Abstract
Aims
To determine the reproducibility and dose–response relationship for urinary salbutamol excretion post inhalation.
Methods
Fifteen volunteers inhaled either one, two, three, four or five doses of 100 µg salbutamol on separate days and then seven of these also repeated each of the one, three and five doses on five occasions. After each study dose urine was collected 30 min post inhalation.
Results
The mean (SD) 30 min urinary salbutamol after one, two, three, four and five doses was 2.61 (1.0.), 5.47 (1.59), 8.68 (2.73), 12.34 (3.96) and 15.99 (4.50) µg, respectively. Statistical analysis revealed a linear relationship (P < 0.001). Mean (SD) coefficient of variation after one, three and five doses was 10.5 (3.6), 10.1 (2.7) and 9.4 (2.3)%.
Conclusions
The 30 min salbutamol urinary excretion post inhalation pharmacokinetic method, to compare inhaled products, is linear with inhaled dose and reproducible.
Keywords: dose–response, inhalation, urinary salbutamol
Introduction
The bioequivalence of inhaled products is complicated because the therapeutic effect is influenced by the topical deposition of drug in the lungs and safety is determined by systemic delivery via the oral and gastrointestinal routes. The FDA has recommended that the therapeutic efficacy between an innovator and generic inhaled product is assessed by a human bioassay experiment that measures the degree of protection to a bronchoprovocation agent [1]. This method measures the amount of inhaled bronchoprovocation agent that is required to reduce the FEV1 by 20% following the inhalation of a placebo and then active drug from the innovator and generic inhalers. Any method used in bioequivalence studies should be reproducible and have a dose–response relationship. Although a dose–response relationship has been reported [2] a bronchoprovocation study comprising two doses from an innovator and generic product suggested that therapeutic equivalence may have been shown due to the plateau of the dose–response curve [3]. This assumption was made because the two products had a different emitted fine particle dose determined by in vitro methods. The reproducibility of this bioequivalence method has not been reported.
The potential of pharmacokinetic methods to determine the bioequivalence of inhaled products has recently been highlighted [4]. We have shown that the amount of salbutamol excreted in the urine during the first 30 min post inhalation represents the amount of drug deposited in the lungs [5]. This urinary salbutamol pharmacokinetic method has been used to compare generic and innovation metered dose inhalers when attached to a large volume spacer [6] and dry powder inhalers [7, 8]. To consolidate the potential of the urinary salbutamol pharmacokinetic method for bioequivalence studies we have assessed its dose–response relationship and reproducibility.
Methods
Ethical approval was obtained from the University of Bradford and all volunteers gave signed informed consent. All subjects were healthy, nonsmoking volunteers older than 18 years with a FEV1 > 90%. They all received training on how to use a metered dose inhaler (MDI). Subjects were trained to exhale slowly as far as comfortable, put the MDI into their mouth and seal their lips round the mouthpiece. They were then instructed to start a slow inhalation through their mouth and activate the MDI immediately after the start of this slow inhalation. This slow inhalation continued until total lung capacity and subjects were trained so that this procedure took at least 5 s. Hence the inhalation rate would be 30–45 l s−1 because inhalation volumes are normally between 2.5 and 4 l. After inhalation they held their breath for 10 s and if another dose was scheduled this was inhaled 30 s later. On each of the 5 study days, following a light breakfast and no caffeine or alcohol containing drinks for at least 12 h, each subject inhaled either one, two, three, four, or five doses from a salbutamol MDI (Ventolin, GlaxoSmithKline), 100 µg per dose, according to a latin square randomization. Each MDI was primed by discharging five doses to waste prior to each study dose(s). Immediately before each study dose(s) each subject voided their urine and then provided a urine sample 30 min after the start of the first dose. The volume was measured and the urinary salbutamol assayed using HPLC [6].
To determine the urinary salbutamol dose–response relationship a two way analysis of variance was used to identify if there was a distinct dose effect. From this the dose of sum of squares was further partitioned into orthogonal polynomial contrasts so that the shape of the dose–response relationship could be determined. Further analysis looked at the linear regression to identify r, intercept and slope, for each subject, together with an inspection to determine if there was a systematic deviation from the overall line of linear regression using the residuals.
