Abstract
Aims
To study the effect of an oral contraceptive (OC) formulation containing ethinyloestradiol and levonorgestrel (LNG) (combination OC) or LNG alone on the CYP2C19-mediated hydroxylation of omeprazole in healthy females.
Methods
This was an open crossover study with three phases. In phase one, 10 healthy females received a single 40-mg dose of omeprazole. Thereafter the subjects received in a random order either 40 µg ethinyloestradiol and 75 µg LNG or 60 µg LNG alone once daily for 10 days. On day 10, 1 h after the last OC dose, subjects received a single 40-mg oral dose of omeprazole. The plasma concentrations of omeprazole, 5′-hydroxyomeprazole and omeprazole sulphone were determined for up to 8 h.
Results
The use of combination OC increased the area under the curve (AUC) of omeprazole by 38% [95% confidence interval (CI) − 3.8, 80; P = 0.040] and caused a 48% increase (95% CI 28, 68) in the AUC ratio of omeprazole/5-hydroxyomeprazole. LNG alone did not effect the 5′-hydroxylation of omeprazole. Neither of the OC preparations seemed to have an inhibitory effect on the formation of omeprazole sulphone by CYP3A4.
Conclusions
Oral contraceptives containing ethinyloestradiol but not those containing only LNG decrease CYP2C19 activity.
Keywords: CYP2C19, cytochrome P450, ethinyloestradiol, levonorgestrel, omeprazole, oral contraceptives
Introduction
Oral contraceptives (OCs) as well as hormone replacement therapy (HRT) are among the most widely prescribed drugs in the world. In vitro studies have shown that several steroids used in these preparations are potent mechanism-based inhibitors of some cytochrome P450 enzymes [1, 2]. These in vitro findings are supported by clinical evidence showing an increased bioavailability of drugs eliminated by CYP1A2 and CYP3A4 when used concomitantly with OCs [3–6], although other studies have found no effect [7, 8]. HRT containing oestradiol and levonorgestrel (LNG) has been found to decrease CYP1A2 activity but not that of CYP3A4 [9–11].
There have been recent reports of inhibition of the polymorphically expressed enzyme CYP2C19 [12, 13] by oral contraceptives. Tamminga et al. examined a database of phenotyped Dutch volunteers and found significantly decreased CYP2C19 activity (based on the mephenytoin S/R ratio) in OC users who were extensive metabolizers [14]. This was confirmed in two later studies, one of them suggesting that CYP2C19 inhibition occurred only in subjects taking combination OCs containing ethinyloestradiol [15, 16].
Omeprazole is extensively metabolized by CYP2C19 via 5′-hydroxylation and to a lesser extent by CYP3A4 via sulphoxidation [17–19]. The further metabolism of omeprazole sulphone is catalysed by CYP2C19 [20, 21], whereas the sulphonation of hydroxyomeprazole seems to be catalysed by CYP3A4 [21] (Figure 1). In individuals with decreased CYP2C19 activity, the hydroxylation of omeprazole is impaired and sulphoxidation by CYP3A4 plays a greater role in overall metabolism [20]. It has been shown that the rate of omeprazole 5′-hydroxylation correlates highly with the mephenytoin S/R ratio [22]. Thus, omeprazole has become widely accepted as a safe probe drug for assessing CYP2C19 activity.
Figure 1.
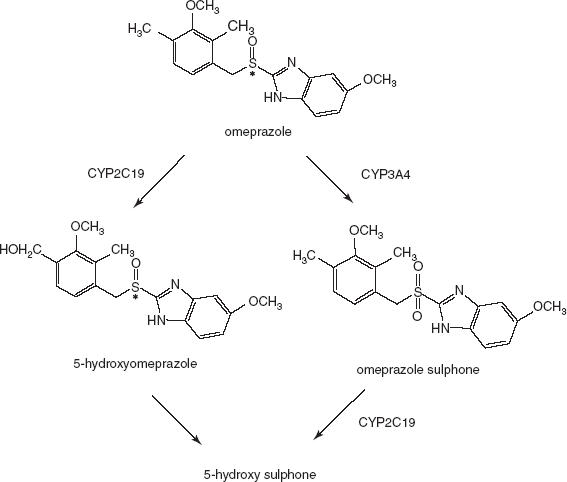
The metabolism of omeprazole and its major metabolites shown schematically.
