Abstract
Aims
To investigate the source of the apparent increased susceptibility of women to develop QT interval prolongation and torsade de pointes after the administration of drugs that delay cardiac repolarization.
Methods
Plasma quinidine concentrations and electrocardiographic changes (QRS and QT intervals) were measured over 24 h following the administration of single oral doses of the QT prolonging drug quinidine (3 mg kg−1) and compared between 27 male and 21 female healthy volunteers.
Results
There were no significant differences between males and females in plasma quinidine concentrations or in calculated pharmacokinetic variables. Maximum quinidine concentrations in males and females were 997 ± 56 and 871 ± 57 ng ml−1, respectively (mean difference (−125, 95% confidence intervals (CI) −239, 11 ng ml−1, P = NS). Quinidine lengthened actual (QTa) and corrected (QTc) QT intervals and the QRS interval to a greater extent in females than males (P < 0.001 for each), but there were no significant sex differences detected in the effects of quinidine on the heart rate corrected JT interval. Maximum prolongation of QTc interval was observed 2 h after quinidine and was significantly greater in women (33 ± 16 vs 24 ± 17 ms, mean difference 9 ± 20 ms, 95% CI 3, 15, P = 0.037). At this time mean differences (95% CI) were 1.0 min−1 (−2.5, 4.4, P = NS) for heart rate, 5.5 ms (3.5, 7.6, P = 0.05) for the QRS and 3.4 ms (−2.5, 9.3, P = NS) for the JTc intervals.
Conclusions
Quinidine-induced increases in QTc were larger in females, but no sex differences in quinidine pharmacokinetics were found. The disparity in prolongation of cardiac repolarization is thus due to a pharmacodynamic difference which appears more complex than simply an increase in repolarization delay in females.
Keywords: cardiac repolarization, long QT syndrome, sex, torsade de pointes
Introduction
Several studies have suggested that women are more prone than men to develop torsade de pointes ventricular tachycardia during administration of drugs that prolong cardiac repolarization [1–5]. The possible explanations for this include the use of higher drug doses in relation to body size in women, or sex differences in the pharmacokinetic or pharmacodynamic properties of the implicated drugs.
There is a paucity of research examining sex differences in the pharmacokinetics of drugs. No consistent and clinically important disparity has been shown for absorption, distribution or renal excretion of drugs and many hepatic metabolic processes appear unaffected by sex [6, 7]. The activity of the hepatic microsomal enzyme CYP3A4 appears higher in women than in men [8] and this isoform is commonly involved in the metabolism of drugs which prolong ventricular repolarization. However, such an effect would reduce rather than increase the effects of drugs on the heart in women, unless these actions were produced by active metabolites.
Less information is available on possible sex-related disparity in drug pharmacodynamics. Differences in cardiac repolarization between males and females have been recognized for many years. Ventricular repolarization, measured as the QT interval on the ECG, is similar between the sexes in childhood [9, 10], but is longer in adult females than adult males until the fifth or sixth decades [11, 12]. Similarly, although inherited as an autosomal dominant trait, there is more pronounced phenotypic expression in women with the Romano–Ward type of long QT syndrome [13, 14]. Female patients in complete heart block appear at increased risk of developing torsade de pointes [15]. The sex dependant mechanism that predisposes women to longer cardiac repolarization and torsade de pointes in these situations remains to be elucidated. It is plausible to postulate that the underlying differences in ion channel physiology responsible may also lead to differences between sexes in the sensitivity to drugs that affect cardiac repolarization.
The purpose of this study was to determine if there is a disparity in the extent of drug-induced repolarization delay between women and men when the same drug dose, adjusted for body weight, was given. It was also designed to establish if such a disparity might result from differences in drug pharmacokinetics, or be due to a pharmacodynamic difference. For these studies the class 1A antiarrhythmic drug quinidine was used since it causes substantial changes in QT interval but has a comparatively short duration of action and does not have major effects on heart rate.
Methods
The study was approved by the Newcastle & North Tyneside Joint Ethics Committee.