Seven of the volunteers also agreed to repeat each of the one, three, and five dose studies, using the MDI, five times to determine the reproducibility of the 30 min urinary salbutamol. Six of these also agreed to inhale one 100 µg salbutamol dose from an Easibreathe MDI on five separate occasions and provide urine samples 30 min post inhalation to determine the reproducibility when a breath-actuated MDI is used. The inhalation technique for the Easibreathe was the same as above except subjects did not need to activate a dose during inhalation.
Results
Fifteen (eight females) healthy subjects with a mean (SD) age of 30.3 (6.8) years weighing 68.5 (11.4) kg, 1.71 (0.11) m tall and FEV1 102.4 (6.3)% predicted completed all five inhalations. Figure 1 shows that although there was an intersubject variability the amount of salbutamol excreted in the urine during the first 30 min was linear (P > 0.001) with dose. The mean (90% of confidence interval) for the r values was 0.969 (0.958, 0.979). The individual regression models revealed that all intercepts except one were not significantly different from zero. Also, without exception, the regression slopes were significantly above zero. Examination of the 15 individual patient regression models was explored for residuals errors and the number of doses. No departure from random plotting was detected. The mean (SD) salbutamol excreted after one, two, three, four and five doses during the 30 min post start of the inhalation was 2.61 (1.01), 5.47 (1.59), 8.68 (2.73), 12.34 (3.96) and 15.99 (4.50) µg, respectively.
Figure 1.
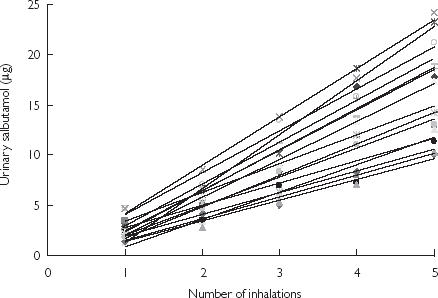
The individual amounts of urinary salbutamol excreted during the first 30 min post inhalation
Seven (four females) with a mean (SD) age, weight and height of 29.1 (4.5) years, 66.1 (14.4) kg and 1.70 (0.13) m, respectively, completed the MDI reproducibility study. Their mean 30 min post inhalation urinary salbutamol excretion and coefficient of variation for the three doses is shown in Table 1. The mean (SD) urinary salbutamol in the 30 min post inhalation was 2.28 (0.81), 7.10 (2.87) and 12.62 (4.54) µg following one, three and five doses and the mean (SD) within subject coefficient of variation was 10.5 (3.6), 10.1 (2.7) and 9.4 (2.3)%, respectively. Following one inhalation from the Easibreathe individual coefficients of variation for the 30 min urinary salbutamol excretion were 5.9, 5.8, 6.2, 9.0, 6.0, 9.6 giving a mean (SD) of 7.1 (1.7)%. Their mean (SD) age, weight and height was 32.0 (2.9) years, 1.73 (0.12) m and 66.8 (14.1) kg and the amount of salbutamol excreted in the urine over the first 30 min post inhalation was 2.52 (0.66) µg.
Table 1.
Each individual's mean urinary salbutamol excretion, in µg, and the (% coefficient of variation) of the five separate inhalations for the three doses used.