In this study, we examined the effect of OCs on the two main metabolic pathways of omeprazole, mediated by CYP2C19 (hydroxylation) and CYP3A4 (sulphoxidation). In particular, we wanted to compare the roles of the ethinyloestradiol and the progestin components of the OCs in the inhibition of CYP2C19 activity.
Methods
Subjects and ethics
Ten female volunteers (aged 19–26 years, body mass index range 18–26) participated in this study. The subjects were nonsmokers and did not use any concomitant medication during the study. All the subjects had regular menstrual cycles and none had used OCs for at least 2 months before the study. They were at various phases of the menstrual cycle in the beginning of the study, but according to a recent study this should have no effect on the results [24]. The subjects were ascertained to be in good health by medical history, clinical examination and standard haematological and clinical chemistry tests. Pregnancy was excluded by a pregnancy test and the subjects were advised to use barrier methods of contraception during the study. Written informed consent was obtained from all the subjects and the study protocol was approved by the Ethics Committee of the Varsinais-Suomi healthcare district.
Protocol
This study had an open crossover design with three phases. In the control phase, after fasting overnight, all the subjects received a single 40-mg dose of omeprazole (Losec® MUPS®; AstraZeneca, Mölndal, Sweden). On the following day the subjects received in randomized fashion a 10-day pretreatment once daily with either combination OC preparation containing 40 µg ethinyloestradiol and 75 µg LNG (EE2 + LNG) (Trikvilar®, white pills; Schering, Berlin, Germany) or 60 µg LNG (two 30-µg tablets, Microluton®; Schering, Berlin, Germany). On day 10 of OC treatment, and 1 h after the last dose, the subjects received a single 40-mg dose of omeprazole. For the determination of omeprazole and its 5′-hydroxy and sulphone metabolites, venous blood samples (10 ml) were drawn immediately before and 1, 1.5, 2, 2.5, 3, 4, 6 and 8 h after the ingestion of drug. Plasma was separated and stored at − 70 °C until analysis. In all the phases subjects continued fasting until 3 h after ingestion of omeprazole, when a standardized lunch was served. There was a wash-out period of 4 weeks between the last two study phases. The hormone preparation was taken under the supervision of the study personnel on the mornings of days 1 and 10. On the other days, the drugs were self-administered between 08.00 and 10.00 h. Alcohol, grapefruit juice and caffeine-containing beverages were not allowed during the study. The compliance of the subjects to the protocol was monitored by tablet counting. Subjects were genotyped for the *2 and *3 mutations in the CYP2C19 gene by using a polymerase chain reaction method with specific primers, as described earlier [15].
Drug and metabolite analyses
Omeprazole, 5-hydroxyomeprazole (OH-omeprazole) and omeprazole sulphone were measured by reversed phase high-performance liquid chromatography with UV-detection as described by Tybring et al.[25] with some minor modifications. Briefly, 50 µl 100 mm sodium dihydrogenephosphate was added to 0.5 ml plasma and the mixture extracted with 4 ml 10% acetonitrile containing dichloromethane for 10 min. After centrifugation, 3 ml of the organic phase were evaporated to dryness and reconstituted in 400 µl 15% acetonitrile in 5 mm dibasic sodium phosphate. The mixture was ultrasonicated for 60 s and 100 µl were injected onto a Zorbax Extend C18 column (150 × 4.6 mm; Agilent Technologies Palo Alto, California, United States). The mobile phase consisted of 23% acetonitrile in 50 mm ammonium acetate buffer pH 7.2. A linear gradient of acetonitrile was applied from 3 min to reach the final concentration of 32% acetonitrile after 13 min. The flow rate was 1 ml min−1 and the detection wavelength 302 nm. Standard curves were analysed in the concentration range 25–2000 nm. The limit of quantification was 25 nm for omeprazole and both metabolites. The within- and between-assay coefficients of variation were < 6.1% for all analytes.
