Abstract
The function of the stomach includes initiation of digestion by exocrine secretions such as acid and pepsin, which are under the control of the endocrine secretion of hormones that also coordinate intestinal motility. The stomach also stores and mechanically disrupts ingested food. Various techniques have been developed to assess gastric physiology, the most important of which is assessment of acid secretion, as well as gastric motility and gastric emptying. The influence of drugs on gastric function and the effect of gastric secretion and mechanical actions on the bioavailability of novel compounds are of critical importance in drug development and hence to clinical pharmacologists. The control of acid secretion is essential in the treatment of peptic ulcer disease as well as gastrooesophageal reflux disease (GORD); pH-metry can be used to determine the necessary dose of an acid suppressant to heal mucosal damage. Disturbed gastric myoelectric activity leading to gastroparesis can cause delayed gastric emptying, often found in patients with diabetes mellitus. Electrogastrography (EGG) may be used to evaluate the influence of prokinetics and other drugs on this condition and aid in determining effective therapy.
Keywords: drug development, gastric function, EGG, gastroparesis, GORD, pH-metry
Introduction
Whilst for many clinicians, acid secretion is the most important aspect of gastric function that is studied in clinical practice, there are other facets that should be considered in drug development and measurements of gastric function. The stomach stores and grinds food to a suitable consistency to pass into the small intestine and initiates the process of digestion. Beyond the exocrine secretions listed in Table 1, endocrine activity coordinates gastric and intestinal tract activity. In addition, the stomach serves as a reservoir with controlled retention and release of chyme after its mechanical disruption by the antrum. The major tests of gastric function are summarized in Table 2.
Table 1. Physiologic functions of gastric exocrine secretions.
| Physiologic function | Secreted product |
|---|---|
| Killing or suppression of growth of ingested micro-organisms | H+ |
| Facilitation of duodenal inorganic iron absorption | H+ |
Stimulation of pancreatic  secretion via secretin release secretion via secretin release |
H+ |
| Suppression of antral gastrin release | H+ |
| Initiation of peptic hydrolysis of dietary proteins | H+, pepsin |
| Liberation of vitamin B12 from dietary protein | H+, pepsin |
| Binding of vitamin B12 for subsequent ileal uptake | Intrinsic factor |
| Initiation of hydrolysis of dietary triglycerides | Gastric lipase |
| Protection against noxious agents | Mucin, NaHCO3−, water, components of the mucus gel |
(adapted from [7])
Table 2. Gastric function tests.
| Exocrine secretory function | Intragastric pH-metry, analysis of gastric juice, Schilling's test |
| Endocrine function | Measurement of plasma hormone concentrations, for example gastrin |
| Myoelectrical and mechanical function | Antroduodenal manometry, gastric barostat, cutaneous electrogastrography (EGG) |
| Storage/emptying | Scintigraphy, 13C breath tests (acetate, octanoate) |
Endoscopy is necessary to exclude gastrointestinal tract pathologies such as ulcers or cancerous lesions; unfortunately, this widely utilized technique does not allow the assessment of gastric function. Several of the listed tests are only rarely used because they are difficult to interpret and results do not influence treatment. This paper discusses the most relevant of the gastric function tests to clinical pharmacologists and evaluates the current role of these tests in clinical practice. Those aspects of gastric function involved in different diseases that can be influenced by drugs or play a role in drug development will be addressed. The influence of changes in gastric function has thus to be taken into account in the development of drugs and their delivery systems.
The rate of gastric emptying plays an important role in the dynamics of drug absorption, especially for drugs classified as Class I of the Biopharmaceutics Classification System [1]. Gastric emptying, for example, determines the absorption rate of theophylline [2]. An important element affecting gastric motility are differences in the fed and fasted state of the stomach, since gastric motility is controlled by migrating motor complexes in the fasted state which regulate gastric emptying [3].
Assessment of gastric function
Exocrine secretory function
Intragastric and oesophageal pH-metry
By analogy to the more popular 24 h pH-metry of the oesophagus, the pH of the stomach can also be monitored by pH-electrodes. The main use of this technique is the assessment of patients with reflux disease with persistent symptoms despite the use of standard doses of a proton pump inhibitor. In these patients, pH-metry is used to exclude nocturnal acid breakthrough that can be treated by the addition of a histamine H2-receptor antagonist at night [4]. In patients with typical symptoms of gastrooesophageal reflux without oesophagitis, oesophageal pH-metry is necessary to establish the diagnosis of reflux disease grade 0 (Savary Miller or Los Angeles classification).
In drug development and evaluation, this technique can be used to determine the length of time during which the pH in the stomach rises above 4.0. In gastrooesophageal reflux disease (GORD), for example, this parameter correlates with treatment success. It is known that that the degree of oesophageal mucosal injury depends on duration of exposure and the pH of the refluxate. Evidence suggests that the duration of an intraoesophageal pH of less than 4.0 correlates with the degree of mucosal injury [5].
Intragastric pH-metry can also be used in drug development to exclude the effect of a drug on gastric acid secretion, for example, pharmacological doses of nonsteroidal anti-inflammatory drugs [6]. To determine transient or localized changes in pH, multiple, rather than single electrodes can be used [7].
