Abstract
Aspirin is widely prescribed and confers considerable benefit to patients by reducing cardiovascular and cerebrovascular morbidity and mortality. Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective analgesics, antipyretics and reduce the inflammatory component in conditions such as rheumatoid arthritis. However, both agents are associated with an increased risk of gastrointestinal symptoms and the potentially serious consequences of gastroduodenal ulceration, bleeding and perforation. The introduction of highly selective cyclo-oxygenase (COX)-2 inhibitors or the coprescription gastroprotective agents with nonselective NSAIDs have offered strategies to reduce the incidence of such events. This review article analyzes the quantitative techniques that can be employed by clinical pharmacologists and the clinical studies performed to assess NSAID damage in the gastrointestinal tract.
Keywords: gastrointestinal tract, NSAIDs, volunteer endoscopy, outcomes studies
Introduction
Aspirin was originally developed as a better tolerated form of sodium salicylate that caused less dyspepsia. However, endoscopic studies established that its use was associated with ulcers and erosions. Ironically, these are not generally seen with sodium salicylate. In the 1960s the nonaspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) were developed as a potentially safer alternative to aspirin, but clinical and endoscopic studies showed that high levels of both nonulcer dyspepsia and of silent ulceration are also characteristic of these drugs. Within 6 months of NSAID treatment 5–15% of patients with rheumatoid arthritis discontinue therapy because of dyspepsia [1], whilst endoscopic studies show a 20% prevalence of ulceration, predominantly in the stomach and predominantly not associated with dyspepsia. The dysjunction between symptoms and ulceration represents one of the main problems in the management of patients taking NSAIDs.
Equally it has become clear that whilst ulcers are very common in patients taking NSAIDs, only a relatively small proportion of these patients progress to ulcer complications. In one prospective study, 13 of every 1000 patients with rheumatoid arthritis who take NSAIDs for 1 year had a serious gastrointestinal complication [2]. Given that patients can have NSAID-associated ulcers without dyspepsia whilst nonulcer dyspepsia is common, the question of whether acute or chronic endoscopic changes are predictive of complications becomes important.
These questions have become prominent with the availability of safer NSAIDs, including cyclo-oxygenase (COX)-2 inhibitors and effective prophylactic strategies such as proton-pump inhibitors (PPIs) or misoprostol. These approaches have been evaluated in acute volunteer endoscopy models, subsequently in 3- to 6-month patient endoscopy studies and in most cases in studies assessing clinically significant outcomes (i.e. ulcers and ulcer complications). This article reviews the methods available to clinical pharmacologists and assesses the relationship between them.
The first study
Initially, aspirin was thought to pass through the stomach unchanged, unlike sodium salicylate, which formed irritant salicylic acid after contact with gastric hydrochloric acid. However, the first report of the injurious effects of aspirin was published in 1938. Douthwaite & Lintott [3] studied the effect of aspirin in 16 patients (13 male, mean age 46.8, range 31–63 years). After aspiration of the gastric contents, 15 grains (1 g) of aspirin was swallowed with 1oz water, followed by gastroscopic examination under local anaesthetic. These were mildly heroic experiments, involving the passage of a rigid gastroscope down the oesophagus of patients with the neck in the fully extended position. In 81% of subjects (n = 13), the gastric mucosa demonstrated a localized inflammatory reaction which varied from slight to intense hyperaemia with the development of submucosal haemorrhage (Figure 1). No correlation was found between the reaction to aspirin and the acidity of the aspirated gastric contents. The inflammatory response was in the immediate vicinity of the drug particles and was mostly confined to the lesser curve. Interestingly, and doubtless of relevance to clinical pharmacologists reading this article, this study also investigated the effects of pink gin and quarter strength mustard on the gastric mucosa. The latter induced an intense flush in the area of exposed stomach, whilst pink gin caused no visible reaction. This led the authors to emphasize ‘the importance of the avoidance of mustard, not only in its ordinary state but also the many masked forms in which it appears as a condiment, by those whose gastric health is not robust’!
Figure 1.
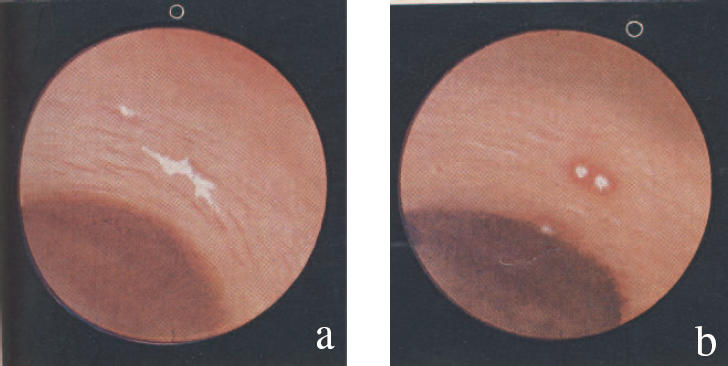
The illustrated endoscopic appearances of (a) inert barium sulphate, showing no reaction, and (b) aspirin, with a surrounding zone of hyperaemia on the gastric mucosa. From [3], with permission.