Statistical analysis
The pharmacokinetic parameters for omeprazole and its metabolites were calculated using standard noncompartmental methods. The maximum concentration in plasma (Cmax) and time to maximum concentration (tmax) for each subject were derived directly from the plasma concentration data. The area under the plasma concentration–time curve was calculated from zero to the last data point (AUC), using the linear trapezoidal rule. When the concentration at the last data point was below the limit of the essay (16/30 samples for omeprazole and 12/30 samples of OH-omeprazole), a value of 12.5 nm was used in the calculation of AUC. The half-lives were estimated by the least squares regression analysis of the terminal linear part of log concentration–time curve by using at least three individually chosen data points with concentrations above the quantification limit.
The AUC ratio was calculated by dividing the AUC of omeprazole by the AUC of OH-omeprazole or by the AUC of omeprazole sulphone. Estimate of the weight corrected apparent oral clearance (CL/F) was obtained by dividing the omeprazole dose by the AUC and body weight.
The absolute changes in the pharmacokinetic parameters were tested using the anova model for repeated measurements. A paired t-test was used for post hoc analysis. Non-normally distributed data, i.e. 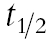 of omeprazole sulphone, omeprazole clearance CL/F, were log transformed prior to statistical analysis. Friedman's test and the Wilcoxon signed rank test for pairwise comparisons were used to compare tmax. A Bonferroni adjustment for repeated significance testing was used to calculate the P-values. A P level < 0.05 was regarded as statistically significant.
of omeprazole sulphone, omeprazole clearance CL/F, were log transformed prior to statistical analysis. Friedman's test and the Wilcoxon signed rank test for pairwise comparisons were used to compare tmax. A Bonferroni adjustment for repeated significance testing was used to calculate the P-values. A P level < 0.05 was regarded as statistically significant.
Results
All 10 subjects completed the study. Three of the subjects were heterozygous for the CYP2C19*2 mutation. No difference was observed between these individuals and the rest of the subjects in the pharmacokinetics of omeprazole.
Compared with the control phase, the AUC of omeprazole was increased by 38% [95% confidence interval (CI) − 3.8, 80; P = 0.040] after the pretreatment with EE2 + LNG, but no change was seen after pretreatment with LNG (Table 1, Figure 2a). The elimination half-life and the CLF of omeprazole was significantly (40%; 95% CI 11, 69) higher in the EE2 + LNG phase. Nevertheless, the reduction in the weight-corrected apparent oral clearance of omeprazole (CL/F) reached only borderline statistical significance (−18%; 95% CI − 38, 1.1). The t½ and the CL/F of omeprazole were unaffected by LNG treatment and no significant differences were seen in Cmax and tmax of omeprazole among the three phases (Table 1).