Analysis of gastric juice
The analysis of gastric juice is a direct technique to assess the acid secretory potential of the stomach. In clinical practice, this method can be utilized to determine achlorhydria in patients with autoimmune type gastritis or to secure the diagnosis of gastrinoma (Zollinger–Ellison syndrome). In both clinical settings, this test is no longer mandatory since either disease can now be diagnosed by noninvasive techniques, for example, the secretin-gastrin provocation test.
This test, however, is useful in the development of acid suppressants drugs such as proton pump inhibitors. Firstly, it can be used to document a normal acid secretory capacity in subjects for assessment of proton pump inhibitor efficacy and secondly, to assess the extent of, or exclude residual acid secretory potential when taking proton pump inhibitors [8].
Schilling's test
Schilling's test is a scintigraphic method to determine the cause of vitamin B12 deficiency. Malabsorption of vitamin B12 may be either caused by the lack of intrinsic factor produced in the parietal cells of the stomach or by malabsorption of the vitamin B12-intrinsic factor complex in the terminal ileum, as in inflammatory bowel disease. Certain drugs may interfere with the utilization of vitamin B12 in the bone marrow, although Schilling's test is not now used in the development of drugs influencing the gastrointestinal tract.
Endocrine function: Measurement of plasma gastrin, secretin-gastrin provocation
The peptide hormone gastrin regulates gastric acid secretion via enterochromaffin-like (ECL) cells that secrete histamine to stimulate acid secretion by parietal cells of the oxyntic (gastric body, acid secreting) mucosa [9]. Raised gastrin concentrations can be found in all patients with achlorhydria regardless of the cause (for example, following treatment with drugs inhibiting gastric acid secretion such as proton pump inhibitors or histamine H2-receptor antagonists; autoimmune gastritis) with the proviso that G cells remain intact. In patients with Zollinger–Ellison syndrome, elevated gastrin concentrations result in acid hypersecretion due to the influence of the regulatory pathway as previously described.
To confirm the diagnosis of gastrinoma, the secretin-gastrin provocation test can be used. Intravenous secretin (1 I.E. kg−1) results in a rise of plasma gastrin by at least 100% or 200 pg ml−1 in patients with this neuroendocrine disorder. Together with the symptoms typical of a gastrinoma and the results of scintigraphic and radiologic studies, the diagnosis of Zollinger–Ellison syndrome can be established without using the invasive procedure of gastric juice analysis.
Neither plasma gastrin concentrations nor secretin-gastrin provocation are of use in drug development, but the rise in plasma gastrin during therapy with a proton pump inhibitor can be used as a surrogate marker of efficacy in acid suppression.
Myoelectrical and mechanical function
Antroduodenal manometry
This technique records motility of the antrum and duodenum. The method is similar to oesophageal manometry, but is not often used in clinical practice. Indications include the assessment of intestinal pseudo-obstruction.
The procedure is only rarely used as it is time consuming (the test can take up to 5 h), requires special equipment, and results have little clinical value. A recently published study reported that the results had influenced treatment and/or yielded a previously unknown diagnosis in less than 30% of patients evaluated by this technique [10]. In about 70% of the studies with an abnormal outcome, only nonspecific abnormalities were found.
Thus, this technique, which is infrequently used in clinical practice, is not applied in drug development.
Gastric barostat
The gastric barostat measures the basal tone and compliance of the gastric wall. This technique can be used to assess relaxation of the gastric wall, which may be abnormal in patients with an autonomic neuropathy (for example, in long standing diabetes mellitus).
Similar to antroduodenal manometry, this technique is only rarely used as it is expensive and the information gained is very limited. Again, this type of study is not routinely used in drug development.
Cutaneous electrogastrography (EGG)
The electrical control activity or ‘pacemaker’ of the stomach can be recorded by surface electrodes, similar to an electrocardiogram. A typical recording of this activity in a healthy subject is shown in Figure 1.
Figure 1.
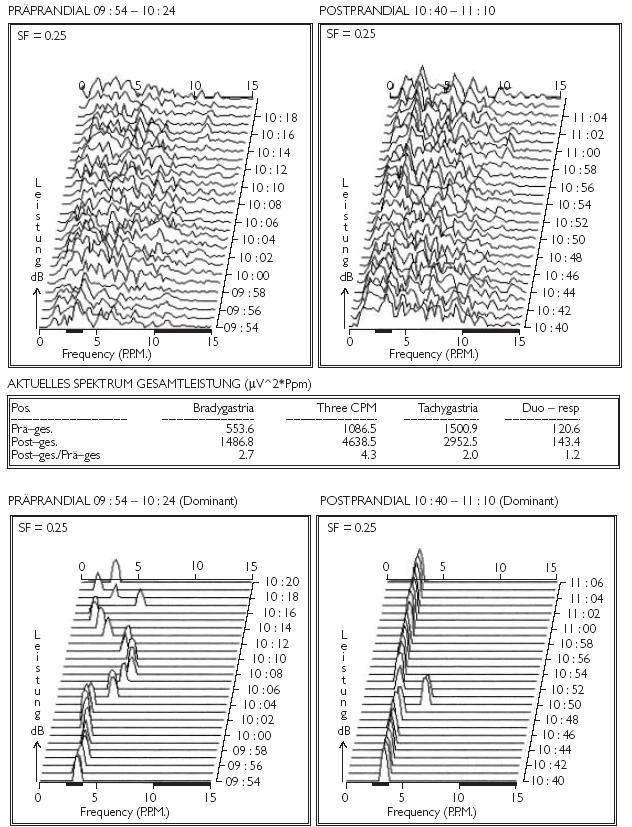
Electrogastrography recordings of a healthy subject: typical images of a regular EGG; rare episodes of preprandial tachygastria which convert to a regular 3 cycles per minute rhythm after ingestion of a test meal with simultaneously increased power
Tachygastria, with frequencies of more than 3 min−1, has been shown to correlate with nausea. Some patients with dyspepsia have an abnormal EGG, in most cases tachygastria [11]. Alterations in frequency (bradygastria, tachygastria, or a mixed pattern) and reduction of amplitude of the postprandial electrical signal are reported in patients with idiopathic and diabetic gastroparesis, anorexia nervosa, motion sickness, and nausea of pregnancy [12, 13]. The precise value of this test in the clinical evaluation of patients or monitoring of therapeutic response to medication remains to be determined and is the subject of ongoing research.
