Abstract
Aims
Inhaled corticosteroids alone or in combination with long acting β2-agonists are indicated for use in mild persistent asthmatics. We set out to evaluate effects on airway hyperresponsiveness (AHR) and airway calibre using hydrofluoroalkane fluticasone/salmeterol (FP/SM) vs. double the dose of fluticasone alone (FP).
Methods
Fourteen mild persistent asthmatics completed a randomized double-blind crossover study with 1-week run-in and washout periods prior to treatments. Subjects received 3 weeks of FP 250 µg or FP 125 µg/SM 25 µg as 1 puff twice daily. Methacholine PD20 and lung function were measured after both baseline and treatment periods.
Results
There were no significant differences in baseline values prior to randomized treatments. Compared with pooled baseline, FP/SM and FP conferred improvements (P < 0.05) on methacholine PD20: 2.5 (95% confidence interval 1.7, 3.2) and 1.6 (0.8–2.3) doubling dose improvements, respectively; between FP/SM vs. FP there was a 0.9 (0.4, 1.4) doubling dose difference (P < 0.05). For forced expiratory volume in 1 s (FEV1), forced mid-expiratory flow (FEF25−75) and morning peak expiratory flow (PEF), FP/SM but not FP conferred improvements (P < 0.05) compared with pooled baseline, with FP/SM being greater than FP (P < 0.05): differences in FEV1 of 7.2% (3.8, 10.6) predicted, FEF25−75 of 11.2% (6.3, 16.1) predicted, and morning PEF of 17 L min−1(1–32).
Conclusions
FP/SM conferred improvements on AHR and airway calibre, while twice the dose of FP improved only AHR in patients with mild asthma. The differential effects of FP/SM and FP suggest separate but complementary actions of the two moieties on airway inflammation and smooth muscle stabilization. This may explain the beneficial effects of combination inhalers on exacerbations.
Keywords: asthma therapy, inflammation, fluticasone, salmeterol
Introduction
Despite asthma being a chronic inflammatory condition of which inhaled corticosteroids (ICS) are the cornerstone of treatment, the addition of a long-acting β2-agonist may be a more beneficial therapeutic option than increasing the dose of ICS in mild persistent asthmatics. In a multicentre trial, it was demonstrated that in mild persistent asthmatics receiving ICS, the addition of formoterol 4.5 µg twice daily reduced the risk of severe exacerbations and improved lung function compared with doubling the dose of budesonide from 100 µg to 200 µg BD [1]. In the same study using steroid-naive asthmatics, budesonide 100 µg BD reduced the risk of severe exacerbations, while adding formoterol only conferred improvements on lung function. Similar benefits on exacerbations have been observed with salmeterol (SM) added to ICS across a range of asthma severities [2].
A combination of endpoints are vital in the overall assessment of asthma control, since measures of airway calibre in isolation do not provide information on the underlying inflammatory process or airway hyperresponsiveness (AHR). Furthermore, despite normal lung function, inflammation of the bronchial mucosa can persist, which left untreated can lead to airway remodelling [3]. Assessment of AHR, which is often the driving force behind bronchoconstriction and subsequent disability of asthma, is therefore an important measure of efficacy of different asthma treatments.
We performed a study to evaluate the effects of new hydrofluoroalkane-134a (HFA) suspension formulations of fluticasone (FP) alone and fluticasone/salmeterol (FP/SM) combination inhaler at half the ICS dose in mild persistent asthmatics. For airway efficacy we evaluated methacholine hyperresponsiveness as the primary outcome variable, as AHR is a fundamental component of the asthmatic disease process and provides complementary information to that of conventional lung function measures [4]. We also measured overnight urinary cortisol corrected for creatinine excretion as a measure of hypothalamic–pituitary–adrenal axis suppression, as this is as sensitive as a full 24-h integrated cortisol collection [5, 6].