Subjects
Forty-eight healthy volunteers (27 men and 21 women) participated in the study after giving written informed consent. The volunteers were ascertained to be healthy by a medical history, a physical examination, ECG (including measurement of QT intervals), full blood count and blood biochemistry. All the subjects were white Europeans with the exception of two (one male, one female) who were of S. Asian origin. With the exception of one female, all were nonsmokers. Their mean ages were 33 years for males (range 19–64) and 31 years (range 18–60) for females and mean body weights were 78 kg for males (range 48–111) and 67 kg for females (range 49–134). The female subjects included 17 premenopausal women, eight of whom were using hormonal contraception (seven oestrogen-containing combined oral contraceptives, one injected progesterone preparation) and four postmenopausal women, one of whom was using hormone replacement therapy. No subject was on any other drug therapy. There were no differences between males and females in plasma electrolytes, or thyroid function.
Study design
An open trial design with blinded analysis of ECGs was used. The volunteers attended in the morning having not eaten for at least 1 h. Premenopausal women were all studied within 10 days of their last menstrual period. An i.v. cannula was inserted, a blood sample taken and an ECG performed. Subjects then rested for 30 min before baseline ECG measurements were made. Subjects then took quinidine sulphate capsules 3 mg kg−1 by mouth. This dose was selected as it is similar to the standard test dose for quinidine (200 mg) that has been associated with significant but modest QTc interval prolongation in previous studies [16]. Weight normalized dosing was used to ensure that the dose of quinidine took into account the smaller body size in females. Capsules were made up individually for each subject. Further ECGs and blood samples for quinidine concentrations were taken over 24 h following drug administration. Subjects were rested supine for 30 min prior to each ECG but otherwise were allowed to move about freely. A meal was allowed 4 h into the study.
Blood sampling and determination of quinidine concentrations
Blood samples were stored on ice prior to centrifugation. Plasma was subsequently stored at −20 °C until assayed. Plasma quinidine concentrations were quantified by HPLC using a previously validated method [17]. This assay used 1 ml of plasma which was mixed with the internal standard quinine and 1 ml of 1 m sodium hydroxide. This was extracted using chloroform, evaporated to dryness and reconstituted in 100 µl methanol. Using an injection of 60 µl, the assay has a lower limit of detection of 25 ng ml−1, which gives a peak to noise ratio of 4 : 1. The interassay coefficient of variation was 3.2% at a concentration of 1044 ng ml−1.
ECG analysis
ECGs from each subject were coded and analysed ‘blind’ to their timing in relation to quinidine administration or the sex of the subject, by a single observer who used a digitizing pad (CalComp 9000, CalComp, Phoenix, AZ, USA) as described previously [18]. The QT interval was measured from the onset of the QRS complex to the end of the T wave, defined as a return to the T-P baseline or, in the presence of a U wave, the T-U nadir. Three representative complexes were analysed from each lead where the T wave was discernible and a mean taken. When the end of the T wave could not be reliably identified, the lead was excluded from analysis. Actual QT intervals (QTa) were calculated as the mean QT across all analysable leads of the 12 lead ECG and were corrected for heart rate using Bazett's formula (QTc = QT/√(RR interval)). JT intervals were calculated as QTa minus QRS duration and JTc intervals as QTc minus QRS. Interlead QT dispersion was calculated as the longest minus the shortest measured QTc on the 12 lead ECG.
Pharmacokinetic analysis
Plasma drug concentrations were analysed by a curve-fitting program for pharmacokinetic analysis (Model–PK version 1.0, McPherson Scientific, Australia). Elimination half-life was calculated from the last four points of the log-linear concentration–time curve. Area under the concentration–time curve was measured using the log-linear trapezoidal rule. The apparent volume of distribution and the oral clearance were also calculated.
Statistical analysis
Results are expressed as mean values ± standard deviation (SD) or standard error of the mean (SEM) with 95% confidence intervals. The plasma concentrations, pharmacokinetic variables and ECG measurements were compared between males and females using a repeated measures analysis of variance using sex and time after quinidine administration as factors. The level of statistical significance used was P < 0.05. Differences between mean quinidine effects in males and females at individual time points, together with 95% confidence intervals, were calculated from the pooled standard deviation of the data having verified that the differences were normally distributed.
Study power
Previous research has shown that the between-subject standard deviation for changes in QTc interval is approximately 18 ms for a drug which increased QTc interval by 24 ms. Thus to detect a 20-ms difference between groups in the change in QTc interval produced by a drug with 90% certainty at the < 0.05 level would require 17 subjects in each group.
Results
There were no significant differences in plasma quinidine concentrations between men and women at any time after quinidine administration (Figure 1) or in any of the pharmacokinetic variables (Table 1).
Figure 1.
