Abstract
Aims
To assess the effects of pseudoephedrine on breast blood flow, temperature and milk production, and to estimate the likely infant dose during breastfeeding.
Methods
Eight lactating women (mean age 35 years and weight 69 kg) participated in a single-blind randomized crossover study of 60 mg pseudoephedrine hydrochloride vs placebo. Breast blood flow and surface temperature were measured from 0 to 4 h following the dose, and change in plasma prolactin was measured as the difference between predose and 1 h postdose concentrations. Milk production was measured for 24 h following placebo and pseudoephedrine. Infant dose of pseudoephedrine for a 60-mg dose administered four times daily to the mother was quantified as the product of average steady-state drug concentration in milk and an estimated milk production rate of 0.15 l kg−1 day−1 and expressed relative to the maternal weight-adjusted dose.
Results
There were no physiologically significant changes in breast blood flow or temperature between the placebo and pseudoephedrine periods. The mean change in plasma prolactin was slightly (13.5%), but not significantly lower (t = 1.245, P = 0.253) after pseudoephedrine (1775 mU l−1) compared with placebo (2014 mU l−1). However, the mean milk volume was reduced by 24% from 784 ml day−1 in the placebo period to 623 ml day−1 in the pseudoephedrine period (difference between means 161 ml day−1 (95% CI: 63, 259 ml day−1); t = 3.9, P = 0.006). Assuming maternal intake of 60 mg pseudoephedrine hydrochloride four times daily, the estimated infant dose of pseudoephedrine was 4.3% (95% CI, 3.2, 5.4%) of the weight-adjusted maternal dose.
Conclusions
A single dose of pseudoephedrine significantly reduced milk production. This effect was not attributable to changes in blood flow, but depression of prolactin secretion may be a contributing factor. At the maximum recommended pseudoephedrine doses, the calculated infant dose delivered via milk was < 10% of the maternal dose, and is unlikely to affect the infant adversely. The ability of pseudoephedrine to suppress lactation suggests a novel use for the drug.
Keywords: human milk, infant dose, M : P ratio, milk production, pseudoephedrine
Introduction
Pseudoephedrine is a sympathomimetic amine (α-adrenoceptor agonist) that enjoys wide use as a nasal mucous membrane and sinus decongestant. The drug has been detected in the milk of three nursing mothers (at 3, 3 and18 months of lactation) after ingestion of a single dose of a combined pseudoephedrine HCl 60 mg and triprolidine HCl 2.5 mg tablet [1]. The average concentration in milk over 24 h was 264 µg l−1. Although milk production was not measured, it was calculated that this would correspond to a relative infant dose of around 5% of the weight-adjusted maternal dose. The American Academy of Paediatrics considers the drug safe during breastfeeding [2], but there are very few studies of the use of decongestants during breastfeeding [3]. Nevertheless, anecdotally, many lactation consultants report that pseudoephedrine use results in decreased milk production [4]. Mammary blood flow in goats [5] and cattle [6] is known to control milk secretion, but no human studies have considered the possibility that an α-adrenoceptor agonist such as pseudoephedrine could constrict the arteriolar and/or capillary bed in the breast and significantly decrease milk secretion. In addition, some sympathomimetic amines have been reported to decrease prolactin secretion [7] by a direct action at dopamine D2 receptors in the pituitary, and thus pseudoephedrine might alter milk production via such a mechanism. Pseudoephedrine at usual doses of 60 mg four times daily has been reported to result in steady-state plasma concentrations of 500–640 µg l−1[8] with peak concentrations occurring 1–2 h after dose [1]. The drug has an average plasma half-life (t1/2) of about 7 h, is metabolized by N-demethylation in the liver, has a volume of distribution of 2.6–6 l kg−1, and some 55–75% is excreted unchanged in the urine [9, 10]. These properties suggest that there is ample opportunity for pseudoephedrine to exert local effects in the breast. The aims of present study were to assess its effects on milk production, breast blood flow and plasma prolactin, and also to quantify the exposure of the breastfed infant to pseudoephedrine via human milk.