Methods
Subjects
Sixteen mild persistent asthmatics were enrolled at random from our volunteer database. Inclusion criteria were: forced expiratory volume in 1 s (FEV1) > 80% predicted and methacholine provocative dose causing 20% fall in FEV1 (PD20) < 500 µg. For 3 months before the screening visit, all subjects were using a short acting β2-agonist alone or maintained on a constant ICS dose < 800 µg day−1 of beclomethasone equivalent, and had no history of respiratory tract infection or oral corticosteroid use. For the entire study period, subjects’ own medication was discontinued, and prior to entry informed written consent was obtained and the Tayside committee on medical research ethics gave ethical approval.
Design
Patients were randomized into a double-blind crossover study with a 1-week run-in and washout period prior to randomized treatments. Canisters were masked and were given as 1 puff BD. Investigators were unaware of the study inhaler administration sequence.
Subjects were given 3 weeks of HFA suspension formulations of 250 µg FP (Flixotide Evohaler 250 µg per actuation labelled dose, 1 puff BD; GlaxoSmithKline, Uxbridge, UK) and 125 µg FP 25 µg−1 SM combination (Advair Evohaler, 1 puff BD; GlaxoSmithKline) via a pressurized metered dose inhaler (pMDI). A run-in and washout of 1 week using an identical placebo inhaler was given prior to randomized treatments. On each visit, subjects were reminded to rinse their mouth after using their pMDI. Subjects required to have PD20 at the end of washout period within 1.5 doubling doses of the value after initial run-in. Following run-in, washout and randomized treatments (i.e. on four occasions, 6 h after last 08.00 h dose), subjects attended the laboratory at 14.00 h for methacholine hyperresponsiveness, spirometry and exhaled tidal nitric oxide (NO) measurements. A peak flow diary card was completed twice daily throughout the study and subjects collected overnight urine after the penultimate evening dose of study inhaler (22.00 h to 08.00 h).
Airway measurements
End-exhaled NO was measured using an integrated LR2000 clinical real-time gas analyser [7]. Spirometry was performed according to American Thoracic Society criteria [8] using a Vitalograph compact spirometer (Vitalograph Ltd, Buckingham Buckinghamshire, UK) which was calibrated daily. The methacholine bronchial challenges were performed using a standardized computer-assisted dosimetric method, in which cumulative doubling doses between 3.125 and 6400 µg were administered [9]. Using this method, a methacholine PD20≤ 100 mg is equivalent to a PC20 of ≤ 1 mg mL−1.
Urine assays
All assays were performed in duplicate. Urinary creatinine was measured on a Cobas-bioautoanalyser (Sigma Pharmaceuticals plc, Watford, UK). Urinary cortisol samples were assayed with a radioimmunoassay kit, having no cross-reactivity for FP (Diasorin Ltd, Wokingham, UK). The coefficient of variation for analytical imprecision of creatinine intra-assay and interassay was 4.2% and 1.7%, respectively; for cortisol the intra- and interassay coefficient of variation was 7.7% and 7.3%, respectively.
Statistical analysis
The study was powered at 80% to show a between-treatment difference of one methacholine doubling dose (the primary outcome variable) with sample size of 12. Data were analysed using ‘Statgraphics’ software (STSC Software Publishing Group, Rockville, MD, USA). The NO, methacholine PD20 and urinary cortisol/creatinine data were logarithmically transformed to normalize their distributions. An overall analysis of variance was performed followed by multiple-range testing with Bonferroni correction, set at 95% confidence limits (two-tailed, P < 0.05). All comparisons are denoted as being significant at P < 0.05 in order not to confound the overall α error. Having shown no significant differences between run-in and washout baseline values prior to each randomized treatment, subsequent comparisons were made between randomized treatments and a pooled baseline (average of baselines after placebo run-in and washout).
Results
Sixteen nonsmoking subjects were initially enrolled, of whom 14 (three males) completed the study. One patient dropped out after the initial washout period because of subsequent unresponsiveness to methacholine, while the other subject dropped out on the second randomized treatment period due to personal reasons. The mean (SE) age of those completing the study was 36 (4) years, with 10 of these maintained on ICS; the others used a short-acting β2-agonist only on an as-required basis. The mean (SE) daily inhaled corticosteroid dose was 243 (48) µg. Eight patients were taking beclomethasone dipropionate and two were taking budesonide.