Table 1. Pharmacokinetics of omeprazole (Ome), OH-omeprazole (OH-Ome) and omeprazole sulphone (Ome-S) after ingestion of 40 mg of omeprazole after 10 days’ treatment with the combination OC or with levonorgestrel (LNG) alone for 10 days, and without pretreatment (control). The results are mean ± SD (median with range for tmax).
| Parameter | Control | EE + LNG | %-diff. (P-value) vs. control | LNG | %-diff. (P-value) vs. control |
|---|---|---|---|---|---|
| Omeprazole | |||||
| AUC (µM*h) | 4.4 ± 2.0 | 5.8 ± 2.4 | 38% (0.040) | 3.9 ± 1.8 | −10% (0.20) |
| 0.8 ± 0.2 | 1.0 ± 0.3 | 40% (0.017) | 0.8 ± 0.2 | −0.3% (1.0) | |
| Cmax (µM) | 2.7 ± 1.3 | 3.0 ± 1.1 | 28% (1.0) | 2.3 ± 1.3 | −16% (0.07) |
| tmax (h) | 1.5 (1.0–2.5) | 2.5 (1.0–4.0) | (0.31) | 2.5 (1.0–4.0) | (0.25) |
| Cl/F (l*h−1*kg−1) | 0.6 ± 0.3 | 0.5 ± 0.4 | −18% (0.11) | 0.6 ± 0.4 | 17% (0.23) |
| OH-Omeprazole | |||||
| AUC (µM*h) | 3.1 ± 0.5 | 2.8 ± 0.7 | −8.9% (0.43) | 2.8 ± 0.6 | −9.1% (0.10) |
| 1.0 ± 0.2 | 1.2 ± 0.2 | 17% (0.063) | 1.1 ± 0.2 | 4.6% (1.0) | |
| Cmax (µM) | 1.5 ± 0.4 | 1.1 ± 0.3 | −21% (0.054) | 1.3 ± 0.4 | −16% (0.034) |
| tmax (h) | 1.5 (1.0–2.5) | 2.25 (1.0–4.0) | (0.27) | 2.5 (1.0–4.0) | (0.19) |
| Omeprazole sulphone | |||||
| AUC (µM*h) | 2.5 ± 1.1 | 3.6 ± 1.6 | 55% (0.066) | 2.3 ± 1.1 | −9.0% (0.54) |
| 2.4 ± 1.1 | 3.9 ± 1.9 | 67% (0.008) | 2.5 ± 0.9 | 8.3% (1.0) | |
| Cmax (µM) | 0.6 ± 0.2 | 0.8 ± 0.3 | 28% (0.27) | 0.6 ± 0.2 | −12% (0.13) |
| tmax (h) | 1.5 (1.0–3.0) | 2.75 (1.5–4.0) | (1.0) | 2.75 (1.5–4.0) | (0.063) |
| AUC ratio | |||||
| AUC(Ome)/AUC(OH-Ome) | 1.5 ± 0.7 | 2.1 ± 0.9 | 48% (<0.001) | 1.4 ± 0.6 | −1.8% (0.63) |
EE, ethinyloestradiol; OC, oral contraceptive.
Figure 2.
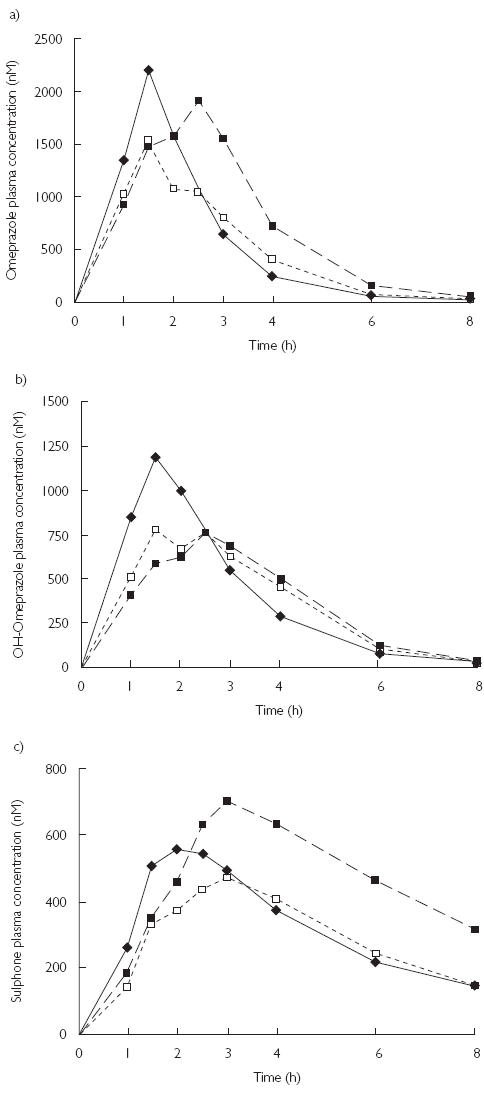
The mean concentration vs. time profiles for (a) omeprazole, (b) OH-omeprazole and (c) omeprazole sulphone in 10 healthy subjects after a single 40-mg oral dose of omeprazole, without pretreatment (♦) and following pretreatment with the combination oral contraceptive (▪) or levonorgestrel alone for 10 days (□). The error bars have been omitted for clarity.
The AUC of OH-omeprazole was not changed by either of the hormone treatments (Table 1, Figure 2b). There was a trend towards reduction in the maximum concentration of OH-omeprazole (−21%, 95% CI − 41, − 0.2) after the EE2 + LNG treatment and LNG treatment decreased the Cmax of OH-omeprazole (−16%, 95% CI − 28, − 3.7). The 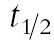 of OH-omeprazole was not significantly altered and there were no changes in the tmax between different the phases.
of OH-omeprazole was not significantly altered and there were no changes in the tmax between different the phases.
