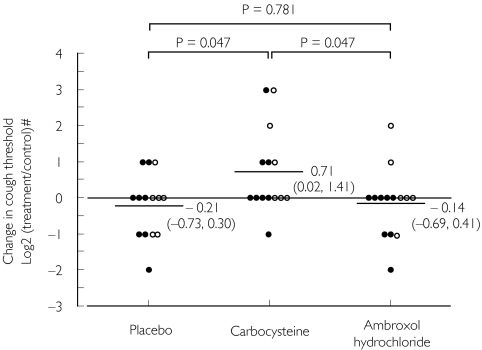
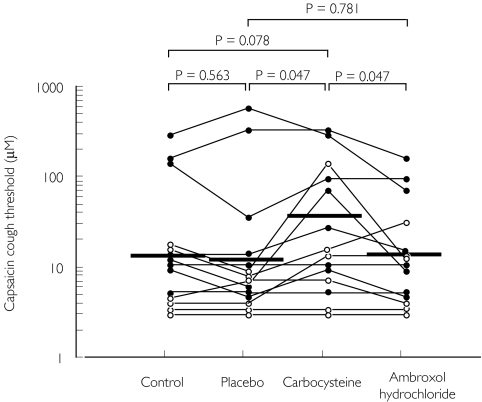
Changes in cough threshold by treatment with carbocysteine, ambroxol hydrochloride or placebo in relation to the control value are shown in Figure 2. The change with carbocysteine (0.71 doubling concentration) was significantly greater than that with ambroxol hydrochloride and placebo (−0.14 and −0.21 doubling concentration; P = 0.047 and P = 0.047, respectively), while the change with ambroxol hydrochloride was not different from that with placebo treatment (P = 0.781). These statistical analyses of cough threshold are as shown in Table 2. Individual cough threshold values for patients are shown in Figure 3. Adverse reactions in serological findings were not detected.
Figure 2.
Change in capsaicin cough threshold by carbocysteine, ambroxol hydrochloride or placebo treatment from the control value in patients with stable bronchial asthma. Capsaicin cough threshold was defined as the lowest concentration of capsaicin solution eliciting five or more coughs. •, Patients undergoing steroid inhalation therapy;, patients without steroid inhalation therapy. #Log 2 (capsaicin cough threshold with each treatment/control capsaicin cough threshold). Each horizontal bar represents mean value of log 2 threshold ratio, and dotted line shows control level. Values in parentheses represent 95% confidence intervals. P-values: Wilcoxon signed ranks test.
Figure 3.
Individual data of capsaicin cough threshold before treatment (control), and after placebo, carbocysteine and ambroxol hydrochloride treatments in patients with stable bronchial asthma. Each horizontal bar represents geometric mean value. •, Patients undergoing steroid inhalation therapy;, patients without steroid inhalation therapy. P-values: Wilcoxon signed ranks test using logarithmically transformed values.
Discussion
The present study shows that 4 weeks’ treatment with a mucoregulatory drug, carbocysteine, increased the cough threshold to inhaled capsaicin in asthmatic patients. In contrast, another mucoregulatory drug, ambroxol hydrochloride, did not affect the cough threshold. No difference could be found in the baseline pulmonary function among the carbocysteine, ambroxol hydrochloride and placebo treatments. From these findings, oral carbocysteine may be useful through its antitussive effect in patients with bronchial asthma, especially in cough variant asthma.