At the initial screening visit prior to run-in, the mean FEV1 (SE) was 2.86 (0.21) L: 96% (2) of predicted and mean FEF25−75 was 2.58 (0.27) L: 65% (5) predicted. Geometric mean end tidal NO was 6.5 (0.9) parts per billion and geometric mean methacholine PD20 was 72 (21) µg.
There were no significant differences in baseline values after the run-in and washout periods prior to FP and FP/SM when analysed by treatment (Table 1). Subsequent comparisons between randomized treatments were therefore made vs. a pooled baseline.
Table 1.
Baseline absolute data prior to randomized treatments.
| Baseline values prior to randomized treatments | ||
|---|---|---|
| FP/SM | FP | |
| Methacholine PD20 (µg) | 105 (30) | 108 (31) |
| Exhaled nitric oxide (p.p.b.) | 9.1 (1.2) | 7.3 (1.5) |
| FEV1 (% predicted) | 90 (2) | 90 (3) |
| FEF25−75 (% predicted) | 60 (5) | 61 (6) |
| Morning expiratory peak flow (L min−1) | 440 (24) | 431 (24) |
| Overnight urinary cortisol/creatinine (nmol mmol−1) | 6.18 (1.03) | 6.11 (1.37) |
Values are means (SE) except methacholine PD20, nitric oxide and overnight urinary cortisol/creatinine, which are expressed as geometric means (SE). FEV1, Forced expiratory volume in 1 s, FEF25–75, forced mid expiratory flow.
Methacholine PD20
Compared with pooled baseline, FP/SM and FP conferred improvements (P < 0.05) on methacholine PD20: 2.5 [95% confidence interval (CI) 1.7, 3.2] and 1.6 (0.8, 2.3) doubling dose improvements, respectively, while between FP/SM vs. FP there was a 0.9 (0.4, 1.4) doubling dose difference (P < 0.05) (Figures 1 and 2, Table 2).
Figure 1.
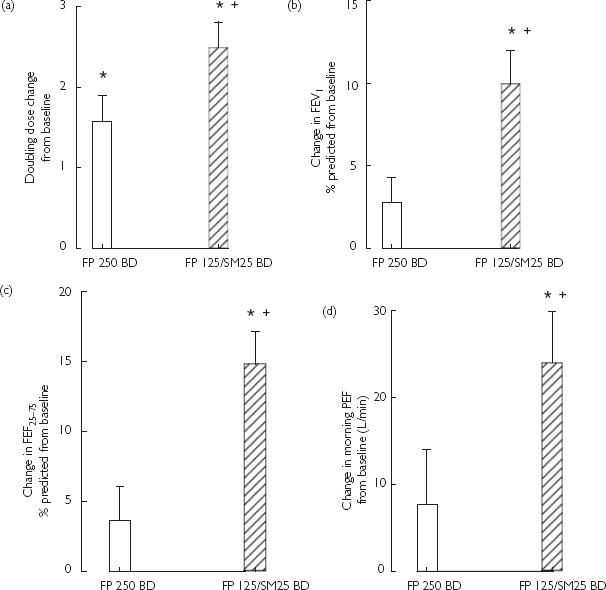
Mean (SE) values as change from pooled baseline after fluticasone (FP) 250 BD (□) and FP 125/salmeterol (SM) 25 BD ( ). *Significant (P < 0.05) difference from pooled baseline. †Significant (P < 0.05) difference between FP 125/SM 25 BD vs. FP 250 BD. (a) Methacholine doubling dose shift. (b) Forced expiratory volume in 1 s (FEV1) (% predicted). (c) FEF25−75 (% pre-dicted). (d) Morning PEF (L min−1).
). *Significant (P < 0.05) difference from pooled baseline. †Significant (P < 0.05) difference between FP 125/SM 25 BD vs. FP 250 BD. (a) Methacholine doubling dose shift. (b) Forced expiratory volume in 1 s (FEV1) (% predicted). (c) FEF25−75 (% pre-dicted). (d) Morning PEF (L min−1).