Methods
Volunteers and experimental design
Eight lactating women were recruited. The study was approved by the Institutional Ethics Committees of the King Edward Memorial and Princess Margaret Hospitals, Curtin University of Technology and The University of Western Australia, and written informed consent was obtained. The women attended at 10.00 h on each of 2 days, approximately 1 week apart, and received either a 60 mg pseudoephedrine HCl tablet (Sudafed®; Warner Lambert Health Care Australia Ltd, Caringbah, Australia) or an identical placebo orally (approximately 3 h after their normal breakfast) in a single-blind randomized cross-over study design.
Volunteers breastfed their infants immediately prior to dose administration. On both study days milk samples (5 ml) were collected by hand expression or breast pump, immediately before dose, and again at 1, 2, 3, 4, 5, 8, 12 and 24 h after dose. In addition, fore- and hind-milk samples (1 ml) were collected at each breastfeed for analysis of fat content. Analysis of pseudoephedrine in milk was performed either on the timed samples, or an equal-volume mixture of fore and hind milk. Serum prolactin and/or plasma pseudoephedrine concentrations were measured in blood samples taken by venepuncture immediately prior to, and 1 h after dose. After the second blood sample, the mothers were permitted to feed their infants on demand for the remainder of the 24 h study.
Measurement of serum prolactin, milk production and milk fat content
Prolactin in serum was measured by Chemiluminescent Microparticle Immunoassay on an Architect® analyser (Abbott Diagnostics, Sydney, Australia). The percentage of fat in the milk was determined using the creamatocrit technique [11] as modified by Lucas et al.[12]. Milk production was measured by test weighing the baby (Baby Weigh® Scale; Medela Inc, McHenry, IL, USA) before and after each feed [13], over 24 h, commencing at the time of the dose. Milk volumes were recorded separately for both breasts, and results normalized to a 24 h period.
Measurement of breast blood flow and surface temperature
Breast blood flow was measured by ultrasonography [14–16] before, and 1, 2, 3 and 4 h after dose. An Acuson 128XP10 ultrasound machine (Acuson Corporation, Mountain View, CA, USA) with a linear array transducer (5 MHz) was used. Unbranched sections of the right internal mammary and lateral thoracic arteries were located and marked to facilitate repeat measurements. Spectral Doppler waveforms (angle < 60°) of the vessels were obtained using a sample gate larger than that of the vessel(s), and blood flow was recorded for 1 min. Average blood flow was expressed as the resistive index [17], calculated as (systolic peak velocity – lowest diastolic velocity)/systolic peak velocity, where velocities are measured in m s−1.
The surface temperature of each breast was measured by thermography using an infra-red camera (Therma- CAM® SC 1000; FLIR Infrared Camera Systems Inc, Boston, MA, USA) essentially as previously described [18]. Measurements (mean of 4–5 thermoframes taken at sequential 0.5-min intervals for each breast) were made at room temperature (25 °C), immediately before the blood flow measurements at 0, 1, 2, 3, 4 h.
Materials
(+)-Pseudoephedrine hydrochloride and phentermine hydrochloride standards were obtained from Sigma Chemical Company, Castle Hill, Australia. All solvents and other chemicals were of analytical or HPLC grade.
High performance liquid chromatographic analysis of pseudoephedrine in plasma and milk
Plasma (1 ml) was spiked with 250 ng phentermine (internal standard), made alkaline with 0.1 ml 1 mol l−1 NaOH and extracted into 10 ml diethylether by shaking for 5 min. After centrifugation at 1500 g for 5 min, the ether phase was back extracted into 0.2 ml 0.05 mol l−1 HCl, by shaking for 1 min. After further centrifugation, 0.04 ml of the acid phase was injected onto the HPLC column. Unknowns were interpolated from a linear standard curve for pseudoephedrine (20–2000 µg l−1, r > 0.998).
