Abstract
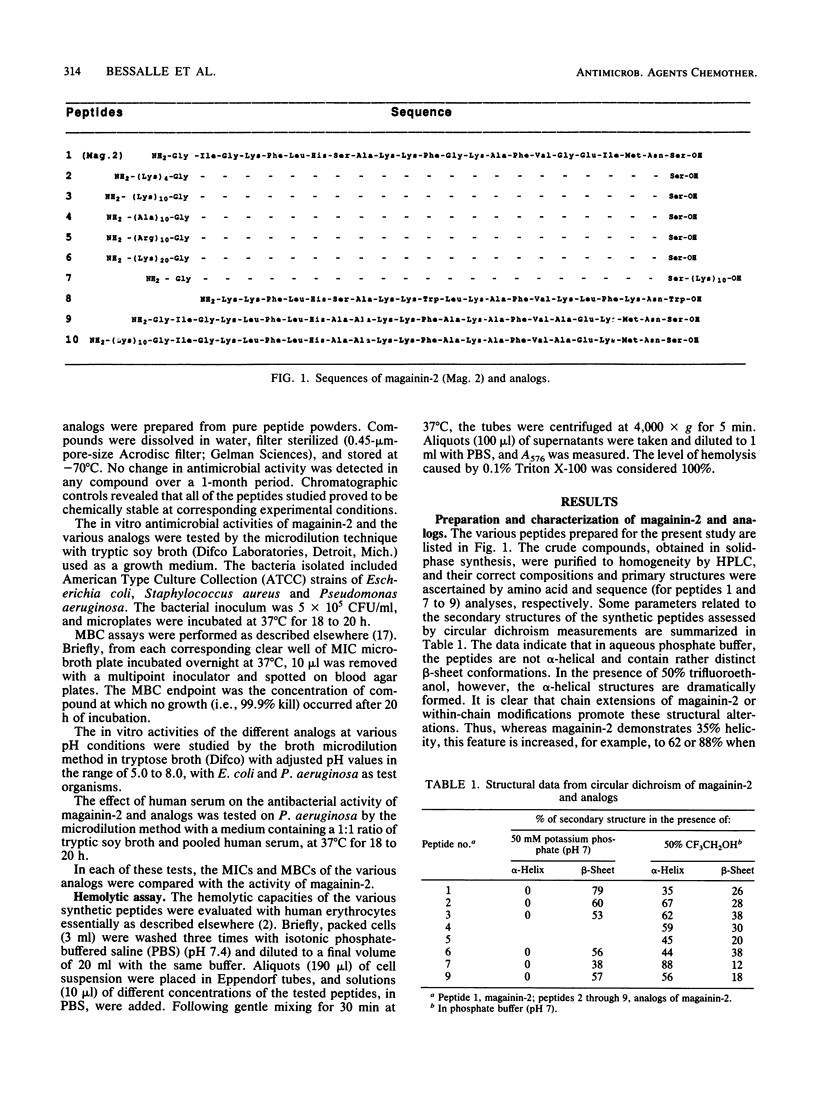
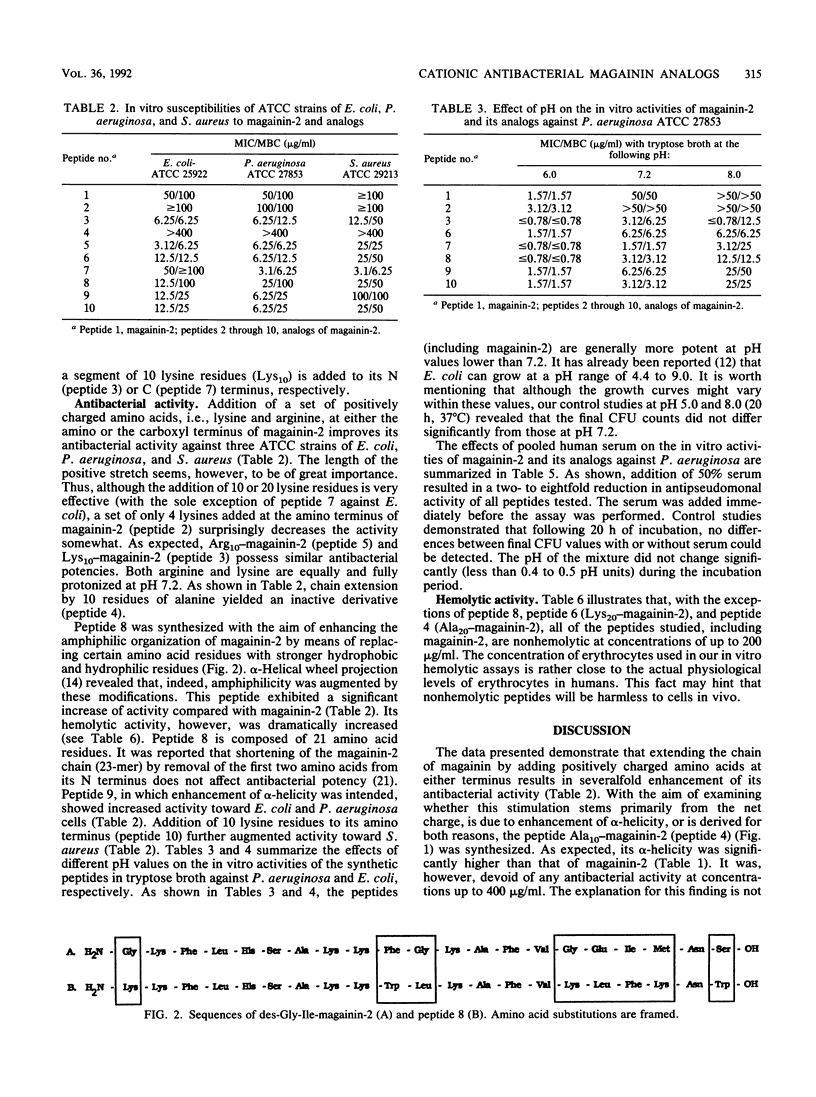
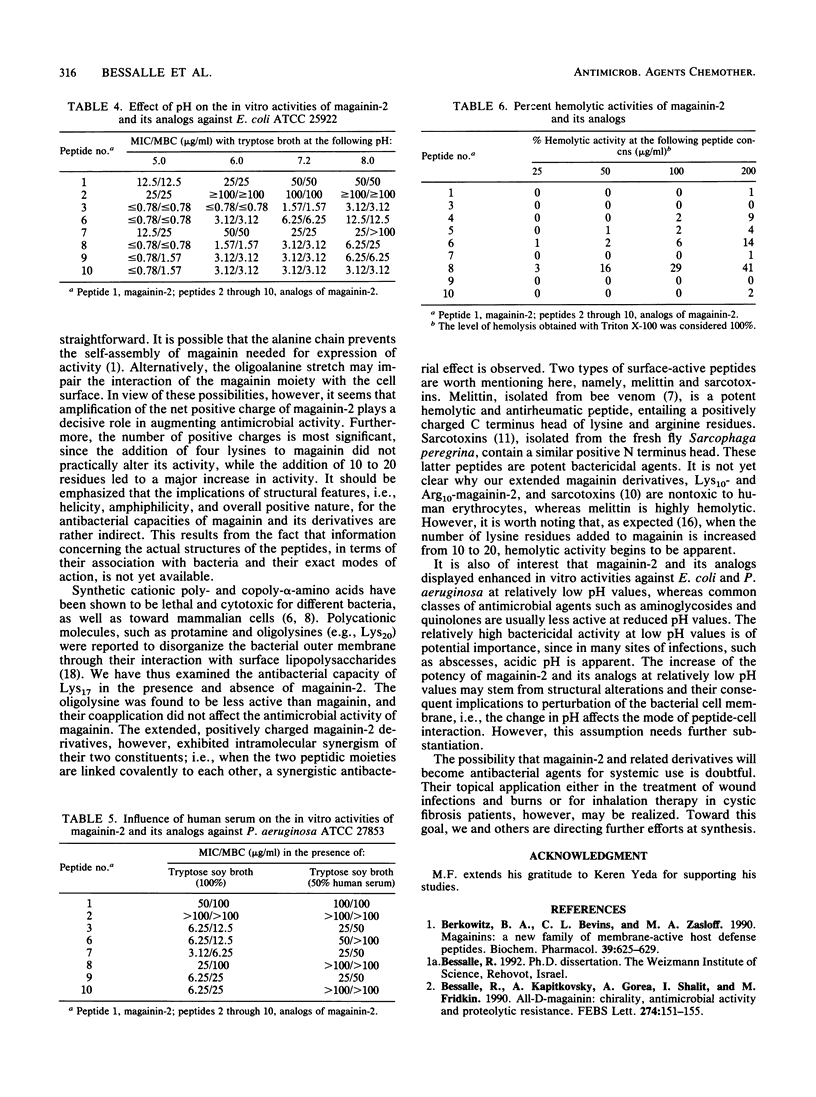
Novel analogs of the broad-spectrum antimicrobial peptide magainin-2 were obtained by extension of its chain through addition of segments of positively charged amino acids to either its N or its C terminus and by increasing its helicity. The activity of magainin-2 toward American Type Culture Collection strains of Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus was most considerably enhanced by these modifications, whereas, in general, its low hemolytic capacity was not or was only slightly affected. The antibacterial potencies of magainin-2 and its derivatives were more evident following decreases of pH from 7.2 to 6 and 5.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz B. A., Bevins C. L., Zasloff M. A. Magainins: a new family of membrane-active host defense peptides. Biochem Pharmacol. 1990 Feb 15;39(4):625–629. doi: 10.1016/0006-2952(90)90138-b. [DOI] [PubMed] [Google Scholar]
- Bessalle R., Kapitkovsky A., Gorea A., Shalit I., Fridkin M. All-D-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 1990 Nov 12;274(1-2):151–155. doi: 10.1016/0014-5793(90)81351-n. [DOI] [PubMed] [Google Scholar]