Subsequent studies
Radio-labelled erythrocytes
Later volunteer studies investigated the effects of drugs on gastric mucosal bleeding using radio-labelled red blood cells [4, 5]. Labelling is relatively simple; red cells are incubated ex vivo with 51chromium and then re-injected into subjects. Stools are collected over periods of time up to about a month (the duration of the experiment being limited by both the sensibilities of the subject and the half life of 51chromium). Initially, the bleeding detected was assumed to come from the stomach. Subsequently, it has become clear that this measure reflects whole gut blood loss and it has been revived for this purpose. Early studies also suggested that some results might have been spurious as some 51chromium is detached from red cells and excreted in the bile. Aspirin influences biliary flow [6, 7], and could conceivably give false results.
Recent painstaking improvements of this technique by Hunt and collegues [8] have re-established its role, though with the correct interpretation that it is measuring whole gut bleeding, probably largely coming from the small intestine [9]. Improvements include compacting of stools to a standardized shape so that the vagaries of stool morphology do not confound results. This technique presents considerable problems of disposal in the modern radiation sensitive world but represents an elegant, highly quantifiable assessment of whole gut microbleeding (Figure 2).
Figure 2.
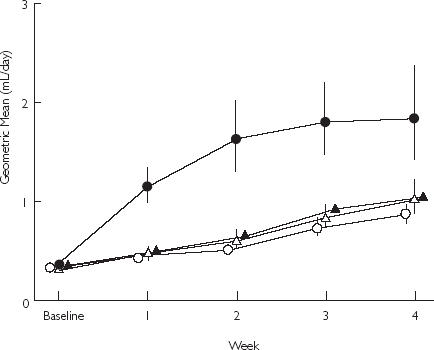
Geometric mean daily faecal blood loss, calculated using 51chromium-labelled erythrocytes, with 84% confidence intervals. From [9], with permission. Placebo (○); Rofecoxib 50 mg (▵); Rofecoxib 25 mg (▴); Ibuprofen 2400 mg (•).
Gastric washings
A more direct approach to acute mucosal bleeding involved measurement of microscopic blood using the peroxidase activity of haemoglobin in timed gastric washings [10–12]. After a period of aspirin ingestion, gastric juice is washed and aspirated through a Salem sump orogastric tube. Gastric mucosal bleeding is assessed by adding aliquots of gastric aspirate to citrate buffer and orthotolidine in cuvettes, with hydrogen peroxide (20 volumes per 100 ml) added after 45 s. The peroxidase activity of haemoglobin liberates oxygen from hydrogen peroxidase, which oxidizes orthotolidine to a blue colour. This is then quantified spectophotometrically 30 and 60 s after the addition of hydrogen peroxide. Two days treatment with aspirin 600 mg twice daily increased mucosal blood loss by a factor of 9.3 (P < 0.001) in one study [12] compared with placebo. The sensitivity threshold for blood detection was determined in vitro as 2 µl l−1 (or 2 parts per million).
Concerns over the possible carcinogenicity of orthotolidine [13] and advances in volunteer endoscopy have limited the recent use of this technique. Nevertheless, the recognition that some NSAIDs such as aspirin and naproxen [14] have a particular ability to influence platelet function and enhance bleeding has restored a role to this technique as an evaluation of intragastric haemostasis to be interpreted in the light of direct endoscopic data [15]. In one recent study, the selective COX-2 inhibitor lumiracoxib caused no endoscopically detectable mucosal injury compared with naproxen [16]. In addition, a modification of the gastric washing technique in which washing was conducted down the endoscope showed that naproxen, as in previous studies, enhanced both spontaneous and biopsy-induced bleeding, like aspirin, whilst lumiracoxib did not.
A recognition that bleeding and mucosal injury are separate phenomena also allows older results to be re-interpreted. Ranitidine causes substantial reductions in intragastric bleeding [17] but actually has limited effects on mucosal injury [18]. One possible explanation for this finding, which may be of value in assessing treatments for intragastric haemorrhage, is that changes in pH reduce bleeding directly. It has become clear that the very limited effect of H2-receptor antagonists on mucosal injury, rather than their effects on intragastric haemostasis, represents the dominant factor in clinical practice. Clinical studies show that histamine H2-receptor antagonists at normal dose are unable to prevent gastric ulcers detected endoscopically [19], consistent with results from acute endoscopic studies of H2-receptor antagonists, and they probably have little or no benefit in ulcer bleeding, making their effects on haemostasis of limited clinical relevance. This may not, however, be the case for PPIs. Like H2-receptor antagonists, PPIs reduce acute intragastric bleeding but also have a direct effect on endoscopically detected lesions. Nevertheless, they are not as effective in reducing gastrointestinal complications as is the substitution of the standard NSAID with a COX-2 inhibitor in the acute situation.
Volunteer endoscopy
This direct approach to the assessment of acute mucosal injury was pioneered by Lanza. In a series of painstaking endoscopic studies in which Lanza himself conducted virtually all the endoscopies, he showed that aspirin and nonaspirin NSAIDs caused acute mucosal erosions, although some NSAIDs such as nabumetone, etodolac and oxaprozin did not [20]. However, other studies showed that azaproprazone caused little acute injury [21], whilst subsequent epidemiological data showed this drug was particularly associated with ulcer haemorrhage. Thus, these studies suggested that acute endoscopic studies were not particularly valuable in predicting which of the nonselective NSAIDs would be safer than others in clinical practice.