Concentrations of pseudoephedrine in milk also were measured by HPLC, using the method of addition [19]. Four equal aliquots of each milk sample (0.1–1.0 ml) were taken, phentermine (250 ng) was added to each, and three were spiked with increasing concentrations of pseudoephedrine (range 20–2000 µg l−1). Analytes were extracted as for plasma, and 0.08 ml of the acid phase injected onto the HPLC. Linear standard curves (r > 0.996), were constructed for each milk sample and the concentration of pseudoephedrine originally present was determined from the negative X-axis intercept.
For HPLC, a LiChrospher RP Select B column (5 µm; 4.6 mm × 250 mm; E. Merck & Co, Damsdart, Germany), and a mobile phase of 5% v/v acetonitrile in 0.045 mol l−1 KH2PO4 buffer (pH 3.0) was used at 1.6 ml min−1. Analytes were quantified at 210 nm using a Waters Associates Model 2487 dual wavelength absorbance detector. For plasma at 25 µg l−1 and 1000 µg l−1, intraday coefficients of variation (CVs) were 2.8% and 0.7%, respectively, while interday CVs were 3.2% and 0.9%, respectively. For milk at 25 µg l−1 and 2000 µg l−1, intraday CVs were 4.6% and 2.9%, while interday CVs were 6% and 3.3%, respectively. The limit of detection for pseudoephedrine was approximately 5 µg l−1 for both plasma and milk.
Data analysis
Data are summarized as mean (95% CI) unless otherwise indicated. Student's paired t-test was used to assess differences between mean values for milk production, change in fat content, and serum prolactin concentration (SigmaStat Ver 2.0; SPSS Inc, Chicago, IL, USA). Breast blood flow and temperature data (mean for both breasts) were analysed using a general linear modelling procedure that allowed for the repeated measures study design (SAS/STAT Software, Version 8, SAS Institute, Cary, NC, USA). Inter-group comparisons were made using a Tukey–Kramer test with P < 0.05 as the level of significance.
Log-linear regression analysis of the last 3–4 pseudoephedrine milk concentration-time data pairs was used to estimate t1/2 in milk, and area under the milk concentration-time curve (AUC0,∞; log trapezoidal rule plus Clast × t1/2/0.693) [20]. The maximum concentration of drug in milk (Cmax) and the time of maximum concentration (tmax) were determined from the data. Average pseudoephedrine concentration in milk at steady-state (Cav) was calculated as the single dose AUC0,∞/τ, where τ = duration of the dose interval in h [21]. An infant milk intake of 0.15 l kg−1 day−1 was assumed [22], and multiplied by milk Cav to give the absolute infant dose in µg kg−1 day−1. The latter value was expressed as a percentage of the weight-normalised maternal dose (µg kg−1 day−1) to yield the measure known as ‘relative infant dose’. Milk : plasma (M : P) ratio was calculated using the measured concentrations in the 1-h milk and plasma samples.
Results
The characteristics of the women who were recruited into the study are summarized in Table 1. All subjects were in good heath. Five took routine medications that remained stable across the study period.
Table 1.
Characteristics of the volunteers
| Volunteer | Age (years) | Weight (kg) | Parity | Number of babies being fed | Lactation stage (weeks) | Other routine medications |
|---|---|---|---|---|---|---|
| 1 | 38 | 70 | 4 | 1 | 64 | Paroxetine |
| 2 | 40 | 74 | 2 | 1 | 76 | Nil |
| 3 | 38 | 93 | 2 | 1 | 13 | Sertraline |
| 4 | 29 | 60 | 1 | 1 | 14 | Salbutamol, salmeterol, fluticasone |
| 5 | 36 | 50 | 3 | 2 | 10 | Nil |
| 6 | 38 | 72 | 2 | 1 | 8 | Salbutamol, beclomethasone, norethisterone |
| 7 | 26 | 74 | 3 | 1 | 32 | Etonogestrel |
| 8 | 33 | 58 | 2 | 1 | 10 | Nil |
| Mean (range) | 35 (26, 40) | 69 (50, 93) | 2 (1, 4) | 1 (1, 2) | 28 (8, 76) |
There were no significant differences between the volumes from the right and left breasts on either study day (data not shown) and only the total 24 h volumes are summarized in Table 2. Mean total production was 24% lower [difference between means = 161 ml day−1 (95% CI = 63, 259 ml day−1); t = 3.9, P = 0.006] after pseudoephedrine compared with placebo. There was a significant linear relationship between pseudoephedrine-induced decrease in 24 h milk production and the stage (weeks) of lactation (% decrease = 1.838 + 0.777 stage; r2 = 0.911), with the largest decreases at 60–80 weeks. Preliminary analysis indicated that the increase in fat content between fore- and hind-milk samples was similar in both breasts, and therefore only mean data are shown in Table 2. The mean increase was similar for placebo and pseudoephedrine, as were the total numbers of breastfeeds day−1 (Table 2).