Gastric storage and emptying
Scintigraphic assay of gastric emptying
Scintigraphy is the most popular technique to confirm the presence of postprandial gastric stasis. Prerequisites are a scintigraphic label that binds effectively to the substrate and this binding should be resistant to pH values in the range of 1–7.5 as well as to high concentrations of bile acid, as under differing pathologic conditions, the substrate can be exposed to this milieu for up to 6 h. The meal must have both a sufficient caloric content (typically> 200 kcal) and a solid consistency to induce the fed motility response. Cost effectiveness analyses have suggested that studies directly following the ingestion of the meal, 2, and 4 h later are sufficient for most clinical indications [14, 15].
13C breath tests
Apart from the above-mentioned ‘gold standard’ procedure to determine gastric emptying, scintigraphy, noninvasive and nonradioactive methods have been devised. 13C-labelled acetate can be used as a marker in liquid and semisolid test meals; 13C-labelled octanoate has been utilized to determine the emptying rate of the stomach for solids.
The results of a 13C-acetate breath test performed in a patient with diabetic gastroparesis before and after treatment are shown in Figure 2.
Figure 2.


13C-acetate breath test results of a patient with diabetic gastroparesis. (a) before treatment – markedly delayed gastric emptying and (b) after treatment – normal gastric emptying.
The advantage to the patient of these labels is the lack of exposure to radiation. 13C-labelled substances have been compared with appropriate scintigraphic labels to establish their accuracy. Evidence suggests that 13C-based breath tests are equivalent in the assessment of clinically relevant disturbances of gastric emptying [16, 17] and can be used in paediatric studies [18].
Even though postabsorptive processing of 13C octanoate has been a matter of concern, this does not seem to influence the validity of this test in the assessment of gastric emptying [19].
All of these tests assess the time a liquid or solid meal is retained in the stomach. Gastric emptying can be significantly shortened (for example, as a result of gastric surgery) or prolonged due to autonomic neuropathy (for example, due to diabetes).
A range of drugs including prokinetic agents may accelerate gastric emptying and hence these techniques can be applied to document the effect of such drugs on gastric motility.
Diseases associated with gastric ‘malfunction’ and their treatment
Gastrooesophageal reflux disease (GORD)
Gastrooesophageal reflux disease (GORD) occurs because of the inability of the lower oesophageal sphincter to protect the oesophagus from the gastric contents. The pathophysiological basis of reflux disease is therefore sphincter malfunction. Nevertheless, by increasing the pH of the refluxate towards neutral, both healing of the mucosal discontinuity and further oesophageal mucosal injury can be avoided. Drugs inhibiting gastric acid secretion such as proton pump inhibitors are the drugs of choice to treat GORD. As mentioned above, the key to successful treatment in GORD is the elevation of the gastric pH to values of at least 4.0.
As a consequence, pH-metry to assess the duration of the time of the pH values above 4.0 is of use in the development of novel drugs, and can be used to compare the efficacy of different doses or preparations. Thus, for example, using this technique, it has been documented that the S-isomer of omeprazole, esomeprazole, maintains gastric pH above 4.0 for a longer period of the day (12.7 h vs 10.5 h; 20 mg each), and more effectively elevates median intragastric pH (4.1 vs 3.6) than its parent substance, omeprazole [20, 21].
However, sequelae of reflux disease are not effectively controlled by the administration of proton pump inhibitors at standard doses in all patients. Recently, it has been suggested that the dose of a proton pump inhibitor should be tailored for each patient; oesophageal pH-metry was utilized to detect oesophageal acid exposure. Treatment was adjusted to ensure that a pH below 4.0 occurred in less than 1.6% of a 24-h period. Indeed, regression of Barrett's oesophagus has been observed to occur using this regimen [22].
Nocturnal acid breakthrough is another difficulty relating to therapeutic control of acid secretion by proton pump inhibitors. The evaluation of pH was utilized to document the effects of single and repeated doses of a histamine H2-receptor antagonist on these episodes; tolerance obscured the initial effectiveness of a combined therapy of proton pump inhibitor administered twice daily along with a histamine H2 receptor antagonist [23].