Although cough is an important mechanism for the protection and clearance of the airway, chronic cough can profoundly and adversely affect the quality of patients’ lives. It is well known that cough can be the sole manifestation in some asthmatic patients [18], however, the mechanisms associated with cough in bronchial asthma remain obscure. Salem and colleagues [19] proposed that cough receptors are stimulated by local bronchoconstriction. This may be one of the causes of cough in bronchial asthma, since another study described asthmatic patients for whom cough was the predominant symptom: pulmonary function tests revealed the narrowing of the central airways, whereas other asthmatic patients with predominant complaints of dyspnoea had narrowing of the peripheral airways [20]. However, recent studies about the variant form of asthma, called cough variant asthma (CVA), revealed normal baseline pulmonary functions and mild bronchial hyper-responsiveness [18, 21, 22]. Another study reported that inhaled prostaglandin E2, which acts as a bronchodilator, enhances cough sensitivity [23, 24]. Our previous study has also demonstrated that inhaled procaterol in a dose enough to produce bronchodilation has no effect on airway cough receptor sensitivity [16]. These findings suggest that other therapeutic options may be available in chronic cough based on bronchial asthma, in addition to bronchodilation therapy suggested by Salem et al.[19]. Cough is considered to result from stimulation of sensory nerve endings in the airway [1]. Two types of cough receptors are present in the larynx: myelinated irregularly firing irritant receptors, and nonmyelinated C-fibre endings. The tracheobronchial tree has also two types of cough receptors: myelinated rapidly adapting receptors (or ‘irritant receptors’), and nonmyelinated bronchial C-fibre endings. It has been postulated that capsaicin, the active ingredient of red pepper, produces cough mainly by stimulating C-fibre endings [3–5]. Thus, cough reflex testing using capsaicin has commonly been used for studies on the pathophysiology of cough reflex and antitussive effects of drugs. In normal subjects, the reproducibility of the dose–response curve for capsaicin-induced cough has been well established when the challenge is repeated at an interval of> 15 min [25]. Successive reports have confirmed the reproducibility of sensitivity as well as of dose–response curve for capsaicin-induced cough, including 15-s inhalation cough challenge used in this study [26, 27]. Our previous study showed that intrinsic thromboxane A2 (TxA2) may be a possible modulator augmenting airway cough sensitivity in stable asthmatics, and the reduction of cough reflex sensitivity might have clinical implications [17]. However, the exact mechanism by which the cough reflex may be altered in humans remains obscure.
Recently, chronic desquamative eosinophilic bronchitis has been considered a fundamental feature of bronchial asthma [6]. Subsequent mucus hypersecretion, mucosal oedema and bronchial hyper-responsiveness are considered the pathological and physiological features contributing to the airway obstruction [8]. In such an inflammatory process, infiltrating and activated cells release a wide spectrum of secretagogues which promote mucus secretion by direct stimulation of secretary cells or by mucus gland hyperplasia. Mucus hypersecretion contributing to airflow limitation seems to result from the release of eicosanoids, platelet-activating factor, histamine and eosinophil cationic protein in asthma [8, 9]. The findings described above indicate the contribution of airway inflammation to abnormal mucus secretion and plugging.
Carbocysteine is a mucoactive and mucoregulatory drug characterized by a spectrum of activities other than a direct effect on mucus secretion [10, 28]. Other researchers have revealed that carbocysteine exhibits a peculiar pharmacological effect in comparison with other mucoactive drugs [29, 30]. Martin and colleagues [29] revealed that carbocysteine has no effect on either mucus properties or mucociliary transport rate, and its reported effectiveness in vivo must be due to some mechanism other than solubilization of mucin. Another study has shown that carbocysteine can ameliorate impairment of mucociliary clearance induced by human neutrophil elastase, possibly through a direct inhibition of the mucus discharge mediated by elastase [11]. Though mucus hypersecretion and injury to airway epithelium are common findings of asthma, studies concerning the effects of carbocysteine in this disorder have been little discussed [31].
We recently established an animal model of cough hypersensitivity induced by antigen challenge and clearly demonstrated the protective effect of carbocysteine on antigen-induced airway cough hypersensitivity [12, 13]. Thus, we conducted this study to evaluate the clinical effect of carbocysteine on cough sensitivity in asthmatic airway and demonstrated that carbocysteine, but not ambroxol hydrochloride, decreased cough sensitivity. The precise mechanisms of this clinical effect of carbocysteine on cough sensitivity remain obscure, because we did not perform histological and/or rheological evaluation. Previous studies indicate that the protective effect associated with carbocysteine includes changes in sputum rheology accompanied by an increased ease of expectoration, improvement in ventilatory function and improvement of physiological barrier against external irritants [30, 32]. This finding may explain some role of carbocysteine, since two patients had a heightened cough threshold after ambroxol hydrochloride. However, failure in the treatment of ambroxol hydrochloride in this study suggests another underlying mechanism. We have already demonstrated that carbocysteine reduces cough sensitivity to inhaled capsaicin with restoration of depressed NEP activity in tracheal tissue following antigen challenge in guinea pigs in vivo[13]. Since we have shown that our animal model has the characteristics of an allergic eosinophilic bronchial disorder [12], we can consider that restoring NEP activity may be responsible for the clinical efficacy of carbocysteine observed in this study. This hypothesis is supported by the previous studies; airway epithelial damage enhances bronchoconstriction through decreasing NEP activity [33, 34].