Table 2.
Daily milk production, changes in fat content from fore to hind milk and feeding frequency for the volunteers
| Milk production on study day (ml day−1) | Change in milk fat content (%)* | Feeding frequency (day−1)2 | |||||
|---|---|---|---|---|---|---|---|
| Volunteer | Placebo | Pseudoephedrine | % decrease in production (placebo-pseudoephedrine) | Placebo | Pseudoephedrine | Placebo | Pseudoephedrine |
| 1 | 651 | 289 | 56 | 6.0 | 2.9 | 12 | 10 |
| 2 | 473 | 192 | 59 | 8.0 | 3.6 | 4 | 7 |
| 3 | 631 | 609 | 4 | 4.9 | 6.5 | 12 | 9 |
| 4 | 841 | 812 | 3 | 5.4 | 2.4 | 7 | 7 |
| 5 | 1438 | 1272 | 12 | 3.1 | 3.3 | 21 | 18 |
| 6 | 815 | 673 | 17 | 2.0 | 1.5 | 12 | 16 |
| 7 | 731 | 546 | 25 | 3.9 | 5.4 | 8 | 9 |
| 8 | 692 | 589 | 15 | 4.0 | 6.0 | 12 | 9 |
| Mean (95% CI) | 784 (543, 1025) | 623 (34, 839)‡ | 24 (6, 42) | 4.2 (1.2, 7.2) | 3.7 (1.0, 5.4) | 11.0 (6.8, 15.2) | 10.6 (7.2, 14.0) |
Values for volunteers are means for samples from both breasts (n = 4–21), while column means (95%CI) are for all samples; †total for both breasts;
P = 0.006.
The change in plasma prolactin (Δ = predose vs 1 h postdose) after placebo or pseudoephedrine is illustrated in Figure 1. In six women, there was a variable decrease in prolactin Δ while in the other two, prolactin Δ increased. However, the mean of the prolactin Δ after pseudoephedrine (1775 ± 2294 mU l−1) was not significantly different (t = 1.245, P = 0.253) from that after placebo (2014 ± 2531 mU l−1).
Figure 1.
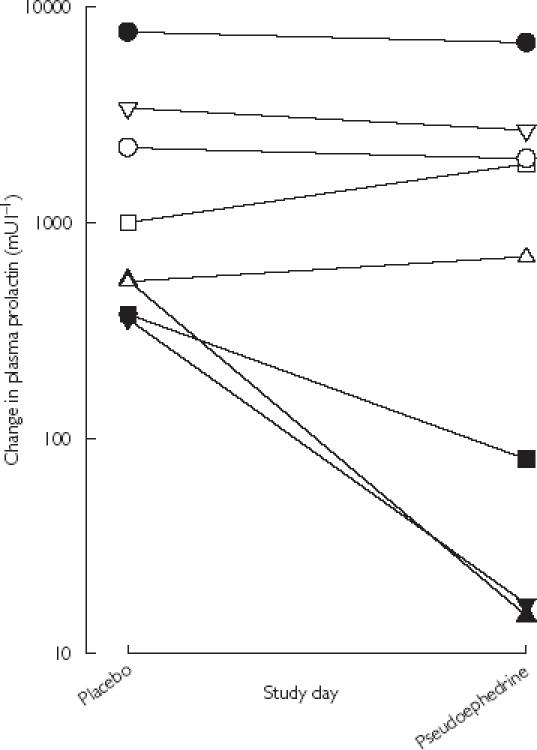
Change in plasma prolactin concentration (log10 scale) between predose and 1 h postdose on placebo and pseudoephedrine study days. Individual volunteers are indicated by the different symbols (1, ▪; 2, ▴; 3, □; 4, ▾; 5, ○; 6, •; 7, ▵; 8, ▿).