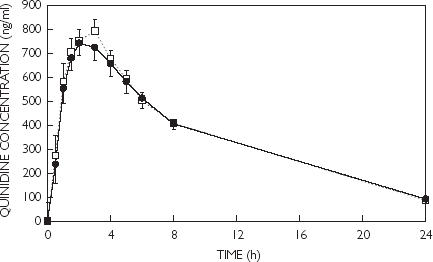
Plasma quinidine concentrations (mean ± SEM) in males (□) and females (•) following a single oral dose (3 mg kg−1).
Table 1. Pharmacokinetic parameters (mean ± SEM (range)) following the administration of quinidine sulphate 3 mg kg−1 in healthy males and females.
| Variable | Males | Females | Difference (females–males, 95% CI) | P value* |
|---|---|---|---|---|
| Half-life (h) | 7.6 ± 0.5 | 7.5 ± 0.7 | −0.1 | NS |
| (2.4–15.7) | (4.0–15.4) | (−1.2, 1.1) | ||
| Cmax (ng l−1) | 997 ± 56 | 871 ± 57 | −125 | NS |
| (582–1593) | (470–1371) | (−239, 11) | ||
| tmax (h) | 1.9 ± 0.2 | 2.0 ± 0.2 | 0.1 | NS |
| (0.5–6.0) | (0.5–4.0) | −0.4, 0.6) | ||
| AUC(0, 24 h) (µg ml−1 h) | 7.7 ± 0.4 | 7.4 ± 0.8 | −0.3 | NS |
| (4.6–15.2) | (2.4–16.5) | (−1.7, 0.9) | ||
| AUC(total) (µg ml−1 h) | 8.9 ± 0.5 | 9.1 ± 1.1 | 0.2 | NS |
| (5.8–17.9) | (4.1–21.5) | (−1.4, 1.9) | ||
| AUC(total) (%) | 14 ± 2 | 18 ± 3 | 4 | NS |
| % extrapolated | (2–43) | (2–57) | (−1, 10) | |
| CL(oral) (ml h−1 kg−1) | 0.37 ± 0.02 | 0.39 ± 0.03 | 0.03 | NS |
| (0.16–0.81) | (0.13–0.72) | (−0.03, 0.08) | ||
| Apparent Vd (l kg−1) | 3.7 ± 0.1 | 4.0 ± 0.4 | 0.2 | NS |
| (2.0–7.1) | (2.9–5.9) | (−0.5, 0.8) |
Statistical comparison by unpaired two-tailed Student's t-test.
There were no significant sex differences in resting heart rate. In the absence of quinidine absolute values for JTa, JTc and QTc intervals were longer and QRS duration shorter in women than men (Table 2).
Table 2. ECG variables in the absence of quinidine in males and females.
| Males Mean | SD | Females Mean | SD | P*(males vs females) | |
|---|---|---|---|---|---|
| Heart rate (beats min−1) | 68.2 | 9.9 | 71.4 | 7.7 | NS |
| QRS interval (ms) | 94.7 | 8.7 | 81.3 | 7.0 | < 0.0001 |
| QTa interval (ms) | 375.5 | 25.5 | 379.9 | 21.4 | NS |
| JTa interval (ms) | 280.8 | 26.1 | 298.7 | 18.9 | < 0.05 |
| JTc interval (ms) | 303.2 | 21.9 | 333.5 | 21.3 | < 0.0001 |
| QTc interval (ms) | 397.9 | 18.9 | 414.8 | 22.8 | < 0.01 |
Statistical comparison by unpaired two-tailed Student's t-test.
Quinidine had no overall effect on heart rate (F = 1.41, P = NS, data not shown.) or QRS interval (Figure 2) but produced significant increases in QTa, JTa, QTc and JTc intervals (Figures 2 and 3). There were no significant sex differences in quinidine effects on the JTc intervals but effects on the QTa, and QTc intervals were larger in females than in males (Figures 2 and 3, Table 3). Quinidine did not affect QRS duration in women but reduced QRS duration in men. As a result, changes in QRS duration were significantly different between the sexes (Figure 2, Table 3). Quinidine had no significant effects on QTc dispersion in males or females (data not shown).
Figure 2.