Another useful test to assess optimal treatment of GORD is oesophageal manometry. This technique records the peristalsis of the tubular oesophagus as well as lower oesophageal sphincter pressure and transient relaxations of the lower oesophageal sphincter (LES); all such aspects are important in the pathophysiology of reflux disease [24, 25]. In addition, acidic reflux may impair the propulsive peristalsis of the tubular oesophagus. Thus, manometry can be used to evaluate the restitution of tubular oesophageal function by drugs. Moreover, oesophageal manometry is mandatory if surgical correction of reflux disease is considered, as the procedure must be tailored to the degree of motility [26].
Use of 24-h ambulatory manometry may be superior to conventional stationary manometry in detecting ‘dropped’ or interrupted contractions, which indicate poor oesophageal motility in patients with GORD. This information may be of value in both design of treatment and prediction of the possible outcome [27].
The value of manometry in the development of novel drugs is to therefore to study their influence on lower oesophageal sphincter pressure and occurrence of transient sphincter relaxations as a pathophysiological basis of reflux disease [28]. Manometry can also be utilized to ensure correct positioning of the tip of the pH-metry probe distal to the lower oesophageal sphincter and is therefore an indirect aid in development of agents to treat gastrooesophageal reflux disease.
Gastroparesis
Gastroparesis is defined as ineffectual gastric emptying, where solids in particular are retained in the stomach. There are many causes of gastroparesis, including idiopathic forms, but the most common cause is long-standing diabetes mellitus. In some patients with gastroparesis, only liquids and particles smaller than 2 mm in diameter can be emptied, a finding that could be of use in the development of drug delivery systems for this disease.
Gastroparesis diabeticorum
Abnormal gastric motility occurs in about 60% of patients with diabetes mellitus [29, 30]. Gastric emptying, controlled via the vagus nerve, is grossly disturbed in these patients. Postprandial antral dysrhythmias such as antral tachygastria are common; in contrast, phase 3 contractions of the interdigestive migrating motor complex (IMMC) are frequently absent which results in poor antral grinding of the lumenal contents. Both maintenance of the gastroduodenal pressure gradient and receptive relaxation of the stomach are abnormal [30]. Pylorospasm may occur and cause functional resistance to gastric outflow [31]. Liquid emptying of the stomach may be normal, but emptying of solids is frequently delayed.
Aside from the symptoms of stasis such as epigastric distress, nausea, bloating, postprandial vomiting, and early satiety which distress the patient, the regulation of blood glucose concentrations can be disturbed and as hyperglycaemia further impairs gastric emptying, this may accelerate the onset of diabetic ketoacidosis.
The pathophysiology underlying the motor disturbances remains unclear. Acute hyperglycaemia can cause delayed gastric emptying [32]. High plasma concentrations of motilin have been reported in patients with gastroparesis. However, this may be a compensatory process, since the treatment with prokinetics can result in a fall of plasma motilin concentrations [33].The diagnosis of diabetic gastropathy should be strongly suspected from the clinical history. Methods to confirm the diagnosis are the exclusion of structural lesions such as ulceration or strictures using oesophago-gastroduodenoscopy, followed by techniques to assess the delay in gastric emptying. In addition, electrogastrography (EGG) may be used to determine the changes in postprandial gastric motor activity such as tachygastria, typical of diabetic gastroparesis. Tests used to confirm the diagnosis and assess the effect of therapy in this disease include radiolabelled scintigraphy, 13C-acetate and octanoate breath tests.
Treatment of gastroparesis
The management of both diabetic and idiopathic gastroparesis remains difficult. In diabetic gastroparesis, glycaemic control is important as lowered blood glucose concentrations may accelerate gastric emptying.
Prokinetic agents are the drugs most commonly used to increase gastric motor activity. Metoclopramide (10 mg 30 min before each meal and at bedtime) and domperidone (10–20 mg four times a day) increase gastric tone and accelerate gastric emptying in gastroparesis, coordinating these effects with pyloric relaxation and duodenal peristalsis [34]. However, the beneficial effect of these drugs often diminishes within 6 weeks, even though some patients continue to be symptom free [35].
Cisapride was clinically used (10–20 mg before each meal and at night), as this drug increases postprandial antral motility, normalizes both the fasting and fed motor patterns [36], and stimulates duodenal motility [37]. Studies have documented that cisapride is superior to metoclopramide in acceleration of gastric emptying in patients with diabetic gastroparesis [38]. Unfortunately, the provocation of cardiac arrhythmias leading to death have been associated with the use of cisapride, which has resulted in its withdrawal from clinical use.
The antibiotic erythromycin, known to be also a motilin agonist, accelerates gastric emptying in patients with diabetic gastroparesis. In an acute setting, infusions of 200 mg erythromycin over 10 min every 8 h seem to be effective in promoting this response [39]. Tachyphylaxis (rapid tolerance) to the drug remains a significant problem and precludes use over longer periods.