This does not, however, seem to be the case for either protective strategies or the evaluation of COX-2 inhibitors [22]. In protective strategy experiments, aspirin or a nonaspirin NSAID are used to invoke injury, and are coprescribed with a gastroprotective agent (e.g. a PPI). Aspirin is particularly useful as the acute response is highly reproducible and virtually all subjects have some mucosal injury within 90 min. However, the endpoint chosen in a protective strategy experiment can affect the result. Typically, the number of erosions and petechial haemorrhages are counted as endpoints, or a Lanza score is derived. Where the latter is done, a binary endpoint is usually presented (typically the proportion of subjects with Lanza grade ≥2, i.e. at least one erosion). Some confusion has arisen in the literature as a number of different but related scores have emanated from Lanza's group. In our unit we prefer to report absolute numbers of erosions as the endpoint rather than converting them to a categorical score.
There has been some controversy about the relative importance of erosions and petechiae and the latter are thought to be less significant forms of injury than erosions. Indeed, it is possible to identify differences between drugs by their effect on erosions, where misoprostol has been quite effective, and their effect on petechiae where standard doses of H2-receptor antagonist can reduce injury scores [23]. Depending on which primary outcome is used, different and potentially misleading conclusions can be drawn. For example, one study reports a significant reduction in petechial haemorrhages in NSAID users coprescribed an H2-receptor antagonist or sucralfate. In the same study there was no reduction in erosions or ulcerations, which are more relevant lesions in gastroduodenal injury. Since H2-receptor antagonists do not prevent long-term NSAID-associated ulcers from developing and may even be associated with increased risk of ulcer complications [1, 24], perhaps because suppressed symptoms prevent early diagnosis, this suggests that use of petechiae as an acute endpoint in the assessment of injury is of limited, if any, value.
A further difficulty with endoscopic studies arises if volunteers develop an acute ulcer, as a contentious issue has been how ulcers are defined. A proposal originally made by Graham and colleagues [25], that has stood the test of time, was that an ulcer is a 3-mm break in the mucosa with depth. Such a distinction from an erosion is probably rather a fine one, particularly in the context of an acute volunteer endoscopy study. Some systems regard a single ulcer as invariably worse than erosions, a situation that may be contentious if one acute ulcer is compared to 20 acute erosions, whilst others give ulcers the same status as erosions. This however, does not appear to invalidate its use in most studies, as the majority of ulcers (probably 80%) are lesions larger than 5 mm and studies with 3 mm ulcers and 5 mm ulcers yield similar results. Moreover, it is now realized that as well as ulcers, erosions are probably predictive of gastrointestinal outcomes.
Volunteer COX-2 studies
Acute volunteer studies show consistently that COX-2 inhibitors even at an exceptionally high dose do not cause any erosions compared with placebo [16, 26, 27] (Figure 3). This is not surprising since the drugs lack an intrinsic mechanism for inducing toxicity. By contrast, studies of NSAIDs with high dose H2-receptor antagonists or PPIs coprescribed (which is probably as effective as use of COX-2 inhibitors in patient studies) generally still show some persisting erosions, although the number is reduced. Although seldom compared directly, prostaglandin analogues such as misoprostol generally result in fewer erosions [28] than an acid suppressive regime during NSAID treatment, a result that is interesting as long-term studies suggest that acid suppression may be more effective against ulcers and less effective against erosions compared to misoprostol. Recently, a third protective strategy has been assessed with the use of nitric oxide (NO)-donating NSAIDs, otherwise known as cyclooxygenase inhibiting NO-donators (CINODs) [29]. These drugs have been very effective in animal models. In human studies, the CINOD AZD3582 (NO-naproxen) behaved like a combination of an NSAID with a PPI in showing reduced but not absent erosions [30].
Figure 3.
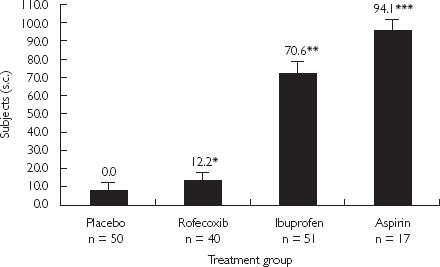
Percentage of subjects with a maximum gastric or duodenal lesion score = 2. (*, P = 0.525 vs placebo. **, P < 0.001 vs placebo and rofecoxib. ***, P < 0.001 vs placebo and rofecoxib (MK-0966); P = 0.054 vs ibuprofen). From [27], with permission.
Chronic patient endoscopic studies
Protection with proton pump inhibitors
The use of PPIs as NSAID prophylaxis has been extensively investigated in chronic patient studies. These have included primary prophylactic studies of patients that did not have an ulcer at baseline [19, 31], pragmatic prophylactic studies in which there was no baseline endoscopy [32] and secondary prophylactic studies in which patients found to have an ulcer at initial endoscopy underwent a course of healing before randomization to maintenance treatment [28, 33] (Figures 4 and 5). All of these studies have been consistent in showing a reduction in NSAID ulceration of approximately three-fold in patients taking PPIs compared with placebo, with reductions in NSAID-associated symptoms as well.
Figure 4.