| Number of salbutamol (100µg) inhalations | |||
|---|---|---|---|
| Subject | 1 | 3 | 5 |
| 1 | 2.60 (3.5) | 10.26 (10.0) | 19.15 (13.4) |
| 2 | 2.47 (12.2) | 9.81 (6.7) | 16.29 (9.5) |
| 3 | 3.60 (7.8) | 10.65 (6.6) | 16.99 (7.4) |
| 4 | 1.70 (12.9) | 5.28 (12.1) | 9.91 (7.4) |
| 5 | 2.56 (10.9) | 5.15 (9.7) | 9.07 (10.3) |
| 6 | 0.94 (12.8) | 3.89 (12.6) | 8.11 (7.2) |
| 7 | 2.09 (13.4) | 4.64 (12.9) | 8.83 (11.0) |
Discussion
Healthy volunteers were used because lung deposition is affected by airway calibre and their renal function should remain stable [9]. The intersubject variability was high (e.g. 38.7% after one dose) due to between subject variability of lung deposition together with their renal excretion. This variability between subjects is consistent with previous reports [5–8]. However the intrasubject variability was much lower and similar to that reported by Hindle & Chrystyn when volunteers inhaled four doses [5]. Hence the method is suitable for cross-over studies. The lower variability using the breath-actuated metered dose inhaler indicates how co-ordination can exert an influence in bioequivalence studies. The dose–response relationship for the 30 min salbutamol urinary excretion was linear and thus all subjects demonstrated first order elimination pharmacokinetics. Simultaneous measurements of 30 min urinary salbutamol and the dose of methacholine to reduce the FEV1 by 20% following one and two doses, of salbutamol from a Easibreathe in 12 asthmatics have shown the two methods to be equal [10]. This positive link to a clinical bioassay together with the reproducibility and dose–response properties highlight the potential of this 30 min index of lung deposition in bioequivalence. The results also highlight the importance of pharmacokinetic studies when determining the bioequivalence of inhaled products. Although this evidence may not convince the Regulatory Bodies who regard a clinical endpoint as the gold standard it is a useful and simple method to identify ‘proof of concept’ when formulating inhaled products or evaluating an inhaled device.
References
- 1.Adams WP, Pouchikian G, Taylor AS, Patel RM, Burke GP, Williams RL. Regulatory aspects of modifications to innovator bronchodilator metered dose inhalers and development of generic substitutes. J Aerosol Med. 1994;0:119–134. doi: 10.1089/jam.1994.7.119. [DOI] [PubMed] [Google Scholar]
- 2.Creticos PS, Petty BG, Khattignavong A, et al. Development of pharmacodynamic–methodology for determination of albuterol MDI bioequivalence. Methacholine challenge study. J Allergy Clin Immunol. 1994;93:249. doi: 10.1067/mai.2002.129036. [DOI] [PubMed] [Google Scholar]
- 3.Mallol J, Aguirre V, Rhem R, Rodriguez J, Dolovich M. Therapeutic equivalence of three metered-dose inhalers containing salbutamol (albuterol) in protecting against meH-induced bronchoconstriction in children with asthma. Pediatric Pulmonol. 2001;32:447–452. doi: 10.1002/ppul.1157. [DOI] [PubMed] [Google Scholar]
- 4.Chrystyn H. Methods to identify drug deposition in the lungs following inhalation. Br J Clin Pharmacol. 2001;51:289–299. doi: 10.1046/j.1365-2125.2001.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hindle M, Chrystyn H. Determination of the relative bioavailability of salbutamol to the lungs following inhalation. Br J Clin Pharmacol. 1992;34:311–315. doi: 10.1111/j.1365-2125.1992.tb05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chege JK, Chrystyn H. Volumatic usage: some generic salbutamol metered dose inhalers can be used. Thorax. 1994;49:1162–1163. doi: 10.1136/thx.49.11.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hindle M, Newton DAG, Chrystyn H. Dry powder inhalers are bioequivalent to metered dose inhalers – a study using a new urinary albuterol (salbutamol) assay technique. Chest. 1995;107:629–633. doi: 10.1378/chest.107.3.629. [DOI] [PubMed] [Google Scholar]
- 8.Hindle M, Parry-Billings M, Peers EM, Chrystyn H. Relative bioavailability of salbutamol to the lungs following inhalation via a novel dry powder inhaler and a standard metered dose inhaler. Br J Clin Pharmacol. 1997;43:336–338. doi: 10.1046/j.1365-2125.1997.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipworth BJ, Clark DJ. Effects of airway calibre on lung delivery of nebulised salbutamol. Thorax. 1997;52:1036–1039. doi: 10.1136/thx.52.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrystyn H, Allen MD, Corlett SA, Tomlinson HS. Simultaneous measurement of pharmacodynamic and pharmacokinetics parameters which can be used to evaluate the equivalence of inhaled salbutamol. Am J Respir Crit Care Med. 1998;157:A636. [Google Scholar]