Tegaserod, a selective partial agonist to the 5-HT4 receptor, has been licensed for the treatment of constipation–predominant irritable bowel syndrome [40]. Studies in vivo have shown that tegaserod enhances motility at all levels of the gastrointestinal tract and stimulates gastric emptying in dogs [41]. This effect has also been demonstrated in human studies [42]. In contrast to cisapride, studies in vitro or in vivo have not demonstrated any relevant QTc prolongation by tegaserod [43]. Tegaserod has not yet been evaluated in humans for diabetic gastroparesis, but the animal studies and the safety profile suggest its potential usefulness.
A new treatment option is the inhibition of cholinergic neurotransmission in the pyloric muscle layers by quadrant injection of botulinum toxin. The body of evidence however, comprises only very few patients in these studies; one study reported six diabetic subjects with gastroparesis, in which the pyloric injection of botulinum toxin resulted in improved gastric emptying in 50%[44]. In a cohort of 10 patients with idiopathic gastroparesis, the injection of botulinum toxin into the pyloric sphincter improved solid emptying (at 4 h: from 27% to 14%[normal: < 10%]) as well as symptom score [15.3–9.0] at 4 weeks, effects that persisted for 3–6 months [45].
All the currently available medical therapies of gastroparesis are hampered by their lack of long-term efficacy. Therefore, long-term treatment modalities have been evaluated to restore the gastric myoelectric activity. Recently, the use of gastric electrical stimulation has been evaluated in the treatment of this disorder. The first report utilizing electrical stimulation to increase motor activity in the human stomach was reported in 1985; Waterfall et al. showed that it is possible to produce entrainment and pacing of gastric smooth muscle in humans, if sufficient energy was used [46]. Familoni et al. [47] were able to improve gastric emptying for up to 1 year in a young woman with diabetic gastroparesis utilizing gastric electrical stimulation.
In our institution, we have performed trials on 19 patients with gastroparesis refractory to medical therapy and found that gastric emptying was substantially accelerated and the severity of vomiting decreased by the use of an implanted permanent electrical stimulator. These effects could be observed at 6 and 12 months following the implant [48]. Side-effects of this treatment are rare and most commonly relate to surgery that is necessary to insert the pacemaker. We have recently observed one perforation of an electrode into the stomach (Figure 3); this is a rare complication (only the second documented according to Medtronic, the manufacturer of this device). Surgical correction can be easily achieved in this situation.
Figure 3.

Gastric pacemaker lead protruding into the lumen of the stomach (left): rare complication of a gastric pacemaker. Endoscopic view with inverted scope (visible on right hand side).
This technique using high-frequency gastric electrical stimulation improves dyspeptic symptoms such as nausea and vomiting, has recently been CE-certified (Enterra®, Medtronic, Tolochenaz/Switzerland). Nevertheless, most clinical studies reported so far are not controlled trials and have mainly published only in abstract form; therefore, further trials are needed to establish the place of these techniques in the treatment of gastroparesis [49].
Gastric function and ‘malfunction’– influence on pharmacokinetics and pharmacodynamics, drug–drug interactions
The speed of gastric emptying greatly influences the transit rate of large particle preparations, that rely on passage through the pylorus by the grinding action of the stomach. Preparations that dissolve or form particles of less than 2 mm in diameter pass through the stomach in a similar fashion to liquids and are less likely to be influenced by gastroparesis. Drugs exposed to acid for longer periods than intended may undergo decomposition and lower quantities of the active component may thus enter the intestine, events that could result in decreased bioavailability.
Knowledge regarding the influence of particle size on gastric retention and release has led to the development of drug delivery systems in the format of microgranules; examples are certain preparations of the proton-pump inhibitors, omeprazole and esomeprazole, as well as mesalazine, used for the therapy of inflammatory bowel disease [50].
Some drugs such as 5-aminosalicylate preparations are released in a pH-dependent fashion; in the case of gastric acid suppression due to atrophic gastritis or due to acid inhibiting drugs (proton pump inhibitors), peak plasma concentrations can be influenced and result in potential toxicity [51]. In a similar fashion, elevation of gastric pH may influence the bioavailability of unchanged digoxin because acid induces the breakdown of digoxin to inactive metabolites [52]. These findings are in contrast to those concerning itraconazole; its bioavailability can even be improved in hypochlorhydric patients by the coadministration of an acidic beverage such as cola [53]. In other cases, acidity does not seem to play a role at all [54].
Aside from gastroparesis due to disease, drugs such as opiates and some sedative agents may cause decreased gastric motility. This may result in indirect drug–drug interactions decreasing plasma peak concentrations. However, prokinetic agents such as metoclopramide can influence bioavailability of other drugs, including ranitidine, as gastric emptying is accelerated [55]. This drug–drug interaction can be expected because of their known (and in most cases) intended effects on gastric transit. On the other hand, other drugs commonly prescribed in clinical practice such as acid inhibitors or aspirin may influence gastric transit of other medication and alcohol [56, 57] resulting in unintended changes in bioavailability of these later substances.
Conclusions
Tests of gastric function are of value for many aspects in the development of drugs and their delivery systems. Intragastric and oesophageal pH-metry and analysis of gastric juice can be used to assess therapy of disorders associated with either increased acid output or gastrooesophageal reflux disease. The assessment of gastric emptying may be useful not only in the development of novel drugs to treat gastroparesis, but also to document the influence of gastric retention on the bioavailability of such medicines.