- Bevins C. L., Zasloff M. Peptides from frog skin. Annu Rev Biochem. 1990;59:395–414. doi: 10.1146/annurev.bi.59.070190.002143. [DOI] [PubMed] [Google Scholar]
- Chen H. C., Brown J. H., Morell J. L., Huang C. M. Synthetic magainin analogues with improved antimicrobial activity. FEBS Lett. 1988 Aug 29;236(2):462–466. doi: 10.1016/0014-5793(88)80077-2. [DOI] [PubMed] [Google Scholar]
- Cuervo J. H., Rodriguez B., Houghten R. A. The Magainins: sequence factors relevant to increased antimicrobial activity and decreased hemolytic activity. Pept Res. 1988 Nov-Dec;1(2):81–86. [PubMed] [Google Scholar]
- Ginsburg I., Borinski R., Malamud D., Struckmeier F., Klimetzek V. Chemiluminescence and superoxide generation by leukocytes stimulated by polyelectrolyte-opsonized bacteria. Role of histones, polyarginine, polylysine, polyhistidine, cytochalasins, and inflammatory exudates as modulators of oxygen burst. Inflammation. 1985 Sep;9(3):245–271. doi: 10.1007/BF00916275. [DOI] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- KATCHALSKI E., BICHOWSKI-SLOMNITZKI L., VOLCANI B. E. The action of some water-soluble poly-alpha-amino acids on bacteria. Biochem J. 1953 Nov;55(4):671–680. doi: 10.1042/bj0550671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Qu X. M., Natori S. Interaction between liposomes and sarcotoxin IA, a potent antibacterial protein of Sarcophaga peregrina (flesh fly). J Biol Chem. 1987 Feb 5;262(4):1665–1669. [PubMed] [Google Scholar]
- Okada M., Natori S. Primary structure of sarcotoxin I, an antibacterial protein induced in the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J Biol Chem. 1985 Jun 25;260(12):7174–7177. [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- SELA M., KATCHALSKI E. Biological properties of poly-alpha-amino acids. Adv Protein Chem. 1959;14:391–478. doi: 10.1016/s0065-3233(08)60614-2. [DOI] [PubMed] [Google Scholar]
- Sakakibara S., Shimonishi Y., Kishida Y., Okada M., Sugihara H. Use of anhydrous hydrogen fluoride in peptide synthesis. I. Behavior of various protective groups in anhydrous hydrogen fluoride. Bull Chem Soc Jpn. 1967 Sep;40(9):2164–2167. doi: 10.1246/bcsj.40.2164. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Martin B., Chen H. C. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc Natl Acad Sci U S A. 1988 Feb;85(3):910–913. doi: 10.1073/pnas.85.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]