During the first 4 h after dose, the mean resistive index (both breasts) for the internal mammary artery was similar within, and also between placebo and pseudoephedrine days (Table 3). Findings for the resistive index of the lateral thoracic arteries were similar (data not shown). Mean temperature (average of both breasts) was similar on both study days (Table 3). A small but significant increase in breast temperature occurred 3 and 4 h after dose on both placebo and pseudoephedrine study days.
Table 3.
Internal mammary artery blood flow (as resistive index), and breast temperature during placebo and pseudoephedrine study days.
| Resistive index on study day* | Temperature (°C) on study day* | |||
|---|---|---|---|---|
| Time after dose (h) | Placebo | Pseudoephedrine | Placebo | Pseudoephedrine |
| 0 | 0.51 (0.47, 0.55) | 0.51 (0.48, 0.54) | 34.2 (33.5, 34.9) | 33.7 (33.2, 34.2) |
| 1 | 0.53 (0.47, 0.59) | 0.49 (0.46, 0.52) | 34.5 (33.6, 35.4) | 34.0 (33.6, 34.4) |
| 2 | 0.54 (0.47, 0.61) | 0.52 (0.49, 0.55) | 35.1 (33.7, 36.5) | 34.2 (33.5, 34.9) |
| 3 | 0.53 (0.50, 0.56) | 0.54 (0.51, 0.58) | 35.0 (33.9, 36.1)† | 34.3 (33.5, 35.1)† |
| 4 | 0.51 (0.48, 0.54) | 0.50 (0.47, 0.53) | 35.8 (34.6, 37.0)† | 35.3 (34.9, 35.7)† |
Mean (95% CI) of average data for both breasts;
P < 0.05 compared with respective 0 h control value (Tukey-Kramer test).
Pseudoephedrine milk concentration-time profiles following the 60 mg oral dose of the HCl salt (49.2 mg base) are shown in Figure 2, and pharmacokinetic descriptors are summarized in Table 4. Drug concentration was not significantly different between fore- and hind-milk (t = 1.89, P = 0.11). The single dose produced maximum concentrations of drug in milk (698 µg l−1) at 1.7 h after dose. The mean t1/2 in milk was 5.5 h and the AUC(0,∞) was 4972 µg l−1 h. Concentrations of pseudoephedrine in milk were low (mean 34 µg l−1; Figure 2) at approximately 24 h after dose and the mean extrapolated portion (AUC 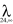 ) of the total AUC
) of the total AUC 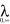 was 5.2%. A mean M : P of 3.4 was calculated from the measured plasma concentration (mean 191 µg l−1 (131, 251 µg l−1) sampled at a mean of 1.1 h after dose) and the concentration in the closest corresponding milk sample (mean 595 µg l−1 (401, 789 µg l−1) taken at a mean of 1.1 h after dose).
was 5.2%. A mean M : P of 3.4 was calculated from the measured plasma concentration (mean 191 µg l−1 (131, 251 µg l−1) sampled at a mean of 1.1 h after dose) and the concentration in the closest corresponding milk sample (mean 595 µg l−1 (401, 789 µg l−1) taken at a mean of 1.1 h after dose).
Figure 2.
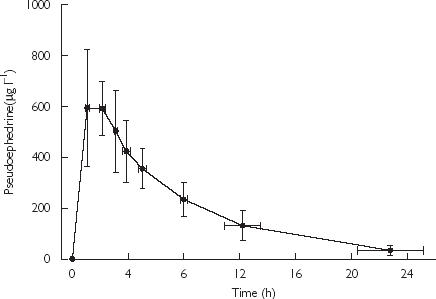
Concentration-time profile for pseudoephedrine in milk from the volunteers following a 60 mg oral dose of pseudoephedrine HCl. Data as mean ± SD (n = 8)
Table 4.