References
- 1.Amidon GL, Lennernas H, Shah VP, Crisoon JR. A theoretical basis for a biopharmaceutic drug classification. the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 2.Kimura T, Higaki K. Gastrointestinal transit and drug absorption. Biol Pharm Bull. 2002;25:149–164. doi: 10.1248/bpb.25.149. [DOI] [PubMed] [Google Scholar]
- 3.Sarna SK. Cyclic motor activity; migrating motor complex. Gastroenterology. 1985;89:894–913. doi: 10.1016/0016-5085(85)90589-x. [DOI] [PubMed] [Google Scholar]
- 4.Peghini P, Katz PO, Gilbert J, et al. Ranitidine controls nocturnal gastric acid breakthrough on omeprazole: a controlled study in normal subjects. Gastroenterology. 1998;115:1335–1339. doi: 10.1016/s0016-5085(98)70010-1. [DOI] [PubMed] [Google Scholar]
- 5.Hunt RH. Importance of pH control in the management of GERD. Ann Intern Med. 1999;159:649–657. doi: 10.1001/archinte.159.7.649. [DOI] [PubMed] [Google Scholar]
- 6.Rademaker JW, Rainsford KD, Stetsko PI, et al. The effect of indomethacin-induced gastric mucosal injury on 24 h intragastric acidity and plasma gastrin concentration in human volunteers. Aliment Pharmacol Ther. 1995;9:625–631. doi: 10.1111/j.1365-2036.1995.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 7.Viani F, Verdu EF, Idstrom JP, et al. Effect of omeprazole on regional and temporal variations in intragastric acidity. Digestion. 2002;65:2–10. doi: 10.1159/000051924. [DOI] [PubMed] [Google Scholar]
- 8.Bell N, Karol MN, Sachs G, Greski-Roses P, Jennings DE, Hunt RH. Duration of effect of lansoprazole on gastric pH and acid secretion in normal male volunteers. Aliment Pharmacol Ther. 2001;15:105–113. doi: 10.1046/j.1365-2036.2001.00831.x. [DOI] [PubMed] [Google Scholar]
- 9.Feldman M. Gastric secretion. normal and abnormal. In: Feldman M, Scharschmidt BF, Sleisenger MH, editors. Sleisenger and Fordtran's Gastrointestinal and liver disease: Pathophysiology/Diagnosis/Management. Philadelphia: W. B. Saunders; 1998. pp. 587–603. [Google Scholar]
- 10.Verhagen MA, Samsom M, Jebbink RJ, Smout AJ. Clinical relevance of antroduodenal manometry. Eur J Gastroenterol Hepatol. 1999;11:523–528. doi: 10.1097/00042737-199905000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Leahy A, Besherdas K, Clayman C, Mason I, Epstein O. Abnormalities of the electrogastrogram in functional gastrointestinal disorders. Am J Gastroenterol. 1999;94:1023–1028. doi: 10.1111/j.1572-0241.1999.01007.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen JDZ, Pan J, McCallum RW. Clinical significance of gastric myoelectric dysrhythmias. Dig Dis. 1995;13:275–290. doi: 10.1159/000171508. [DOI] [PubMed] [Google Scholar]
- 13.Stern RM, Koch KL, Stewart WR, Wasey MW. Electrogastrography – Current issues in validation and methodology. Psychophysiology. 1987;24:55–64. doi: 10.1111/j.1469-8986.1987.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri M, Zinsmeister AR, Greydanus MP, Brown ML, Proano M. Towards a less costly but accurate test of gastric emptying and small bowel transit. Dig Dis Sci. 1991;36:609–615. doi: 10.1007/BF01297027. [DOI] [PubMed] [Google Scholar]
- 15.Thomforde GM, Camilleri M, Phillips SF, Forstrom LA. Evaluation of an inexpensive screening scintigraphic test of gastric emptying. J Nucl Med. 1995;36:93–96. [PubMed] [Google Scholar]
- 16.Braden B, Adams S, Duan LP, et al. The 13C acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108:1048–1055. doi: 10.1016/0016-5085(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 17.Bromer MQ, Kantor SB, Wagner DA, Knight LC, Maurer AH, Parkmer HP. Simultaneous measurement of gastric emptying with a simple muffin meal using 13C octanoate breath test and scintigraphy in normal subjects and patients with dyspeptic symptoms. Dig Dis Sci. 2002;47:1657–1663. doi: 10.1023/a:1015856211261. [DOI] [PubMed] [Google Scholar]
- 18.Gatti C, di Abriola FF, Dall'Oglio L, Villa M, Franchini F, Amarri S. Is the 13C-acetate breath test a valid procedure to analyse gastric emptying in children? J Pediatr Surg. 2000;35:62–65. doi: 10.1016/s0022-3468(00)80015-9. [DOI] [PubMed] [Google Scholar]
- 19.Bluck LJ, Harding M, French S, Wright A, Halliday D, Coward WA. Measurement of gastric emptying in man using deuterated octanoic acid. Rapid Commun Mass Spectrom. 2002;16:127–133. doi: 10.1002/rcm.541. [DOI] [PubMed] [Google Scholar]