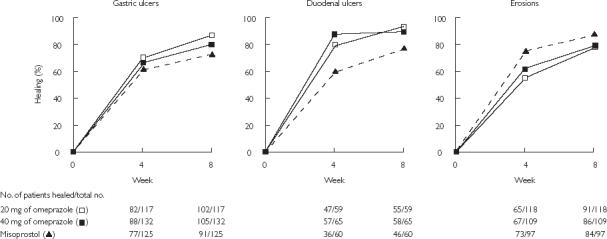
Cumulative healing rates of gastric ulcers, duodenal ulcers, and erosions at 4 and 8 weeks during treatment with 20 mg omeprazole daily, 40 mg omeprazole daily, or 200 µg misoprostol four times daily. From [28], with permission
Figure 5.
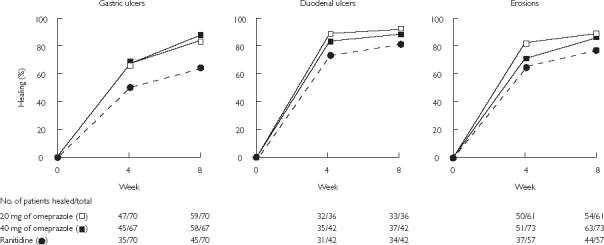
Cumulative healing rates of gastric ulcers, duodenal ulcers, and erosions at 4 and 8 weeks during treatment with 20 mg omeprazole daily, 40 mg omeprazole daily, or 150 mg ranitidine twice daily. From [33], with permission.
Misoprostol protection
Most studies of misoprostol coprescribed with NSAIDs have been in patients with an undamaged mucosa at baseline. In these studies, patients who are already using NSAIDs typically undergo a washout period, or those who start NSAIDs are assessed by prior endoscopy. Patients with osteoarthritis are usually studied, although sometimes a mixed population or those with rheumatoid arthritis comprise the study group. Patients typically undergo endoscopy at baseline and after 13 weeks, often with an interval endoscopy in between. It is important to realise that those with interval endoscopies report a cumulative ulcer incidence, which is, for obvious reasons, higher than those with an endoscopy at the end of the study. These studies have shown a reduction in the development of ulcers detected endoscopically with misoprostol. In a secondary prophylactic setting, patients managed with a relatively low dose of misoprostol (200 mg twice daily) did less well than those managed with omeprazole 20 mg with regards ulcer recurrence or symptoms [28].
Outcomes studies
The first outcomes study to be conducted was the MUCOSA study [34]. In this study patients were randomized to receive misoprostol 200 mg four times daily or placebo. Because of tolerability problems, patients only took on average 600 mg daily of misoprostol. There was a high drop out rate because of adverse effects (42%, mostly due to gastrointestinal side-effects) but nevertheless a reduction in admission to hospital for clinically significant ulcer complications was shown (Figure 6). The MUCOSA study vividly highlighted the problems of conducting outcomes studies. Endpoint precision is difficult to achieve because the unpredictable nature of ulcer complications means that gastroenterologists not aware of the trial assessed the majority of patients. The assessments were therefore not done prospectively to a protocol. This led to some controversy about the validity of the MUCOSA study but it was accepted as showing a significant reduction and the authors concluded that the trial had validated endoscopic assessment with which it correlated. Although one major conclusion of this study was that misoprostol reduced serious NSAID-induced upper GI complications by 40%, the absolute risk of a serious complication in the placebo group was only 0.95% (42/4439) and the number needed to treat (NNT) with misoprostol to prevent one complication was 263.
Figure 6.
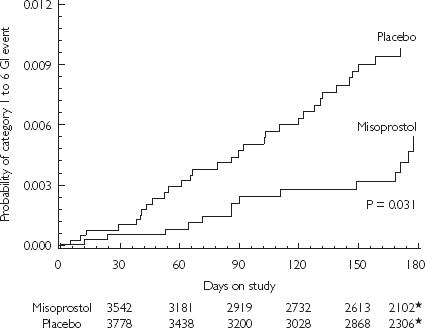
Kaplan-Meier curves for gastrointestinal (GI) events for the misoprostol and placebo groups. The numbers below the horizontal axis are the numbers of patients remaining in the study at the times shown. *= the number of patients in each group remaining in the study at 170 days. From [34], with permission
Outcomes studies of COX-2 inhibitors
Given the correlations between endoscopy and outcomes the MUCOSA study established, it is somewhat ironic, when COX-2 inhibitors were shown to have ulcer rates similar to placebo in endoscopic studies, that regulatory authorities pointed to a need for further outcomes studies to show directly that ulcer complications were reduced, before accepting a label change about ulcer complications. Two outcomes studies were set-up, both of which have lead to considerable controversy. In these studies, patients were randomized to a COX-2 inhibitor or a comparator nonselective NSAID, without screening endoscopy.