The AUC of omeprazole sulphone was 55% higher after EE2 + LNG treatment, but statistically this change reached only marginal significance (95% CI − 6.5, 116; P = 0.066) (Table 1, Figure 2c). No change in the AUC of omeprazole sulphone was seen after treatment with LNG. The elimination half-life of omeprazole sulphone was increased by 67% (95% CI 31, 103) in the EE2 + LNG phase, whereas LNG treatment had no effect. The Cmax or tmax of omeprazole sulphone were not affected by either hormone treatment.
There was a highly significant increase of 48% (95% CI 28, 68) in the AUC (omeprazole)/AUC (OH-omeprazole) ratio after the EE2 + LNG treatment compared with control phase. The change was evident in all 10 subjects. There was no change in the AUC ratio after LNG treatment (Table 1).
Discussion
In this study, the use of a combination OC preparation, containing 40 µg ethinyloestradiol and 75 µg LNG, caused a significant inhibition of the CYP2C19-mediated hydroxylation of omeprazole. This increase was observed in all 10 subjects. Also, the inhibition of the further metabolism of omeprazole sulphone by CYP2C19 was reflected in the longer elimination half-life of omeprazole sulphone. In contrast, the dosing with 60 µg LNG had no significant effect on the CYP2C19- or CYP3A4-catalysed metabolism of omeprazole. Accordingly, the results of our study support the conclusion by Hägg et al. that the inhibition of CYP2C19 activity is caused by the ethinyloestradiol component of OCs [16]. No decrease was seen in the Cmax of omeprazole sulphone and the elimination half-life of hydroxyomeprazole remained unchanged, which suggests that neither OC preparation affected CYP3A4 activity. This finding is in line with an earlier report by Belle et al.[8].
In a previous study, the use of an OC preparation containing 30 µg ethinyloestradiol and 75 µg gestodene inhibited the CYP3A4-mediated hydroxylation of midazolam in healthy volunteers [26]. However, another study found that a preparation containing 50 µg ethinyloestradiol and 500 µg norgestrel had no effect on this reaction [8]. These results suggest that the inhibition of CYP3A4 activity could be progestin related, but may depend on the progestins used. The effect of combination OCs on another CYP3A4 substrate, alprazolam, has been studied with conflicting results. Stoehr et al.[6] found that alprazolam clearance was diminished in the OC users, but this effect could not be confirmed in two later clinical studies on alprazolam pharmacokinetics [27, 28]. However, two of these three studies did not identify the progestin used in the OC preparations.
Ethinyloestradiol and gestodene are known to cause relatively potent mechanism-based inhibition of CYP3A4 and CYP2C19 activity in vitro[1, 2, 29, 30]. Levonorgestrel is clearly less potent an inhibitor of both CYP2C19 and CYP3A4 than other progestins such as gestodene and 3-ketodesogestrel [30]. Several of the progestin-only OCs in clinical use contain LNG and this may explain the finding that progestin-only preparations do not alter CYP2C19 activity [16]. In our study, the dose of LNG was somewhat higher in the combination pill, but even in the progestin-only pill, the dose of LNG was twice as high as used in clinical practice, and should thus exclude clinically relevant inhibition of CYP2C19.
Based on results from this and earlier studies it seems that the inhibition of CYP2C19 activity by OCs is caused by ethinyloestradiol, and preparations containing only levonorgestrel do not affect the activity of CYP2C19. However, inhibition of CYP2C19 activity by oral contraceptives containing progestin other than levonorgestrol cannot be ruled out.
Acknowledgments
Mrs Margareta Lind and Mrs Lilleba Bohman are thanked for their skilful assistance in drug and metabolite analyses and genotyping. Grants: supported by Karolinska Institutet, the Swedish Medical Research Council (3902) (G.T.) National Institutes of Health, USA, 1R01 GM60548-01A2, and Turku University Hospital research fund EVO13390 (K.L.)