Figure 2.
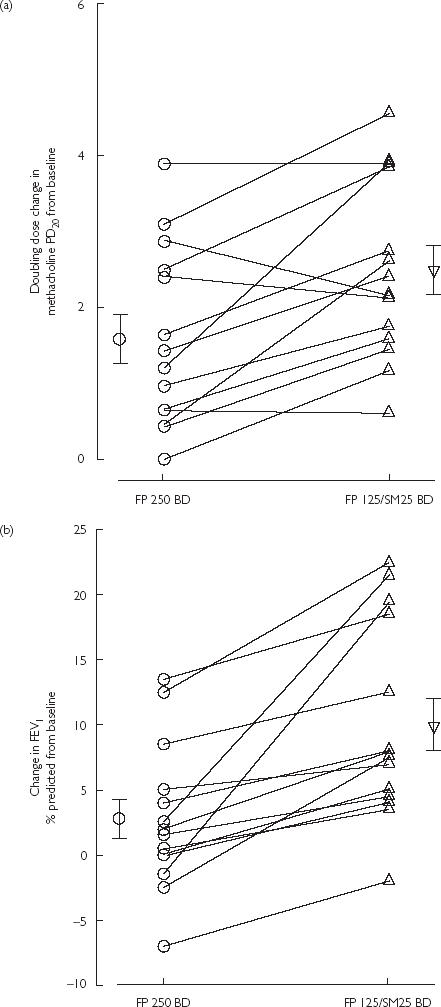
Scatterplots illustrating individual changes and means (SE) from pooled baseline after fluticasone (FP) 250 BD or FP 125/salmeterol (SM) 25 BD for (a) methacholine doubling dose shift, and (b) forced expiratory volume in 1 s (% predicted).
Table 2.
Airway and systemic data.
| Pooled baseline | FP/SM | FP | |
|---|---|---|---|
| Airway data | |||
| Methacholine PD20 (µg) | 107 (28) | 597 (182)*† | 319 (97)* |
| Exhaled tidal nitric oxide (p.p.b.) | 8.2 (1.2) | 4.0 (0.4)* | 4.3 (0.9)* |
| FEV1 (% predicted) | 90 (2) | 100 (2)*† | 93 (2) |
| FEF25−75 (% predicted) | 61 (6) | 75 (6)*† | 65 (5) |
| Morning peak expiratory flow (L min−1) | 435 (24) | 460 (26)*† | 443 (24) |
| Evening peak expiratory flow (L min−1) | 438 (26) | 456 (25)* | 446 (24) |
| Systemic data | |||
| Overnight urinary cortisol/creatinine (nmol/mmol) | 6.14 (0.90) | 4.89 (1.04) | 4.79 (0.91) |
Values are expressed as means (SE) except methacholine PD20, nitric oxide and overnight urinary cortisol/creatinine which are geometric means (SE).
Significant (P < 0.05) difference vs. pooled baseline.
Significant (P < 0.05) difference between FP/SM vs. FP.
Lung function
For FEV1, FEF25−75and morning PEF, FP/SM but not FP conferred improvements (P < 0.05) compared with pooled baseline, with FP/SM being greater than FP (P < 0.05): a difference in FEV1 of 7.2% (3.8, 10.6) predicted, FEF25−75 of 11.2% (6.3, 16.1) predicted, and morning PEF of 17 (1, 32) L min−1. For evening PEF, FP/SM was greater (P < 0.05) than pooled baseline amounting to a difference of 18 (3, 32) L min−1, but not different from FP alone, with the latter being no different from pooled baseline (Figures 1 and 2, Table 2).
Exhaled NO
Both FP/SM and FP suppressed (P < 0.05) exhaled NO compared with pooled baseline amounting to a 2.0 (95% CI 1.4, 3.1)-fold and 1.9 (1.3, 2.9)-fold fall, respectively, with no significant difference between treatments (Table 2).
Urine cortisol/creatinine
Compared with pooled baseline, both treatments were not significantly different, with there also being no difference between treatments (Table 2).