VIGOR study
The VIOXX In Gastrointestinal Outcome Research (VIGOR) study [35] compared a supratherapeutic dose of rofecoxib (50 mg daily) with a normal dose of naproxen (500 mg twice daily). The primary endpoint of this study was perforations, ulcers and bleeds (PUBs), which includes considerable numbers of uncomplicated ulcers diagnosed because of symptoms. A secondary endpoint included complicated events (largely but not exclusively ulcer complications of perforation, pyloric obstruction and bleeding). The VIGOR study in addition recorded lower GI events although these were evaluated less formally than upper GI events. The study showed an approximate halving in the rate of both upper and lower GI events (Figure 7). The NNT with rofecoxib compared with naproxen to reduce uncomplicated gastrointestinal events was 62, while this rose to 192 to reduce complicated GI events. Because there was no placebo arm, it is not known whether the reduction was to a placebo level.
Figure 7.
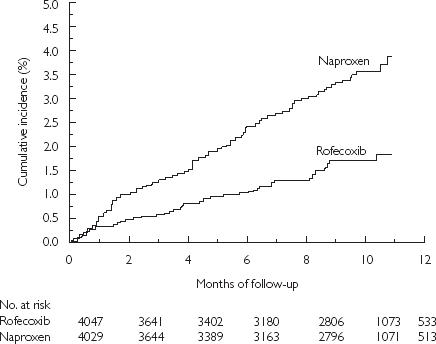
Cumulative incidence of the primary end point of a confirmed upper gastrointestinal event among all randomised patients. From [35], with permission
Although the VIGOR study was not powered to show a reduction in all-cause mortality, there were somewhat more deaths in the rofecoxib group. In addition the number of cardiovascular events including myocardial infarction (MI) was increased on rofecoxib (0.4%) compared with naproxen (0.1%). This difference was primarily accounted for by the rate of MI in the group of patients (4% of total) who met the FDA criteria for aspirin secondary prophylaxis, but who were not treated during the trial. The use of low dose aspirin was contraindicated in the VIGOR study and many events occurred in patients who had a prima facia indication for its use. Subsequently, all studies of COX-2 inhibitors have allowed low dose aspirin use. There was no significant difference in MI rates between the two treatment groups among patients without indications for aspirin therapy. It is not clear whether the differences between naproxen and rofecoxib arose because of an increase in events with rofecoxib or a reduction with naproxen.
CLASS
The Celecoxib Long-Term Assessment of Safety Study (CLASS) [36] was also controversial for different reasons. Much criticism has attached to the fact that 6-month data from a study lasting up to 13 months were published [37, 38]. It has been suggested that this represented an attempt by the authors to distort their results, but the journal that published the paper is not immune from criticism since most in the field knew that CLASS was not a 6-month study.
The 6-month data showed that there was a trend towards reduction in complicated events on celecoxib compared with the nonselective NSAIDs ibuprofen or diclofenac but this did not reach statistical significance (P = 0.09). It is very difficult to know whether CLASS was truly a positive or negative study. Some have concluded that it showed that COX-2 inhibitors do not reduce clinically significant outcomes [39] but this seems an unreasonable conclusion in view of a wealth of other endoscopic trial and epidemiological data to the contrary. Rather, CLASS was a badly designed, underpowered study that failed to deliver a clear message. An ad hoc analysis of patients not taking aspirin did show a reduction whilst those taking aspirin appeared to have a similar event rate on celecoxib and nonselective NSAIDs.
Secondary outcomes
It is somewhat ironic that the term ‘outcomes study’ has become synonymous with very large studies of supposedly unselected populations, since a large number of clinical decisions have to be taken at the point where a patient on an NSAID presents with an ulcer complication in the first place. Those patients where continued use of an NSAID is important for the quality of life or continued use of aspirin for longevity, present a particularly acute diagnostic dilemma. A large body of data now exists in support of the use of proton pump inhibitor prophylaxis for such patients. A recent study also suggests benefits of COX-2 inhibitors.
In one study, Helicobacter pylori positive patients taking aspirin or NSAIDS who presented with a bleeding gastric ulcer were randomized either to H. pylori eradication or treatment with standard dose of omeprazole (20 mg) [40]. After ulcer healing, naproxen 500 mg twice daily or aspirin were re-started according to original presentation. The rate of further presentation with ulcer re-bleeding over a further 6 months was 18.8% in those who received H. pylori eradication vs 4.4% in those treated with omeprazole alone (Figure 8). These are very important results as they establish that the strategy of gastric prophylaxis is clinically valid in patients who have presented with an ulcer bleed. Very recently a comparison, after H. pylori eradication, between diclofenac use with a PPI and substitution of a COX-2 inhibitor (celecoxib) shows that both strategies are of apparently equal value in this situation [41]. However, it could be argued that this comparison is a little loaded against PPI strategy because the NSAID used may destabilize haemostasis as well as causing ulcers [42] and the policy of H. pylori eradication may have mitigated against the full effectiveness of the PPI.
Figure 8.