References
- 1.Guengerich FP. Metabolism of 17 alpha-ethynylestradiol in humans. Life Sci. 1990;47:1981–1988. doi: 10.1016/0024-3205(90)90431-p. [DOI] [PubMed] [Google Scholar]
- 2.Guengerich FP. Mechanism-based inactivation of human liver microsomal cytochrome P-450 IIIA4 by gestodene. Chem Res Toxicol. 1990;3:363–371. doi: 10.1021/tx00016a015. [DOI] [PubMed] [Google Scholar]
- 3.Balogh A, Klinger G, Henschel L, Borner A, Vollanth R, Kuhnz W. Influence of ethinylestradiol-containing combination oral contraceptives with gestodene or levonorgestrel on caffeine elimination. Eur J Clin Pharmacol. 1995;48:161–166. doi: 10.1007/BF00192743. [DOI] [PubMed] [Google Scholar]
- 4.Abernethy DR, Todd EL. Impairment of caffeine clearance by chronic use of low-dose oestrogen-containing oral contraceptives. Eur J Clin Pharmacol. 1985;28:425–428. doi: 10.1007/BF00544361. [DOI] [PubMed] [Google Scholar]
- 5.Slayter KL, Ludwig EA, Lew KH, Middleton E, Jr, Ferry JJ, Jusko WJ. Oral contraceptive effects on methylprednisolone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 1996;59:312–321. doi: 10.1016/S0009-9236(96)80009-9. [DOI] [PubMed] [Google Scholar]
- 6.Stoehr GP, Kroboth PD, Juhl RP, Wender DB, Phillips JP, Smith RB. Effect of oral contraceptives on triazolam, temazepam, alprazolam, and lorazepam kinetics. Clin Pharmacol Ther. 1984;36:683–690. doi: 10.1038/clpt.1984.240. [DOI] [PubMed] [Google Scholar]
- 7.Schrenk D, Brockmeier D, Morike K, Bock KW, Eichelbaum M. A distribution study of CYP1A2 phenotypes among smokers and non-smokers in a cohort of healthy Caucasian volunteers. Eur J Clin Pharmacol. 1998;53:361–367. doi: 10.1007/s002280050394. [DOI] [PubMed] [Google Scholar]
- 8.Belle DJ, Callaghan JT, Gorski JC, et al. The effects of an oral contraceptive containing ethinyloestradiol and norgestrel on CYP3A activity. Br J Clin Pharmacol. 2002;53:67–74. doi: 10.1046/j.0306-5251.2001.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laine K, Palovaara S, Tapanainen P, Manninen P. Plasma tacrine concentrations are significantly increased by concomitant hormone replacement therapy. Clin Pharmacol Ther. 1999;66:602–608. doi: 10.1053/cp.1999.v66.103404001. [DOI] [PubMed] [Google Scholar]
- 10.Gorski JC, Wang Z, Haehner-Daniels BD, Wrighton SA, Hall SD. The effect of hormone replacement therapy on CYP3A activity. Clin Pharmacol Ther. 2000;68:412–417. doi: 10.1067/mcp.2000.110560. [DOI] [PubMed] [Google Scholar]
- 11.Krecic-Shepard ME, Barnas CR, Slimko J, Jones MP, Schwartz JB. Gender-specific effects on verapamil pharmacokinetics and pharmacodynamics in humans. J Clin Pharmacol. 2000;40:219–230. doi: 10.1177/00912700022008883. [DOI] [PubMed] [Google Scholar]
- 12.De Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–15422. [PubMed] [Google Scholar]
- 13.De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594–598. [PubMed] [Google Scholar]
- 14.Tamminga WJ, Wemer J, Oosterhuis B, et al. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive- related gender differences. Eur J Clin Pharmacol. 1999;55:177–184. doi: 10.1007/s002280050615. [DOI] [PubMed] [Google Scholar]
- 15.Laine K, Tybring G, Bertilsson L. No sex-related differences but significant inhibition by oral contraceptives of CYP2C19 activity as measured by the probe drugs mephenytoin and omeprazole in healthy Swedish white subjects. Clin Pharmacol Ther. 2000;68:151–159. doi: 10.1067/mcp.2000.108949. [DOI] [PubMed] [Google Scholar]