- 20.Lind T, Rydberg L, Kyleback A, et al. Esomeprazole provides improved acid control vs. omeprazole in patients with symptoms of gastro-esophageal reflux disease. Aliment Pharmacol Ther. 2000;14:861–867. doi: 10.1046/j.1365-2036.2000.00813.x. [DOI] [PubMed] [Google Scholar]
- 21.Rohss K, Hasselgren G, Hedenstrom H. Effect of esomeprazole 40 mg vs omeprazole 40 mg on 24-hour intragastric pH in patients with symptoms of gastroesophageal reflux disease. Dig Dis Sci. 2002;47:954–958. doi: 10.1023/a:1015009300955. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan R, Katz PO, Ramakrishnan A, Katzka DA, Vela MF, Castel DO. Maximal acid reflux control for Barrett's oesophagus: feasible and effective. Aliment Pharmacol Ther. 2001;15:519–524. doi: 10.1046/j.1365-2036.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 23.Fackler WK, Ours TM, Vaezi MF, Richter JE. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology. 2002;122:625–632. doi: 10.1053/gast.2002.31876. [DOI] [PubMed] [Google Scholar]
- 24.Storr M, Meining A, Allescher HD. Pathophysiology and pharmacological treatment of gastroesophageal reflux disease. Dig Dis. 2000;18:93–102. doi: 10.1159/000016970. [DOI] [PubMed] [Google Scholar]
- 25.Pohle T, Domschke W. Results of short-and long-term medical treatment of gastroesophageal reflux disease (GERD) Langenbecks Arch Surg. 2000;385:317–323. doi: 10.1007/s004230000139. [DOI] [PubMed] [Google Scholar]
- 26.Freys SM, Fuchs KH, Heimbucher J, Thiede A. Tailored augmentation of the lower esophageal sphincter in experimental antireflux operations. Surg Endosc. 1997;11:1183–1188. doi: 10.1007/s004649900565. [DOI] [PubMed] [Google Scholar]
- 27.Chrysos E, Athanasakis E, Zoras OJ, Tsiaoussis J, Xynos E. Twenty-four-hour ambulatory versus stationary esophageal manometry in the evaluation of esophageal motility in patients with gastroesophageal reflux disease. Digestion. 2002;66:1–8. doi: 10.1159/000064422. [DOI] [PubMed] [Google Scholar]
- 28.Corazziari E, Bontempo I, Anzini F. Effects of cisapride on distal esophageal motility in humans. Dig Dis Sci. 1989;34:1600–1605. doi: 10.1007/BF01537117. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz M, Edelbroek M, Fraser R, Maddox A, Wishart J. Disordered gastric motor function in diabetes mellitus. Scand J Gastroenterol. 1991;26:673–684. doi: 10.3109/00365529108998583. [DOI] [PubMed] [Google Scholar]
- 30.Malagelada JR. Diabetic gastroparesis. Semin Gastrointest Dis. 1995;4:181–186. [PubMed] [Google Scholar]
- 31.Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90:1919–1925. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- 32.MacGregor IL, Gueller R, Watts HD, Meyer JH. The effect of acute hyperglycemia on gastric emptying in man. Gastroenterology. 1976;70:190–196. [PubMed] [Google Scholar]
- 33.Achem-Karam SR, Funakoshi A, Vinik AI, Owyang C. Plasma motilin concentrations and interdigestive migrating motor complex in diabetic gastroparesis: effect of metoclopramide. Gastroenterology. 1985;88:492–499. doi: 10.1016/0016-5085(85)90512-8. [DOI] [PubMed] [Google Scholar]
- 34.Brown CK, Khanderia U. Use of metoclopramide, domperidone, and cisapride in the management of diabetic gastroparesis. Clin Pharmacol. 1990;9:357–365. [PubMed] [Google Scholar]
- 35.Horowitz M, Harding PE, Chatterton BE, Collins PJ, Shearman DJ. Acute and chronic effects of domperidone on gastric emptying in diabetic autonomic neuropathy. Dig Dis Sci. 1985;30:1–9. doi: 10.1007/BF01318363. [DOI] [PubMed] [Google Scholar]
- 36.Camilleri N, Malagelada JR, Abell TL, Rown ML, Hench V, Zinsmeister AR. Effect of six weeks of treatment with cisapride on gastroparesis and intestinal pseudoobstruction. Gastroenterology. 1989;96:704–712. [PubMed] [Google Scholar]
- 37.Johnson AG. The effects of cisapride on antroduodenal co-ordination and gastric emptying. Scand J Gastroenterol Suppl. 1989;165:36–43. doi: 10.3109/00365528909091229. [DOI] [PubMed] [Google Scholar]
- 38.Feldman M, Smith HJ. Effect of cisapride on gastric emptying of indigestible solids in patients with gastroparesis diabeticorum. A comparison with metoclopramide and placebo. Gastroenterology. 1987;92:171–174. doi: 10.1016/0016-5085(87)90854-7. [DOI] [PubMed] [Google Scholar]