Measured pharmacokinetic parameters for pseudoephedrine in milk, and simulated average milk concentrations and infant doses
| Volunteer | Cmax*(µg l–1) | tmax*(h) | t1/2*(h) | AUC(0,∞)*(µg l−1 h) | M : P*† | Cav(µg l−1)‡ | Absolute infant dose (µg kg−1 day−1) | Relative infant dose (%)‡ |
|---|---|---|---|---|---|---|---|---|
| 1 | 679 | 1.3 | 3.9 | 3240 | 3.1 | 540 | 81 | 3.8 |
| 2 | 593 | 1.5 | 4.8 | 3639 | 1.5 | 607 | 91 | 3.2 |
| 3 | 496 | 2.0 | 4.5 | 3531 | 2.8 | 589 | 88 | 2.2 |
| 4 | 614 | 1.0 | 4.4 | 4518 | 2.5 | 753 | 113 | 4.3 |
| 5 | 750 | 1.0 | 7.2 | 6443 | 3.4 | 1074 | 161 | 4.8 |
| 6 | 682 | 3.1 | 5.8 | 5673 | 3.8 | 945 | 142 | 4.3 |
| 7 | 1003 | 1.4 | 6.9 | 7365 | 4.1 | 1227 | 184 | 6.7 |
| 8 | 767 | 2.5 | 4.5 | 5365 | 5.6 | 894 | 134 | 5.0 |
| Mean (95% CI) | 698 (572, 824) | 1.7 (1.1, 2.3) | 5.3 (4.3, 6.3) | 4972 (3544, 6040) | 3.4 (2.4, 4.4) | 829 (621, 1038) | 124 (93, 155) | 4.3 (3.3, 5.4) |
Measured or calculated from the 60 mg single dose of pseudoephedrine HCl;
estimated from single paired milk and plasma samples 1 h after dose;
calculated at steady-state for a 60 mg dose of pseudoephedrine HCl given four times daily.
To calculate the likely infant exposure to pseudoephedrine in breastmilk, we simulated a maximum recommended dose scenario of 60 mg taken four times daily (mean pseudoephedrine daily dose = 2946 µg kg−1 for our volunteers) to arrive at the Cav milk concentrations, and infant doses shown in Table 4. Mean absolute infant dose at this level of maternal intake would be 124 µg kg−1 day−1, and mean relative infant dose 4.3% of the weight-adjusted maternal dose.
Discussion
The major finding of this study was that 24-h milk production was significantly decreased by some 24% after administration of a single 60 mg dose of pseudoephedrine. Since there were no consistent trends in the change in the fat content from fore to hind milk (Table 2), the degree of emptying of the breast at a breastfeed [23] did not explain the decrease in milk production on the pseudoephedrine day. This, combined with the similar feeding frequencies between both study days (Table 2) suggests that the babies were successfully removing the available milk and that the decrease in production was not related to altered feeding behaviour. One explanation for this finding could reside in the ability of the drug to constrict small blood vessels and thereby reduce blood flow and temperature in the breast. However, the data (Table 3) clearly show that blood flow in the breast was unaffected by pseudoephedrine during the time when peak concentrations of the drug occurred in milk (1–4 h after dose). In addition, breast surface temperature was similar after pseudoephedrine or placebo. Another possible explanation for the reduced milk production after pseudoephedrine is an alteration in prolactin due to dopaminergic actions of the drug in the pituitary. Here, our data indicated a variable decrease in the prolactin surge in response to suckling in six of the eight subjects and a modest increase in the other two. While the overall mean was some 13.5% lower, the change did not achieve statistical significance. The data also suggested that the size of the decrease in prolactin was greater in women who were in late lactation (60–80 weeks). However, the number of subjects studied was small and this finding should be interpreted cautiously.