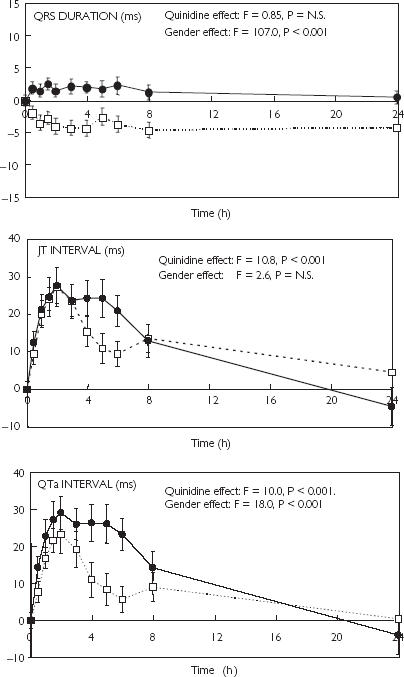
Changes (mean ± SEM) induced by oral quinidine (3 mg kg−1) in the QRS, JTa and QTa intervals.in male (□) and female (•) healthy volunteers.
Figure 3.
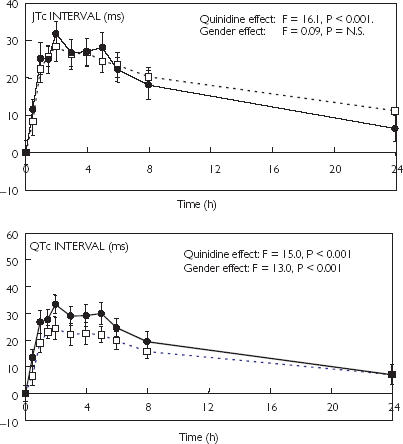
Changes (mean ± SEM) induced by oral quinidine (3 mg kg−1) in the JTc and QTc intervals in male (□) and female (•) healthy volunteers
Table 3. Mean sex differences (females–males) in quinidine-induced effects on ECG variables (95% CI).
| Time (h) | Heart rate (beats min−1) | QRS (ms) | JT (ms) | QT (ms) | JTc (ms) | QTc (ms) |
|---|---|---|---|---|---|---|
| 0.5 | 0.0 | 3.8** | 2.9 | 6.7* | 3.2 | 6.9* |
| (−2.0, 2.0) | (2.0, 5.6) | (−2.2, 8.1) | 1.5, 12.0) | (−2.8, 9.2) | (1.0, 12.9) | |
| 1 | 0.4 | 5.0** | 1.2 | 6.0 | 2.8 | 7.8 |
| (−2.4, 3.2) | (3.1, 6.9) | (−5.7, 8.2) | (−1.3, 13.3) | (−5.3, 11.0) | (−0.4, 16.0) | |
| 1.5 | −0.1 | 5.4** | 0.6 | 5.6 | −0.8 | 4.6 |
| (−3.6, 3.4) | (3.3, 7.4) | (−7.7, 8.8) | (−2.3, 13.5) | (−8.1, 6.6) | (−2.7, 11.9) | |
| 2 | 1.0 | 5.5*** | 0.3 | 5.8 | 3.4 | 8.9* |
| (−2.5, 4.4) | (3.5, 7.6) | (−7.6, 8.2) | (−1.9, 13.5) | (−2.5, 9.3) | (3.0, 14.9) | |
| 3 | −0.5 | 6.4** | 0.3 | 6.8 | 0.5 | 6.90 |
| (−4.1, 3.2) | (4.0, 8.9) | −9.3, 10.0) | (−2.9, 16.4) | (−7.3, 8.2) | (−1.0, 14.8) | |
| 4 | −3.8 | 6.4*** | 8.9 | 15.3* | 0.2 | 6.6 |
| (−7.4, −0.1) | (4.1, 8.6) | (−0.3, 18.2) | (5.4, 25.3) | (−7.4, 7.8) | (−1.4 (14.6) | |
| 5 | −4.3 | 4.4** | 13.5* | 17.8** | 3.6 | 8.0 |
| (−9.6, 1.1) | (1.9, 6.8) | (4.5, 22.4) | (8.0, 27.7) | (−4.3, 11.5) | (−0.1, 16.1) | |
| 6 | −4.9 | 6.1** | 11.5* | 17.6** | −1.2 | 4.8 |
| (−10.7, 1.2) | (3.3, 8.8) | (3.4, 19.6) | (9.0, 26.1) | (−7.6, 5.2) | (−1.5, 11.1) | |
| 8 | −0.4 | 5.9** | −0.6 | 5.2 | −2.2 | 3.7 |
| (−4.1, 3.2) | (3.5, 8.3) | (−8.3, 7.0) | (−2.7, 13.2) | (−9.4, 5.0) | (−3.4, 10.8) | |
| 24 | 2.1 | 4.7** | −9.1 | −4.4 | −4.7 | 0.0 |
| (−1.3, 5.5) | (2.5, 6.9) | (−18.1, −0.2) | (−13.6, 4.8) | (−10.7, 1.2) | (−6.3, 6.2) |
P < 0.05,
P < 0.01,
P < 0.001.