Discussion
Our findings have demonstrated that the combination of SM 25 µg with FP 125 µg BD resulted in greater improvements on AHR and measures of airway calibre than FP 250 µg BD alone. While FP/SM improved both airway calibre and AHR, the effects of FP alone were dissociated, being evident only on AHR. Thus, there was a clear disconnect between lung function and AHR following randomized treatments. In other words, in asthmatics with a well-preserved FEV1 but with evidence of moderate to severe AHR, the effect upon AHR was a more sensitive measure of anti-inflammatory activity than lung function.
When used in addition to ICS, long-acting β2-agonists have been shown to improve lung function and symptoms, in addition to reducing exacerbations and reliever inhaler requirements [2,10,11]. They exert beneficial effects by direct action upon airway smooth muscle causing bronchodilation and exhibit a protective effect due to functional antagonism in the presence of increased bronchomotor tone. This functional antagonism can be considered to be a surrogate for stabilization of airway smooth muscle and determines the degree of protection when exposed to a bronchoconstrictor stimulus. This in turn can be quantified from methacholine bronchoprovocation. The superiority of FP/SM vs. FP alone on AHR is therefore likely to be explained by an additive airway stabilizing effect, since SM has been shown to have no clinically meaningful in vivo anti-inflammatory activity [12–14]. This would also be consistent with the same trend showing superiority of FP/SM vs. FP on measures of airway calibre.
Current guidelines emphasize the importance of establishing asthmatics on ICS prior to addition of long-acting β2-agonist [15]. In the study by Lazarus et al. patients previously controlled on triamcinolone 800 µg daily were randomly switched to use SM as monotherapy or continue with triamcinolone for 4 months [16]. Patients who were assigned the former treatment experienced more exacerbations and showed an increase in sputum and blood inflammatory markers, despite maintaining their lung function. Likewise in the study by Lemanske et al.[17] in patients not controlled on triamcinolone 800 µg day−1, halving the ICS dose followed by elimination over 4 months with or without SM as add-on showed no significant impact on exacerbations with SM, in contrast to a significant reduction when the dose was left unchanged. These two studies highlight the different but complementary actions of long-acting β2-agonists and ICS on airway smooth muscle and inflammation, respectively. Our results are also in keeping with larger studies which have shown that adding a long-acting β2-agonist to ICS compared with doubling the dose of ICS is superior on markers of asthma control, especially exacerbations. Our findings would therefore support the hypothesis that effects of SM on exacerbations may be due to its stabilizing effects on airway smooth muscle.
It is important to note that the mean FEV1 of enrolled patients was 96% of predicted and 70% were maintained on ICS; however, all demonstrated moderate to severe AHR to methacholine according to American Thoracic Society guidelines [18]. It may be considered surprising that despite conferring a significant reduction in AHR to methacholine and exhaled NO, FP alone had no significant effect on airway calibre. This may be explained by the well-preserved FEV1 (allowing little further ‘room for improvement’) and relatively short duration of treatment. Moreover, our study was not powered on FEV1 (which would have required a larger study with more severe patients), but was powered on AHR to methacholine. In this respect, the relative insensitivity of FEV1 as a measure underlying airway inflammation is highlighted. It can also be observed in the study by Holt et al.[19] that in terms of FEV1, effects of ICS alone tend not to be marked and encounter the plateau of the dose–response curve at relatively low doses. In contrast, effects upon FEV1 are much more sensitive to bronchodilator therapy with long-acting β2-agonists.
This study investigated the effects of double the dose of ICS compared with the addition of a long-acting β2-agonist in mild persistent asthma on several important clinical end-points. We elected to use AHR to methacholine, which acts directly upon bronchial smooth muscle causing bronchoconstriction, as our primary endpoint. AHR has been shown to be a surrogate marker of underlying airway inflammation [20, 21] and following treatment with ICS, AHR to methacholine is reduced in conjunction with airway eosinophils [4,22, 23]. Our results are especially pertinent to current therapy, since both inhalers studied used HFA suspension formulations. In view of their ozone-depleting potential, pMDIs relying on chlorofluorocarbon (CFC) as the propellant are being phased out and the non-CFC propellant HFA is an increasingly used alternative for FP and FP/SM. The HFA pMDIs have been formulated to deliver an identical fine particle profile for FP as the CFC pMDIs, and have been shown to be therapeutically equivalent [24, 25].