The study also provided an opportunity to investigate the likely infant dose of pseudoephedrine in mothers who may take it at manufacturer's recommended daily dose as a nasal decongestant. The milk concentration–time profile for pseudoephedrine in our volunteers was similar to that reported by Findlay et al.[1], but our milk AUC data additionally demonstrate a two-fold interpatient variability in drug concentration. The mean M : P of 3.4 was within the previously reported range (2.2–3.9) [1], while the wide range of M : P values in our study (1.5–5.6) was consistent with derivation from single paired data. The relative infant dose of pseudoephedrine in milk was calculated (from milk AUC data and not requiring the use of M : P) to be a mean of 4.3% (range 2.2, 6.7%) for the usual maximum recommended daily dose of the drug (240 mg as a decongestant). The calculation should be reasonably robust given that the plasma pharmacokinetics of pseudoephedrine are linear at a normal acid urine pH [10], and that we and others [1] have shown that transfer into milk is proportional. The calculated dose was in agreement with the 5% dose exposure that we calculated using limited earlier data [1]. The notional safety limit for drug exposure in breast milk is < 10% of the weight-adjusted maternal dose [22], and hence pseudoephedrine is likely to be safe, even when given at maximum dose rates. The mean absolute infant dose of 124 µg kg−1 day−1 is relatively low compared with the recommended clinical dose of 4 mg kg−1 day−1 orally for children < 12 years of age [24]. However, our study clearly demonstrates that the drug can significantly decrease milk production even after a single dose, and for this reason alone, it should be used cautiously during lactation. Use of a topical nasal decongestant could provide a preferable alternative pharmacotherapeutic option.
Our study suggests that, in addition to its use as a mucous membrane decongestant, pseudoephedrine may be a useful drug for suppressing excess milk production and further studies on this prospect are presently in progress in our laboratories.
Acknowledgments
We are grateful to Professor Bruce Sunderland, Pharmacy School, Curtin University of Technology for the manufacture and supply of placebo tablets used in the study, to Steve Fletcher, Clinical Biochemistry, The Western Australian Centre for Pathology and Medical Research for assistance with the plasma prolactin assays and to Max K. Bulsara and Helman Alfonso for their assistance with the statistical analysis of blood flow and temperature data. We acknowledge financial support for part of the project from the Women's and Infants Research Foundation WA and Medela A.G, Switzerland.
References
- 1.Findlay JW, Butz RF, Sailstad JM, Warren JT, Welch RM. Pseudoephedrine and triprolidine in plasma and breast milk of nursing mothers. Br J Clin Pharmacol. 1984;18:901–906. doi: 10.1111/j.1365-2125.1984.tb02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee on Drugs of the American Academy of Pediatrics. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137–150. [PubMed] [Google Scholar]
- 3.Mitchell JL. Use of cough and cold preparations during breastfeeding. J Hum Lact. 1999;15:347–349. doi: 10.1177/089033449901500417. [DOI] [PubMed] [Google Scholar]
- 4.Anderson PO. Decongestants and milk production. J Hum Lact. 2000;16:294. [PubMed] [Google Scholar]
- 5.Mepham B. Physiological aspects of lactation. In: Mepham B, editor. Biochemistry of Lactation. New York: Elsevier; 1983. pp. 2–28. [Google Scholar]
- 6.Gorewit RC, Aromando MC. Mechanisms involved in the adrenaline-induced blockade of milk ejection in dairy cattle. Proc Soc Exp Biol Med. 1985;180:340–347. doi: 10.3181/00379727-180-42186. [DOI] [PubMed] [Google Scholar]