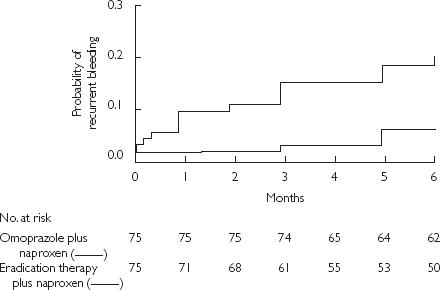
Cumulative probability of recurrent bleeding in users of nonaspirin NSAIDs who received eradication therapy for Helicobacter pylori plus naproxen, or maintenance omeprazole plus naproxen. The difference between groups was significant (P = 0.005 by the log-rank test). From [40], with permission
With regard to aspirin use for cardiovascular protection, the situation is also becoming clear. An initial study showed a surprisingly low rate of re-bleeding over 6 months following H. pylori eradication alone (1.9%) in patients with a bleeding peptic ulcer who restarted aspirin [40]. Therefore, further significant reductions in re-bleeding with PPI therapy were impossible to show in this study. A second study investigating patients with ulcer complications taking aspirin, all of whom had H. pylori eradicated, showed a higher rate of recurrent ulcer complications over 12 months in those receiving aspirin alone (14.8%) but a substantial, significant fall with PPI cotreatment (to 1.6%) [43].
These studies clearly show that proton pump inhibitors are valuable for patients who have presented with ulcer bleeding. The data mean, for example, that the common practice of stopping aspirin after an ulcer bleed is wrong and almost certainly dangerous since, where aspirin is indicated, its withdrawal is likely to lead to a higher rate of further serious cardiovascular events.
Discussion
This review has thus highlighted areas that remain contentious and raises the following interesting issues regarding the investigation of NSAID damage in the gastrointestinal tract:
Are erosions important?
On the basis of very little evidence, short-term erosions, or erosions found clinically at endoscopy, have been dismissed as being of little significance. In fact, the available evidence suggests that in clinical trials patients with erosions are more likely to develop ulcers and ulcer complications (a five-fold increase was recently reported [44]). Thus erosions, whilst relatively harmless in their own right, should be considered as indicating patients at higher risk for ulcers or complications.
Do endoscopy studies predict outcomes studies?
This question can be re-phrased ‘why is there a four-fold reduction in ulcers with COX-2 inhibitors in endoscopic studies compared with nonselective NSAIDs, but only a two-fold reduction in outcomes?’ At present this question cannot be answered. One possibility is that endoscopic studies paint an unduly optimistic picture. Alternatively, the difference in outcomes may be compromised by the imprecision surrounding outcome studies.
What should be the endpoint of outcomes studies?
The recent controversy over cardiovascular events in patients on COX-2 inhibitors [35] has highlighted the need to include critical events other than ulcer complications. How to weight the importance of hypertension, where events may follow after several years is particularly challenging.
How could outcomes studies be improved?
A major problem with outcomes studies is the imprecision of the endpoint. This may well be the reason why they have failed to detect as substantial a reduction as might have been predicted. Greater precision of endpoints can be achieved if clinicians or clinical pharmacologists assessing the endpoints are aware of the trial protocol. This requires that when patients on such trials fall ill, they almost inevitably go to a hospital where individuals trained in the trial protocol assess them. This requirement could be achieved if outcomes studies were conducted in countries, such as the United Kingdom, with a well-developed primary care system and a national health service where emergency hospital admissions are to a predictable hospital [22].
References
- 1.Singh G, Ramey DR, Morfeld D, Shi H, Hatoum HT, Fries JF. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Int Med. 1996;156:1530–1536. [PubMed] [Google Scholar]
- 2.Singh G, Triadafilopoulos G. Epidemiology of NSAID-induced gastrointestinal complications. J Rheumatol. 1999;26(Suppl 56):18–24. [PubMed] [Google Scholar]
- 3.Douthwaite AH, Lintott GAM. Gastroscopic observation of the effect of aspirin and certain other substances on the stomach. Lancet. 1938:1222–1225. [Google Scholar]
- 4.Arvidsson B, Magnusson B, Solvell L, Mangusson A. Acetylsalicylic acid and gastrointestinal bleeding measurement of blood loss using a modified radioactive chromium method. Scand J Gastroenterol. 1975;10:155–160. [PubMed] [Google Scholar]
- 5.Hawkey CJ. Review: acute human models of gastric mucosal injury. Aliment Pharmacol Ther. 1987;1:593–606. doi: 10.1111/j.1365-2036.1987.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 6.Hawkey CJ. Review article: aspirin and gastrointestinal bleeding. Aliment Pharmacol Ther. 1994;8:141–146. doi: 10.1111/j.1365-2036.1994.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt OR, Beazell JM, Atkinson AJ, Ivey AC. The effect of therapeutic agents on the constituents of bile. Am J Dig Dis. 1938;5:613–617. [Google Scholar]