No statistically significant differences were observed between pre and postmenopausal women in baseline electrocardiographic measurements or in changes following quinidine administration, although these comparisons lack power due to the small numbers of postmenopausal subjects involved. Statistically significant differences between males and females in electrocardiographic responses to quinidine persisted if the data from the four postmenopausal women were excluded from the analysis (data not shown).
The slope of the relationship between quinidine concentration and the change in QTc interval was used as a measure of the cardiac sensitivity to quinidine in individual subjects where the relationship was sufficiently strong. A decision was made in advance of the analysis to exclude data where the correlation coefficient (r2) was greater than 0.5. The rationale for this was to reduce the risk of high quality data being diluted by unreliable slopes calculated using data where correlation was poor, leading to spurious results. Before excluding these data, median r2 values were 0.63 (range 0.04–0.89) for males and 0.69 (range 0.40–0.84) for females. Mean values for the QTc/[Quinidine] slope were significantly higher in women (P < 0.038), although there was considerable overlap between the groups (Figure 4).
Figure 4.
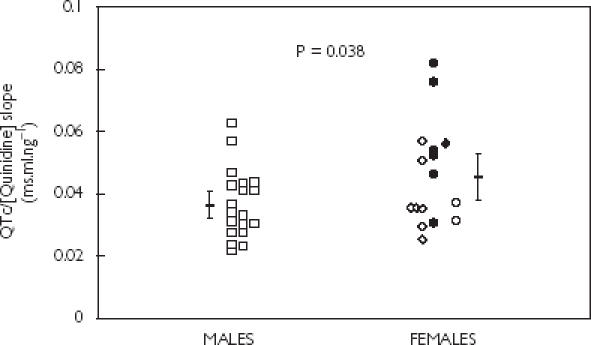
Effects of sex on the slope of the line of best fit describing the relationship between plasma quinidine concentration (ng ml−1) and change in QTc interval (ms). Males (□), premenopausal females taking (•) and not taking (◊) oral contraceptives, and postmenopausal females taking (♦) and not taking (○) hormone replacement therapy. Data from five males and five females have been excluded because of a poor correlation (r2 > 0.5). Mean values ± 95% CI are also shown. Statistical comparison between males and females was by the unpaired Student's t-test.
Discussion
In this study quinidine produced more marked prolongation of cardiac repolarization in women than in men. The doses used were adjusted for body weight, since drug-induced QT prolongation is a concentration-related effect [19]. The study does not provide any evidence for significant sex-related differences in total plasma quinidine concentrations after a single oral dose, neither did pharmacokinetic analysis identify any sex differences. However, the numbers of blood samples taken and the duration of the study were restricted in order to encourage greater recruitment of subjects. The absence of data points between 8 and 24 h after the administration will reduce the accuracy of estimation of pharmacokinetic variables, although this is unlikely to produce a systematic bias since it applies equally to males and females. The single dose nature of the study was necessary because the risk of torsade de pointes from multiple doses cannot be justified in healthy volunteers. However, single dosing does not produce concentrations as high as those achieved during clinical use of the drug and it is possible that sex differences in ECG effects would be more marked under those circumstances.
These findings are consistent with those of Benton et al. [20] who demonstrated more marked QTc prolongation in women following intravenous quinidine and at equivalent serum drug concentrations. A trend towards increased weight-adjusted clearance was observed in women in that study but this was not statistically significant. Similarly no significant effect was seen in our study, which involved a larger number of subjects. In any event, increased clearance would not explain the enhanced effects of quinidine on repolarization in women. Neither can the differences in ECG effects be due to sex differences in plasma electrolytes or thyroid function.
In our research, no attempt was made to determine free quinidine concentrations or metabolites. However, it is unlikely that differences in these parameters would explain the different pattern of ECG effects observed between males and females. Furthermore Benton et al. did not detect sex-related differences in free quinidine or quinidine metabolite concentrations [20].