In conclusion, the present study clearly indicates that carbocysteine can attenuate the cough sensitivity of asthmatic airway. Oral administration of carbocysteine may be a novel therapeutic option in patients with bronchial asthma, especially in CVA. This is the first report demonstrating the efficacy of carbocysteine in cough sensitivity in asthmatics through clinical trial. Further studies may be required to investigate the role of NEP in the treatment of carbocysteine for the asthmatic airway in vivo.
References
- 1.Irwin RS, Boulet LP, Cloutier MM, et al. Managing cough as a defense mechanism and as a symptom. Chest. 1998;114:133S–181S. doi: 10.1378/chest.114.2_supplement.133s. [DOI] [PubMed] [Google Scholar]
- 2.Giles GG, Gibson HB, Lickiss N, Shaw K. Respiratory symptoms in Tasmanian adolescents: a follow-up of the 1961 birth cohort. Aust NZ J Med. 1994;14:631–637. doi: 10.1111/j.1445-5994.1984.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 3.Coleridge HM, Coleridge JCG. Impulse activity in afferent vagal C-fibers with endings in the intrapulmonary airways of dogs. Respir Physiol. 1977;29:125–142. doi: 10.1016/0034-5687(77)90086-x. [DOI] [PubMed] [Google Scholar]
- 4.Fuller RW, Jackson DM. Physiology and treatment of cough. Thorax. 1990;45:425–430. doi: 10.1136/thx.45.6.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundberg JM, Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature. 1983;302:251–253. doi: 10.1038/302251a0. [DOI] [PubMed] [Google Scholar]
- 6.Bjornsdottir US, Quan SF, Busse WW. Eosinophils and asthma. In: Busse WW, Holgate ST, editors. Asthma and Rhinitis. Boston: Blackwell Scientific Publications; 1995. pp. 328–346. [Google Scholar]
- 7.Wardlaw AJ, Dunnetts S, Gleich GJ, Collins JV, Kay AB. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Am Rev Respir Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- 8.Lundgren JD, Baraniuk JN. Mucus secretion and inflammation. Pulmonary Pharmacol. 1992;5:81–96. doi: 10.1016/0952-0600(92)90024-b. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren JD, Shelhamer JH. Pathogenesis of airway mucus hypersecretion. J Allergy Clin Immunol. 1990;85:399–417. doi: 10.1016/0091-6749(90)90147-v. [DOI] [PubMed] [Google Scholar]
- 10.Colombo B, Turconi P, Daffonchino L, Fedele G, Omini C, Cremaschi D. Stimulation of Cl¯ secretion by the mucoactive drug S-carboxymethylcysteine lysine-salt in the isolated rabbit trachea. Eur Respir J. 1994;7:1622–1628. doi: 10.1183/09031936.94.07091622. [DOI] [PubMed] [Google Scholar]
- 11.Daffonchio L, De Santi MM, Gardi C, Lungarella G, Omini C. Effect of S-carboxymethylcysteine lysine salt on mucociliary clearance in rabbits with secretory cell metaplasia. Res Commun Mol Pathol Pharmacol. 1994;86:59–74. [PubMed] [Google Scholar]
- 12.Liu Q, Fujimura M, Tachibana H, Myou S, Kasahara K, Yasui M. Characterization of increased cough sensitivity after antigen challenge in guinea pigs. Clin Exp Allergy. 2001;31:474–484. doi: 10.1046/j.1365-2222.2001.00989.x. [DOI] [PubMed] [Google Scholar]
- 13.Katayama N, Fujimura M, Ueda A, et al. Effects of carbocysteine on antigen-induced increases in cough sensitivity and bronchial responsiveness in guinea pigs. J Pharmacol Exp Ther. 2001;297:975–980. [PubMed] [Google Scholar]
- 14.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 15.Fujimura M, Sakamoto S, Kamio Y, Matsuda T. Effects of methacholine-induced bronchoconstriction and procaterol-induced bronchodilation on cough receptor sensitivity to inhaled capsaicin and tartaric acid. Thorax. 1992;47:441–445. doi: 10.1136/thx.47.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimura M, Sakamoto S, Kamio Y, Bando T, Kurashima K, Matsuda T. Effect of inhaled procaterol on cough receptor sensitivity in patients with asthma or chronic bronchitis and in normal subjects. Thorax. 1993;48:615–618. doi: 10.1136/thx.48.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimura M, Kamio Y, Kasahara K, Bando T, Hashimoto T, Matsuda T. Prostanoids and cough response to capsaicin in asthma and chronic bronchitis. Eur Respir J. 1995;8:1499–1505. [PubMed] [Google Scholar]