- 7.Walsh CT, Neville MC. Effect of xenobiotics on milk secretion and composition. J Nutrit Biochem. 1994;5:418–441. doi: 10.1093/ajcn/61.3.687S. [DOI] [PubMed] [Google Scholar]
- 8.Bye C, Hill HM, Hughes DTD, Peck AW. A comparison of plasma levels of L (+) pseudoephedrine following different formulations, and their relation to cardiovascular and subjective effects in man. Eur J Clin Pharmacol. 1975;8:47–53. doi: 10.1007/BF00616414. [DOI] [PubMed] [Google Scholar]
- 9.E-MIMS Ver 4000597. St Leonards, Australia: MultiMedia Australia Pty Limited; 2002. Sudafed Product Information. [Google Scholar]
- 10.Kanfer I, Dowse R, Vuma V. Pharmacokinetics of oral decongestants. Pharmacotherapy. 1993;13:116S–128S. [PubMed] [Google Scholar]
- 11.Fleet IR, Linzell JL. A rapid method of estimating fat in very small quantities of milk. J Physiol. 1964;175:15–17. [Google Scholar]
- 12.Lucas A, Gibbs JA, Lyster RL, Baum JD. Creamatocrit simple clinical technique for estimating fat concentration and energy value of human milk. Br Med J (Clin Res Edn) 1978;1:1018–1020. doi: 10.1136/bmj.1.6119.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur PG, Hartmann PE, Smith M. Measurement of the milk intake of breast-fed infants. J Pediat Gastroenterol Nutrit. 1987;6:758–763. doi: 10.1097/00005176-198709000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Janbu T, Koss KS, Thoresen M, Wesche J. Blood velocities to the female breast during lactation and following oxytocin injections. J Dev Physiol. 1985;7:373–380. [PubMed] [Google Scholar]
- 15.Sambrook M, Bamber JC, Minasian H, Hill CR. Ultrasonic Doppler study of the hormonal response of blood flow in the normal human breast. Ultrasound Med Biol. 1987;13:121–129. doi: 10.1016/0301-5629(87)90139-6. [DOI] [PubMed] [Google Scholar]
- 16.Scatarige JC, Hamper UM, Sheth S, Allen HA., III Parasternal sonography of the internal mammary vessels: technique, normal anatomy, and lymphadenopathy. Radiology. 1989;172:453–457. doi: 10.1148/radiology.172.2.2664869. [DOI] [PubMed] [Google Scholar]
- 17.Rettenbacher T, Hollerweger A, Macheiner P, Gritzmann N. Color Doppler sonography of normal breasts: detectability of arterial blood vessels and typical flow patterns. Ultrasound Med Biol. 1998;24:1307–1311. doi: 10.1016/s0301-5629(98)00124-0. [DOI] [PubMed] [Google Scholar]
- 18.Mitoulas LR, Lai CT, Gurrin LC, Larsson M, Hartmann PE. Efficiacy of breast milk expsression using an electric pump. J Hum Lact. 2002;18:340–348. doi: 10.1177/089033402237907. [DOI] [PubMed] [Google Scholar]
- 19.Begg EJ, Duffull SB, Hackett LP, Ilett KF. Studying drugs in human milk: time to unify the approach. J Hum Lact. 2002;18:319–328. doi: 10.1177/089033402237904. [DOI] [PubMed] [Google Scholar]
- 20.Thomann P. Non-compartmental analysis methods manual. In: Heinzel G, Woloszcak R, Thomann P, editors. TopFit 20 Pharmacokinetic, pharmacodynamic data analysis system for the PC. Stuttgart: Gustav Fischer; 1993. pp. 5–66. [Google Scholar]
- 21.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York: Marcel Dekker Inc; 1982. pp. 119–120. [Google Scholar]
- 22.Bennett PN. Use of the monographs on drugs. In: Bennett PN, editor. Drugs and Human Lactation. 2. Amsterdam: Elsevier; 1996. pp. 67–74. [Google Scholar]
- 23.Daly SEJ, Dirosso A, Owens RA, Hartmann PE. Degree of breast emptying explains changes in the fat-content, but not fatty-acid composition, of human-milk. Exp Physiol. 1993;78:741–755. doi: 10.1113/expphysiol.1993.sp003722. [DOI] [PubMed] [Google Scholar]
- 24.Hirshfeld AB, Getachew A, Sessions J. Drug doses. In: Siberry GK, Iannone R, editors. The Harriet Lane HandbookA Manual for Pediatric House Officers. 15. St Louis: Mosby; 2000. p. 831. [Google Scholar]