- 8.Hunt RH, Harper S, Callegari PYuC, Quan H, Evans J, James C, Bowen B, Rashid F. Complementary studies of the gastrointestinal safety of the cyclo-oxygenase-2-selective inhibitor etoricoxib. Aliment Pharmacol Ther. 2003;17:201–210. doi: 10.1046/j.1365-2036.2003.01407.x. [DOI] [PubMed] [Google Scholar]
- 9.Hunt RH, Bowen B, Mortensen ER, Simon TJ, James C, Cagliola A, Quan H, Bolognese JA. A randomized trial measuring faecal blood loss after treatment with rofecoxib, ibuprofen, or placebo in healthy subjects. Am J Med. 2000;109:201–206. doi: 10.1016/s0002-9343(00)00470-8. [DOI] [PubMed] [Google Scholar]
- 10.Hunt JN. A procedure for measuring gastric bleeding caused by drugs. Dig Dis Sci. 1979;24:525–528. doi: 10.1007/BF01489320. [DOI] [PubMed] [Google Scholar]
- 11.Fisher MA, Hunt JN. A sensitive method for measuring haemoglobin in gastric contents. Digestion. 1976;14:409–414. doi: 10.1159/000197964. [DOI] [PubMed] [Google Scholar]
- 12.Hawkey CJ, Simpson G, Somerville KW. Reduction by enprostil of aspirin-induced blood loss from human gastric mucosa. Am J Med. 1986;81:50–53. doi: 10.1016/s0002-9343(86)80011-0. 2A. [DOI] [PubMed] [Google Scholar]
- 13.Walker B, Jr, Gerber A. Occupational exposure to aromatic amines: benzidine and benzidine-based dyes. Natl Cancer Inst Monogr. 1981;58:11–13. [PubMed] [Google Scholar]
- 14.Wight NJ, Gottesdiener K, Garlick NM, Atherton CT, Novak S, Gertz BJ, Calder NA, Cote J, Wong P, Dallob A, Hawkey CJ. Rofecoxib, a COX-2 inhibitor, does not inhibit human gastric mucosal prostaglandin production. Gastroenterology. 2001;120:867–873. doi: 10.1053/gast.2001.22432. [DOI] [PubMed] [Google Scholar]
- 15.Hawkey CJ, Hawthorne AB, Hudson N, Cole AT, Mahida YR, Daneshmend TK. Separation of the impairment of haemostasis by aspirin from mucosal injury in the human stomach. Clin Sci. 1991;81:565–573. doi: 10.1042/cs0810565. [DOI] [PubMed] [Google Scholar]
- 16.Hawkey CJ. PUCCINI (Prevention of Ulcers with COX-189, lumiracoxib, Compared with Ibuprofen in NSAID investigation). Reduced cumulative incidence of gastroduodenal ulcers with two doses of a new coxib, COX-189 (lumiracoxib) compared with standard therapeutic doses of ibuprofen in osteoarthritis patients. Gastroenterology. 2002;122:AM1732. [Google Scholar]
- 17.Hawkey CJ, Somerville KW, Marshall S. Prophylaxis of aspirin-induced gastric mucosal bleeding with ranitidine. Aliment Pharmacol Ther. 1988;2:245–252. doi: 10.1111/j.1365-2036.1988.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 18.Cole AT, Brundell S, Hudson N, Hawthorne AB, Mahida YR, Hawkey CJ. Ranitidine. differential effects on gastric bleeding and mucosal damage induced by aspirin. Aliment Pharmacol Ther. 1992;6:707–715. doi: 10.1111/j.1365-2036.1992.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 19.Taha AS, Hudson N, Hawkey CJ, Swannell AJ, Trye PN, Cottrell J, Mann SG, Simon TJ, Sturrock RD, Russell RI. Famotidine for the prevention of gastric and duodenal ulcers caused by non-steroidal anti-inflammatory drugs. N Engl J Med. 1996;334:1435–1439. doi: 10.1056/NEJM199605303342204. [DOI] [PubMed] [Google Scholar]
- 20.Lanza FL. Gastrointestinal toxicity of newer NSAIDs. Am J Gastroenterol. 1993;9:1318–1323. [PubMed] [Google Scholar]
- 21.Rainsford KD, James C, Johnson DM, Stetsko PI, Hill RE, Salena BJ, Hunt RH. Effects of chronic NSAIDs on gastric mucosal injury related to mucosal prostanoids, and plasma drug concentrations in human volunteers. Agents Actions. 1993;39 doi: 10.1007/BF01972708. Spec. no: C21–C23. [DOI] [PubMed] [Google Scholar]
- 22.Hawkey CJ. Outcomes studies of drug induced ulcer complications: do we need them and how should they be done? Br Med J. 2000;321:291–293. doi: 10.1136/bmj.321.7256.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanza FL, Graham DY, Davis RE, Rack MF. Endoscopic comparison of cimetidine and sucralfate for prevention of naproxen-induced acute gastroduodenal injury. Effect of a scoring method. Dig Dis Sci. 1990;35:1494–1499. doi: 10.1007/BF01540567. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of non-steroidal anti-inflammatory drugs. N Engl J Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 25.Larkai EN, Smith JL, Lidsky MD, Graham DY. astroduodenal mucosa and dyspeptic symptoms in arthritic patients during chronic non-steroidal anti-inflammatory drug use. Am J Gastroenterol. 1987;82:1153–1158. [PubMed] [Google Scholar]