- 16.Hägg S, Spigset O, Dahlqvist R. Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. Br J Clin Pharmacol. 2001;51:169–173. doi: 10.1111/j.1365-2125.2001.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson T, Regardh CG, Dahl-Puustinen ML, Bertilsson L. Slow omeprazole metabolizers are also poor S-mephenytoin hydroxylators. Ther Drug Monit. 1990;12:415–416. doi: 10.1097/00007691-199007000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Andersson T, Regardh CG, Lou YC, Zhang Y, Dahl ML, Bertilsson L. Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics. 1992;2:25–31. doi: 10.1097/00008571-199202000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Andersson T, Miners JO, Veronese M, et al. Identification of human liver cytochrome P450 isoforms mediating omeprazole metabolism. Br J Clin Pharmacol. 1993;36:521–530. doi: 10.1111/j.1365-2125.1993.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Böttiger Y, Tybring G, Gotharson E, Bertilsson L. Inhibition of the sulfoxidation of omeprazole by ketoconazole in poor and extensive metabolizers of S-mephenytoin. Clin Pharmacol Ther. 1997;62:384–391. doi: 10.1016/S0009-9236(97)90116-8. [DOI] [PubMed] [Google Scholar]
- 21.Andersson T, Miners JO, Veronese ME, Birkett DJ. Identification of human liver cytochrome P450 isoforms mediating secondary omeprazole metabolism. Br J Clin Pharmacol. 1994;37:597–604. doi: 10.1111/j.1365-2125.1994.tb04310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang M, Dahl ML, Tybring G, Gotharson E, Bertilsson L. Use of omeprazole as a probe drug for CYP2C19 phenotype in Swedish Caucasians: comparison with S-mephenytoin hydroxylation phenotype and CYP2C19 genotype. Pharmacogenetics. 1995;5:358–363. doi: 10.1097/00008571-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Bertilsson L, Tybring G, Widen J, Chang M, Tomson T. Carbamazepine treatment induces the CYP3A4 catalysed sulphoxidation of omeprazole, but has no or less effect on hydroxylation via CYP2C19. Br J Clin Pharmacol. 1997;44:86–189. doi: 10.1046/j.1365-2125.1997.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MJ, Bertino JS, Jr, Gaedigk A, Zhang Y, Sellers EM, Nafziger AN. Effect of sex and menstrual cycle phase on cytochrome P450 2C19 activity with omeprazole used as a biomarker. Clin Pharmacol Ther. 2002;72:192–199. doi: 10.1067/mcp.2002.126174. [DOI] [PubMed] [Google Scholar]
- 25.Tybring G, Bottiger Y, Widen J, Bertilsson L. Enantioselective hydroxylation of omeprazole catalyzed by CYP2C19 in Swedish white subjects. Clin Pharmacol Ther. 1997;62:129–137. doi: 10.1016/S0009-9236(97)90060-6. [DOI] [PubMed] [Google Scholar]
- 26.Palovaara S, Kivisto KT, Tapanainen P, Manninen P, Neuvonen PJ, Laine K. Effect of an oral contraceptive preparation containing ethinylestradiol and gestodene on CYP3A4 activity as measured by midazolam 1′-hydroxylation. Br J Clin Pharmacol. 2000;50:333–337. doi: 10.1046/j.1365-2125.2000.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scavone JM, Greenblatt DJ, Locniskar A, Shader RI. Alprazolam pharmacokinetics in women on low-dose oral contraceptives. J Clin Pharmacol. 1988;28:454–457. doi: 10.1002/j.1552-4604.1988.tb05759.x. [DOI] [PubMed] [Google Scholar]
- 28.Kroboth PD, Smith RB, Stoehr GP, Juhl RP. Pharmacodynamic evaluation of the benzodiazepine–oral contraceptive interaction. Clin Pharmacol Ther. 1985;38:525–532. doi: 10.1038/clpt.1985.218. [DOI] [PubMed] [Google Scholar]
- 29.Jurima M, Inaba T, Kalow W. Mephenytoin hydroxylase activity in human liver: inhibition by steroids. Drug Metab Dispos. 1985;13:746–749. [PubMed] [Google Scholar]
- 30.Back DJ, Houlgrave R, Tjia JF, Ward S, Orme ML. Effect of the progestogens, gestodene, 3-keto desogestrel, levonorgestrel, norethisterone and norgestimate on the oxidation of ethinyloestradiol and other substrates by human liver microsomes. J Steroid Biochem Mol Biol. 1991;38:219–225. doi: 10.1016/0960-0760(91)90129-s. [DOI] [PubMed] [Google Scholar]