The longer baseline QTc and JTc intervals in women are consistent with previous reports [12]. The underlying reason for this difference in repolarization is still being elucidated. In rabbit myocytes, female cells have reduced outward Ikr and Ikl current densities compared with cells from males [21]. Studies in female rabbits following oophorectomy have shown that both oestradiol and dihydrotestosterone down-regulate the expression of mRNA for the HK2 and 0.7 kb IsK potassium channels. For both hormones this is associated with an increase in unmedicated QT interval, with larger effects observed for oestradiol. In comparison with control animals, oestradiol had no effect and dihydrotestosterone decreased the extent of QT prolongation induced by quinidine [22]. These findings from animal studies suggest a role for sex hormones in modulating relevant cardiac ion channels and implicate them in the sex differences in cardiac repolarization in the presence and absence of drugs.
If a hormonal mechanism is responsible for the sex differences, the currently available evidence does not suggest that oestrogens are involved. The propensity of (±)-sotalol to produce JTc prolongation is similar in women of premenopausal and postmenopausal age [3]. Consistent with this, hormonal replacement therapy does not affect QTc interval in postmenopausal women [23] or in women with coronary artery disease [24].
A recent study has shown that ibutilide-induced increases in QTc were larger in females than males and most marked during menses and the ovulation phase of the menstrual cycle, although the differences with respect to the luteal phase were not statistically significant. QT prolongation is inversely correlated with serum concentrations of progesterone and the progesterone : oestradiol ratio [25]. If this effect is confirmed, it might indicate that progestogens attenuate increases in QT interval. However, if this were the case, some QT interval shortening following the menopause might be expected.
The shortening of cardiac repolarization at puberty in males suggests a possible role for testosterone. Consistent with this, repolarization is prolonged in castrated men and shorter in women with virilization [26].
Perhaps the most interesting finding of this study is the novel observation that sex differences in quinidine effects appear to be concentrated on the QRS portion of the QT interval, which might be attributable to sex differences in the effects of quinidine on sodium rather than potassium channels. Quinidine is a blocker of the rapid inward sodium current (INa) [27] and it is possible that the drug has differential effects on this channel in males and females. However, this observation needs to be confirmed since in males QRS duration had not returned to baseline values 24 h after quinidine administration.
The implications of this study are that differences between men and women in response to drugs that prolonging cardiac repolarization are attributable to pharmacodynamic differences. It is possible that these are mediated via sex steroids although this remains to be confirmed and the precise mechanisms elucidated.
The previously demonstrated increased risk of torsade de pointes [1–5] in women may result from their longer QTc interval in the absence of drugs and their increased sensitivity to drugs that prolong QT interval. Physicians prescribing drugs prolonging cardiac repolarization should exercise particular caution in women, especially in the presence of other risk factors such as hypokalaemia or slow heart rate.
Acknowledgments
Dr El Eraky received financial support from the Education and Culture Bureau of the Embassy of the Arab Republic of Egypt.
References
- 1.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female sex as a risk factor for torsade de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 2.Reinoehl J, Frankovich D, Machado C, et al. Probucol-associated tachyarrhythmic events and QT prolongation: importance of sex. Am Heart J. 1996;13:1184–1191. doi: 10.1016/s0002-8703(96)90095-2. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann MH, Hardy S, Archibald D, Quart B. Sex difference in risk of torsades de pointes with d,l-sotalol. Circulation. 1996;94:2535–2541. doi: 10.1161/01.cir.94.10.2535. [DOI] [PubMed] [Google Scholar]
- 4.Drici MD, Knollman BC, Wang WX, Woosley RL. Cardiac actions of erythromycin: influence of female sex. JAMA. 1998;280:1774–1776. doi: 10.1001/jama.280.20.1774. [DOI] [PubMed] [Google Scholar]