In combination products the bronchodilator component might conceivably result in improved peripheral lung deposition of the ICS, thereby producing enhanced anti-inflammatory activity in smaller airways. In turn this raises the possibility that greater alveolar absorption of ICS may occur, resulting in increased systemic adverse effects. We elected to use overnight urinary cortisol/creatinine as a marker for systemic adverse effects, as this is a sensitive measure of basal hypothalamic–pituitary–adrenal axis activity, which is as sensitive as an integrated 24-h plasma or urine cortisol profile [5, 6]. Our results demonstrate that at a dose of 250 µg BD of FP alone or FP 125 µg/SM 25 µg BD in combination, no significant adrenal suppression occurred compared with baseline. Interestingly, although the HFA–FP and CFC–FP formulations have a similar fine particle profile, HFA–FP has been shown to exhibit lower systemic bioavailability and adrenal suppression [26, 27]. Our findings do not exclude the possibility of detectable adrenal suppression occurring at higher doses of the FP/SM combination, coinciding with the steep part of the systemic dose–response curve.
In conclusion, the FP/SM combination exhibited improvements on both AHR and airway calibre, while twice the dose of FP improved only the former in patients with mild persistent asthma. The differential effects of FP/SM and FP alone suggest separate but complementary actions of the two moieties on airway inflammation and stabilization of airway smooth muscle. This may explain the beneficial effects of combination inhalers on exacerbations. However, it is important to point out that our findings cannot be extrapolated to more severe patients, as the observed disconnect between airway calibre and AHR might not be so apparent. Further long-term larger studies are required to evaluate this fully.
Acknowledgments
The authors wish to acknowledge the nursing and technical assistance of Louise M. Cowan and Lesley C. McFarlane. This study was funded by a University of Dundee research grant.
References
- 1.O'Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392–1397. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- 2.Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA) Br Med J. 2000;320:1368–1373. doi: 10.1136/bmj.320.7246.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sont JK, Han J, van Krieken JM, et al. Relationship between the inflammatory infiltrate in bronchial biopsy specimens and clinical severity of asthma in patients treated with inhaled steroids. Thorax. 1996;51:496–502. doi: 10.1136/thx.51.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sont JK, Willems LN, Bel EH, et al. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. The AMPUL Study Group. Am J Respir Crit Care Med. 1999;159:1043–1051. doi: 10.1164/ajrccm.159.4.9806052. [DOI] [PubMed] [Google Scholar]
- 5.Wilson AM, Lipworth BJ. 24 hour and fractionated profiles of adrenocortical activity in asthmatic patients receiving inhaled and intranasal corticosteroids. Thorax. 1999;54:20–26. doi: 10.1136/thx.54.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntyre HD, Mitchell CA, Bowler SD, et al. Measuring the systemic effects of inhaled beclomethasone: timed morning urine collections compared with 24 hour specimens. Thorax. 1995;50:1280–1284. doi: 10.1136/thx.50.12.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharitonov SA, Chung KF, Evans D, et al. Increased exhaled nitric oxide in asthma is mainly derived from the lower respiratory tract. Am J Respir Crit Care Med. 1996;153:1773–1780. doi: 10.1164/ajrccm.153.6.8665033. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 9.Beach JR, Young CL, Avery AJ, et al. Measurement of airway responsiveness to methacholine: relative importance of the precision of drug delivery and the method of assessing response. Thorax. 1993;48:239–243. doi: 10.1136/thx.48.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–1411. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 11.Tattersfield AE, Lofdahl CG, Postma DS, et al. Comparison of formoterol and terbutaline for as-needed treatment of asthma: a randomised trial. Lancet. 2001;357:257–261. doi: 10.1016/S0140-6736(00)03611-4. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JA, Bradding P, Britten KM, et al. The long-acting beta2-agonist salmeterol xinafoate: effects on airway inflammation in asthma. Eur Respir J. 1999;14:275–282. doi: 10.1034/j.1399-3003.1999.14b07.x. [DOI] [PubMed] [Google Scholar]