- 39.Janssens J, Peeters TL, Vantrappen G, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med. 1990;322:1028–1031. doi: 10.1056/NEJM199004123221502. [DOI] [PubMed] [Google Scholar]
- 40.Camilleri M. Review article: tegaserod. Aliment Pharmacol Ther. 2001;15:277–289. doi: 10.1046/j.1365-2036.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- 41.Fioramonti J, Million M, Bueno L. Investigations on a 5HT4 agonist (SDZ HTF 919) and its main metabolite in conscious dogs: effects on gastrointestinal motility and impaired gastric emptying. Gastroenterology. 1998;114:A752. (Abstract) [Google Scholar]
- 42.Degen L, Matzinger D, Merz M, et al. Tegaserod (HTF 919), a 5-HT4 receptor partial agonist, accelerates gastrointestinal transit. Gastroenterology. 2000;118:A845. doi: 10.1046/j.1365-2036.2001.01103.x. (Abstract) [DOI] [PubMed] [Google Scholar]
- 43.Appel S, Kumle A, Hubert M, Duvauchelle T. First pharmacokinetic-pharmacodynamic study in humans with a selective 5-hydroxytryptamine 4 receptor agonist. J Clin Pharmacol. 1997;37:229–237. doi: 10.1002/j.1552-4604.1997.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 44.Ezzeddine D, Jit R, Katz N, Gopalswamy N, Bhutani MS. Pyloric injection of botulinum toxin for treatment of diabetic gastroparesis. Gastrointest Endosc. 2002;55:920–923. doi: 10.1067/mge.2002.124739. [DOI] [PubMed] [Google Scholar]
- 45.Miller LS, Szych GA, Kantor SB, et al. Treatment of idiopathic gastroparesis with injection of botulinum toxin into the pyloric sphincter muscle. Am J Gastroenterol. 2002;97:1653–1660. doi: 10.1111/j.1572-0241.2002.05823.x. [DOI] [PubMed] [Google Scholar]
- 46.Waterfall WE, Miller D, Ghista DN. Electrical stimulation of the human stomach. Dig Dis Sci. 1985;30:799. (Abstract) [Google Scholar]
- 47.Familoni BO, Abell TL, Voeller G, Salem A, Garbere O. Electrical stimulation at a higher frequency than basal rate in human stomach. Dig Dis Sci. 1997;42:885–891. doi: 10.1023/a:1018852011857. [DOI] [PubMed] [Google Scholar]
- 48.van der Voort IR, Dietl KH, Menzel J, Pohle T, Senninger N, Domschke W. Therapie der Gastroparese – Langzeitergebnisse der elektrischen Stimulation. Med Klin. 2002;97:91. (Abstract-Band, I) (Abstract) [Google Scholar]
- 49.Bortolotti M. The ‘electrical way’ to cure gastroparesis. Am J Gastroenterol. 2002;97:1874–1883. doi: 10.1111/j.1572-0241.2002.05898.x. [DOI] [PubMed] [Google Scholar]
- 50.Wilding IR, Kenyon CJ, Hooper G. Gastrointestinal spread of oral prolonged-release mesalazine microgranules (Pentasa®) dosed as either tablet or sachet. Aliment Pharmacol Ther. 2000;14:163–169. doi: 10.1046/j.1365-2036.2000.00696.x. [DOI] [PubMed] [Google Scholar]
- 51.Mulder CJJ, van den Hazel SJ. Drug therapy: dose–response relationship of oral mesalazine in inflammatory bowel disease. Med Inflamm. 1998;7:135–136. doi: 10.1080/09629359891027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen AF, Kroon R, Schoemaker R, Hoogkamer H, van Vliet A. Influence of gastric acidity on the bioavailability of digoxin. Ann Intern Med. 1991;115:540–545. doi: 10.7326/0003-4819-115-7-540. [DOI] [PubMed] [Google Scholar]
- 53.Lange D, Pavao JH, Wu J, Klausner M. Effect of a cola beverage on the bioavailability of itraconazole in the presence of H2 blockers. J Clin Pharmacol. 1997;37:535–540. doi: 10.1002/j.1552-4604.1997.tb04332.x. [DOI] [PubMed] [Google Scholar]
- 54.Shelton MJ, Adams JM, Hewitt RG, Steinwandel C, DeRemer M, Cousins S, Morse GD. Effects of spontaneous gastric hypoacidity on the pharmacokinetics of zidovudine and didanosine. Pharmacotherapy. 1997;17:438–444. [PubMed] [Google Scholar]
- 55.Lee HT, Lee YJ, Chung SJ, Shim CK. Effect of prokinetic agents, cisapride and metoclopramide, on the bioavailability in humans and intestinal permeability in rats of ranitidine, and intestinal charcoal transit in rats. Res Commun Mol Pathol Pharmacol. 2000;108:311–323. [PubMed] [Google Scholar]
- 56.Amir I, Anwar N, Baraona E, Lieber CS. Ranitidine increases the bioavailability of imbibed alcohol by accelerating gastric emptying. Life Sci. 1996;58:511–518. doi: 10.1016/0024-3205(95)02316-x. [DOI] [PubMed] [Google Scholar]
- 57.Kechagias S, Jonsson KA, Norlander B, Carlsson B, Jones AW. Low-dose aspirin decreases blood alcohol concentrations by delaying gastric emptying. Eur J Clin Pharmacol. 1997;53:241–246. doi: 10.1007/s002280050369. [DOI] [PubMed] [Google Scholar]