- 18.Corrao WM, Braman SS, Irwin RS. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med. 1979;300:633–637. doi: 10.1056/NEJM197903223001201. [DOI] [PubMed] [Google Scholar]
- 19.Salem H, Aviado DM. Antitussive drugs. Am J Med Sci. 1964;247:585–600. [PubMed] [Google Scholar]
- 20.McFadden ER., Jr Exertional dyspnoea and cough as preludes to acute attacks of bronchial asthma. N Engl J Med. 1975;292:555–559. doi: 10.1056/NEJM197503132921103. [DOI] [PubMed] [Google Scholar]
- 21.O’Connell EJ, Rojas AR, Sachs MI. Cough-type asthma: a review. Ann Allergy. 1991;66:278–285. [PubMed] [Google Scholar]
- 22.Koh YY, Chae SA, Min KU. Cough variant asthma is associated with a higher wheezing threshold than classic asthma. Clin Exp Allergy. 1993;23:696–701. doi: 10.1111/j.1365-2222.1993.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 23.Stones R, Barnes PJ, Fuller RW. Contrasting effects of prostaglandins E2 and F2a on sensitivity of the human cough reflex. J Appl Physiol. 1992;73:649–653. doi: 10.1152/jappl.1992.73.2.649. [DOI] [PubMed] [Google Scholar]
- 24.Wasserman MA, Griffin RL, Marsalisi FB. Inhibition of bronchoconstriction by aerosols of prostaglandins E1 and E2. J Pharmacol Exp Ther. 1980;214:68–73. [PubMed] [Google Scholar]
- 25.Collier JG, Fuller RW. Capsaicin inhalation in man and the effects of sodium cromoglycate. Br J Pharmacol. 1984;81:113–117. doi: 10.1111/j.1476-5381.1984.tb10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Midgren B, Hansson L, Karlsson J, Simonsson BG, Persson CGA. Capsaicin-induced cough in humans. Am Rev Respir Dis. 1992;146:347–351. doi: 10.1164/ajrccm/146.2.347. [DOI] [PubMed] [Google Scholar]
- 27.Songur N, Fujimura M, Kamio Y. Comparison between tidal breathing and dosimeter methods in assessing cough receptor sensitivity to capsaicin. Respirology. 2000;5:337–342. [PubMed] [Google Scholar]
- 28.Brga PC, Allegra L, Rampoldi C, Ornaghi A, Beghi G. Long-lasting effect on rheology and clearance of bronchial mucus after short-term administration of high-doses of carbocysteine-lysine to patients with chronic bronchitis. Respiration. 1990;57:353–358. doi: 10.1159/000195871. [DOI] [PubMed] [Google Scholar]
- 29.Martin R, Litt M, Marriott C. The effect of mucolytic agents on the rheologic and transport properties of canine tracheal mucus. Am Rev Respir Dis. 1980;121:495–500. doi: 10.1164/arrd.1980.121.3.495. [DOI] [PubMed] [Google Scholar]
- 30.Brown DT. Carbocysteine. Drug Intell Clin Pharmacol. 1988;22:603–608. doi: 10.1177/106002808802200721. [DOI] [PubMed] [Google Scholar]
- 31.Asti C, Melillo G, Caselli GF, et al. Effectiveness of carbocysteine lysine salt monohydrate on models of airway inflammation and hyperresponsiveness. Pharmacol Res. 1995;31:387–392. doi: 10.1016/1043-6618(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 32.Allegra L, Cordaro CI, Grassi C. Prevention of acute exacerbation of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: a multi, double-blind, placebo-controlled trial. Respiration. 1996;63:174–180. doi: 10.1159/000196540. [DOI] [PubMed] [Google Scholar]
- 33.Cheung D, Bel EF, Hartigh JD, Dijkman JH, Sterk PJ. The effect of an inhaled neutral endopeptidase inhibitor, thiorphan, on airway responses to neurokinin A in normal human in vivo. Am Rev Respir Dis. 1992;145:1275–1280. doi: 10.1164/ajrccm/145.6.1275. [DOI] [PubMed] [Google Scholar]
- 34.Cheung D, Timmers MC, Zwinderman AH, Hartigh JD, Dijkman JH, Sterk PJ. Neural endopeptidase activity and airway hyperresponsiveness to neurokinin A in asthmatic subjects in vivo. Am Rev Respir Dis. 1993;148:1467–1473. doi: 10.1164/ajrccm/148.6_Pt_1.1467. [DOI] [PubMed] [Google Scholar]