- 5.Coumel P. Safety of bepredil: from review of the European data. Am J Cardiol. 1992;69:75D–78D. doi: 10.1016/0002-9149(92)90963-y. [DOI] [PubMed] [Google Scholar]
- 6.Gleiter CH, Gundert-Remy U. Gender differences in pharmacokinetics. Eur J Drug Metab Pharmacokin. 1996;21:123–128. doi: 10.1007/BF03190260. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CV, Acosta EP, Strykowski JM. Gender differences in human pharmacokinetics and pharmacodynamics. J Adolescent Health. 1994;15:619–629. doi: 10.1016/s1054-139x(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 8.Hunt CM, Westerkam WR, Steve GM. Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol. 1992;44:275–283. doi: 10.1016/0006-2952(92)90010-g. [DOI] [PubMed] [Google Scholar]
- 9.Pearl W. Effects of gender, age and heart rate on QT intervals in children. Pediatr Cardiol. 1996;17:135–136. doi: 10.1007/BF02505201. [DOI] [PubMed] [Google Scholar]
- 10.McCammorr RW. A longitudinal study on electrocardiographic intervals in healthy children. Acta Pediatr. 1961;50(Suppl. 126):3–52. [Google Scholar]
- 11.Merri M, Benhorin J, Alberti M, Locati E, Moss AJ. Electrocardiographic quantitation of ventricular repolarization. Circulation. 1989;80:1301–1308. doi: 10.1161/01.cir.80.5.1301. [DOI] [PubMed] [Google Scholar]
- 12.Rautaharju P, Zhou SH, Wong S, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–695. [PubMed] [Google Scholar]
- 13.Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome: Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 14.Jiang C, Atikinson D, Towbin J, et al. Two long QT syndrome loci map to chromosomes 3 and 7 with evidence for further heterogeneity. Nature Genet. 1994;8:141–147. doi: 10.1038/ng1094-141. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki R, Machando C, Reinoehl J, et al. Increased propensity of women to develop torsades de pointes during complete heart block. J Cardiovasc Electrophysiol. 1995;6:1032–1038. doi: 10.1111/j.1540-8167.1995.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 16.Laganiere S, Davies RF, Carignan G, et al. Pharmacokinetic and pharmacodynamic interactions between diltiazem and quinidine. Clin Pharmacol Ther. 1996;60:255–264. doi: 10.1016/S0009-9236(96)90052-1. [DOI] [PubMed] [Google Scholar]
- 17.Karbwang J, Bangchang KN, Molunto P, et al. Determination of quinine and quinidine in biological fluids by high performance liquid chromatography. South East Asian J Trop Med Public Health. 1989;20:65–69. [PubMed] [Google Scholar]
- 18.Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHL. QTc interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048–1052. doi: 10.1016/s0140-6736(00)02035-3. [DOI] [PubMed] [Google Scholar]
- 19.Thomas SHL. Drugs, QT interval abnormalities and ventricular arrhythmias. Adverse Drug Reactions Toxicol Rev. 1994;13:77–102. [PubMed] [Google Scholar]
- 20.Benton RE, Sale M, Flockhart DA, Woosley RL. Greater quinidine–induced QTc interval prolongation in women. Clin Pharmacol Ther. 2000;67:413–418. doi: 10.1067/mcp.2000.105761. [DOI] [PubMed] [Google Scholar]
- 21.Liu XK, Katchman A, Drici MD, et al. Gender difference in the cycle length-dependent QT and potassium currents in rabbits. J Pharmacol Exp Ther. 1998;285:672–679. [PubMed] [Google Scholar]
- 22.Drici MD, Burklow TR, Haridasse V, Vedanandam DVM, Glazer R, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–1474. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- 23.Larsen JA, Tung RH, Sadananda R, et al. Effect of hormone replacement therapy on QT interval. Am J Cardiol. 1998;82:993–995. doi: 10.1016/s0002-9149(98)00523-2. [DOI] [PubMed] [Google Scholar]
- 24.Sbarouni E, Zarvalis E, Kyriakides ZS, Kremastinos DT. Ansence of effects of short term estrogen replacement therapy on resting and exertional QT and QTc dispersion in postmenopausal women. Pacing Clin Electrophysiol. 1998;21:2392–2395. doi: 10.1111/j.1540-8159.1998.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez I, Kilbourn MJ, Liu X-K, Pezzullo J, Woosley RL. Drug-induced prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 26.Bidoggia H, Maciel JP, Capalozza N, et al. Sex differences on the electrocardiographic pattern of cardiac repolarisation: possible effect of testosterone. Am Heart J. 2000;140:678–683. doi: 10.1067/mhj.2000.109918. [DOI] [PubMed] [Google Scholar]
- 27.Weidmann S. Effects of calcium ions and local anaesthetics on electrical properties of Purkinje fibres. J Physiol (Lond) 1955;129:568–582. doi: 10.1113/jphysiol.1955.sp005379. [DOI] [PMC free article] [PubMed] [Google Scholar]