- 26.Simon LS, Lanza FL, Lipsky PE, Hubbard RC, Talwalker S, Schwartz BD, Isakson PC, Geis GS. Preliminary study of the safety and efficacy of SC-58635, a novel cyclooxygenase 2 inhibitor: efficacy and safety in two placebo-controlled trials in osteoarthritis and rheumatoid arthritis, and studies of gastrointestinal and platelet effects. Arthritis Rheum. 1998;41:1591–1602. doi: 10.1002/1529-0131(199809)41:9<1591::AID-ART9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Lanza FL, Rack MF, Simon TJ, Quan H, Bolognese JA, Hoover ME, Wilson FR, Harper SE. Specific inhibition of cyclooxygenase-2 with MK-0966 is associated with less gastroduodenal damage than either aspirin or ibuprofen. Aliment Pharmacol Ther. 1999;13:761–767. doi: 10.1046/j.1365-2036.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 28.Hawkey CJ, Karrasch JA, Szczepanski L, Walker DG, Barkun A, Swannell AJ, Yeomans ND. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group. N Engl J Med. 1998;338:727–734. doi: 10.1056/NEJM199803123381105. [DOI] [PubMed] [Google Scholar]
- 29.Davies NM, Roseth AG, Appleyard CB, McKnight W, Del Soldato P, Calignano A, Cirino G, Wallace JL. NO-naproxen vs. naproxen: ulcerogenic, analgesic and anti-inflammatory effects. Aliment Pharmacol Ther. 1997;11:69–79. doi: 10.1046/j.1365-2036.1997.115286000.x. [DOI] [PubMed] [Google Scholar]
- 30.Hawkey CJ, Jones JIW, Atherton CT, Skelly MM, Bebb JR, Fagerholm U, Jonzon B, Karlsson P, Bjarnason IT. Gastrointestinal safety of AZD 3582: a new chemical entity with a novel multi-pathway mechanism of action. Gastroenterology. 2002;122:A446. [Google Scholar]
- 31.Hawkey CJ. Progress in prophylaxis against nonsteroidal anti-inflammatory drug-associated ulcers and erosions. Omeprazole NSAID Steering Committee. Am J Med. 1998;104:67S–74S. doi: 10.1016/s0002-9343(97)00215-5. [DOI] [PubMed] [Google Scholar]
- 32.Ekström P, Carling L, Wetterhus S, Wingren PE, Anker-Hanson O, Lundegardin G, Thorhailsson E, Unge P. Prevention of peptic ulcer and dyspeptic symptoms with omeprazole in patients receiving continuous non-steroidal anti-inflammatory drug therapy. A Nordic multicentre study. Scand J Gastroenterol. 1996;31:753–758. doi: 10.3109/00365529609010347. [DOI] [PubMed] [Google Scholar]
- 33.Yeomans ND, Tulassay Z, Juhasz L, Racz I, Howard JM, van Rensburg CJ, Swannell AJ, Hawkey CJ. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid Suppression Trial: Ranitidine versus Omeprazole for NSAID-associated Ulcer Treatment (ASTRONAUT) Study Group. N Engl J Med. 1998;338:719–726. doi: 10.1056/NEJM199803123381104. [DOI] [PubMed] [Google Scholar]
- 34.Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, Geis GS. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. The MUCOSA Study. Ann Intern Med. 1995;123:241–249. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]
- 35.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 36.Siverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburgh KM, Geis S. Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. The CLASS Study. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 37.Jüni P, Rutjes AWS, Dieppe PA. Are selective COX 2 inhibitors superior to traditional non steroidal anti-inflammatory drugs? Adequate analysis of the CLASS trial indicates that this may not be the case. Br Med J. 2002;324:1287–1288. doi: 10.1136/bmj.324.7349.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverstein F, Simon L, Faich G. Reporting of 6-month vs 12-month data in a clinical trial of celecoxib. JAMA. 2001;286:2399. [PubMed] [Google Scholar]
- 39.Micklewright R, Lane S, Linley W, McQuade C, Thompson F, Maskery N. Review article. NSAIDs, gastroprotection and cyclooxygenase-II-selective inhibitors. Aliment Pharmacol Ther. 2003;17:321–332. doi: 10.1046/j.1365-2036.2003.01454.x. [DOI] [PubMed] [Google Scholar]
- 40.Chan FK, Chung SC, Suen BY, Lee YT, Leung WK, Leung VK, Wu JC, Lau JY, Hui Y, Lai MS, Chan HL, Sung JJ. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N Engl J Med. 2001;344:967–973. doi: 10.1056/NEJM200103293441304. [DOI] [PubMed] [Google Scholar]
- 41.Chan FK, Hung LC, Suen BY, Wu JC, Lee KC, Leung VK, Hui AJ, To KF, Leung WK, Wong VW, Chung SC, Sung JJ. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med. 2002;347:2104–2110. doi: 10.1056/NEJMoa021907. [DOI] [PubMed] [Google Scholar]
- 42.Labenz J, Tillenburg B, Peitz U, Verdu E, Stolte M, Borsch G, Blum AL. Effect of curing Helicobacter pylori infection on intragastric acidity during treatment with ranitidine in patients with duodenal ulcer. Gut. 1997;41:33–36. doi: 10.1136/gut.41.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai KC, Lam SK, Chu KM, Wong BC, Hui WM, Hu WH, Lau GK, Wong WM, Yuen MF, Chan AO, Lai CL, Wong J. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346:2033–2038. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 44.Hawkey CJ, Laine L, Harper SE, Quan HU, Bolognese JA, Mortensen E. Rofecoxib Osteoarthritis Endoscopy Multinational Study Group. Influence of risk factors on endoscopic and clinical ulcers in patients taking rofecoxib or ibuprofen in two randomized controlled trials. Aliment Pharmacol Ther. 2001;15:1593–1601. doi: 10.1046/j.1365-2036.2001.01007.x. [DOI] [PubMed] [Google Scholar]