- 13.Calhoun WJ, Hinton KL, Kratzenberg JJ. The effect of salmeterol on markers of airway inflammation following segmental allergen challenge. Am J Respir Crit Care Med. 2001;163:881–886. doi: 10.1164/ajrccm.163.4.2001060. [DOI] [PubMed] [Google Scholar]
- 14.Gardiner PV, Ward C, Booth H, et al. Effect of eight weeks of treatment with salmeterol on bronchoalveolar lavage inflammatory indices in asthmatics. Am J Respir Crit Care Med. 1994;150:1006–1011. doi: 10.1164/ajrccm.150.4.7921429. [DOI] [PubMed] [Google Scholar]
- 15.The British Thoracic Society guidelines on asthma management: 1995 review and position statement. Thorax. 1997;52(Suppl 1):S1–S21. [Google Scholar]
- 16.Lazarus SC, Boushey HA, Fahy JV, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285:2583–2593. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 17.Lemanske RF, Jr, Sorkness CA, Mauger EA, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA. 2001;285:2594–2603. doi: 10.1001/jama.285.20.2594. [DOI] [PubMed] [Google Scholar]
- 18.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 19.Holt S, Suder A, Weatherall M, et al. Dose–response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis. Br Med J. 2001;323:253–256. doi: 10.1136/bmj.323.7307.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson PG, Saltos N, Borgas T. Airway mast cells and eosinophils correlate with clinical severity and airway hyperresponsiveness in corticosteroid-treated asthma. J Allergy Clin Immunol. 2000;105:752–759. doi: 10.1067/mai.2000.105319. [DOI] [PubMed] [Google Scholar]
- 21.Jatakanon A, Lim S, Chung KF, et al. An inhaled steroid improves markers of airway inflammation in patients with mild asthma. Eur Respir J. 1998;12:1084–1088. doi: 10.1183/09031936.98.12051084. [DOI] [PubMed] [Google Scholar]
- 22.Lim S, Jatakanon A, John M, et al. Effect of inhaled budesonide on lung function and airway inflammation. Assessment by various inflammatory markers in mild asthma. Am J Respir Crit Care Med. 1999;159:22–30. doi: 10.1164/ajrccm.159.1.9706006. [DOI] [PubMed] [Google Scholar]
- 23.Van Den Berge M, Meijer RJ, Kerstjens HA, et al. PC(20) adenosine 5′-monophosphate is more closely associated with airway inflammation in asthma than PC(20) methacholine. Am J Respir Crit Care Med. 2001;163:1546–1550. doi: 10.1164/ajrccm.163.7.2010145. [DOI] [PubMed] [Google Scholar]
- 24.Tonnel AB, Bons J, Legendre M, et al. Clinical efficacy and safety of fluticasone propionate 250 µg twice daily administered via a HFA 134a pressurized metered dose inhaler to patients with mild to moderate asthma. French study group. Respir Med. 2000;94(Suppl B):S29–S34. [PubMed] [Google Scholar]
- 25.Cripps A, Riebe M, Schulze M, et al. Pharmaceutical transition to non-CFC pressurized metered dose inhalers. Respir Med. 2000;94(Suppl B):S3–S9. [PubMed] [Google Scholar]
- 26.Wilson AM, Sims EJ, Orr LC, et al. Differences in lung bioavailability between different propellants for fluticasone propionate. Lancet. 1999;354:1357–1358. doi: 10.1016/S0140-6736(99)03581-3. [DOI] [PubMed] [Google Scholar]
- 27.Kunka R, Andrews S, Pimazzoni M, et al. Dose proportionality of fluticasone propionate from hydrofluoroalkane pressurized metered dose inhalers (pMDIs) and comparability with chlorofluorocarbon pMDIs. Respir Med. 2000;94(Suppl B):S10–S16. [PubMed] [Google Scholar]


