Abstract
Aims
To investigate the extent of dose-related off-label antibiotic paediatric prescribing in primary care and to identify any potential clinical effects, particularly of lower than recommended dose prescribing.
Methods
Assessment of antibiotic prescribing in 168 396 children aged 0–16 years for the year 1999–2000 from data retrieved from 158 general practices using the national Scottish primary care computer system GPASS. The setting was general practices in Scotland.
Results
During the study period at least one course of antibiotics was prescribed to 23 911 children (14.2%). A total of 4582 (19.2%) children were prescribed an antibiotic dose of less than that recommended in the Summary of Product Characteristics (SPC). The number of children prescribed an antibiotic at less than recommended dose increased with age from 1154 (11.8%) aged 0–4 years to 1827 (30.0%) in the 12–16 years age group. For each antibiotic, prescribing lower than recommended dose occurred most frequently at those ages at which a dose increase was recommended in the SPC. Antibiotic prescribing at less than the recommended dose was not associated with an increased return rate for further antibiotic prescriptions during the following month, but in 5–11-year-olds was associated with a significant 48% increase in the total number of antibiotic courses prescribed during the study year {mean = 2.09 [95% confidence interval (CI) 1.79, 2.39] vs. 1.41 [95% CI 1.35, 1.47]}. Antibiotic prescribing at doses higher than recommended occurred less frequently (1.6%) and decreased steadily with age.
Conclusions
Off-label prescribing of antibiotics at less than the recommended dose in children is common in primary care and occurs primarily as the result of a failure to increase antibiotic dosage with age in line with SPC recommendations. Adoption of a uniform approach to SPC age banding for antibiotic dose increments would reduce the frequency of dose-related off-label antibiotic prescribing in children and help minimize the potential for the development of antibiotic resistance.
Keywords: antibiotics, children, off-label, prescribing
Introduction
Antibiotics are amongst the most common medicines prescribed to children of all ages and account for the majority of off-label prescriptions in primary care [1]. The use of off-label medicines in children is less than desirable and may expose children to unknown risks [2, 3].
The success and widespread use of antibiotic therapy has led to the development of antimicrobial resistance, now a significant problem reflecting in part the arguably inappropriate use of antibiotics [4, 5]. In children lower than recommended dose (off-label) antibiotic therapy has been associated with increased pharyngeal carriage of penicillin-resistant Streptococcus pneumoniae[6]. The primary strategies for preventing resistance focus on prescribing antibiotics only when likely to be beneficial, and targeting treatment at specific susceptible pathogens. A further key mechanism for limiting the development of bacterial resistance is to ensure maximum efficacy by prescribing an appropriate dose for an appropriate treatment duration [7].
Although all marketed antibiotic preparations have identical formulation strengths (e.g. 125/5 mL syrup, 250/5 mL syrup, 250 mg and 500 mg tablets), prescribing an appropriate dose for an individual child can be difficult as the individual Summary of Product Characteristics (SPC) recommends differing ages at which a dose increase should occur. For example, a significant dose increase for amoxycillin occurs at 10 years of age, while for phenoxymethylpenicillin step increases are recommended at 1 and 6 years of age. Such wide variations in dosage recommendations make antibiotic prescribing complicated and potentially confusing.
In General Practice the off-label use of medicines in children is common [1, 8], with prescriptions for antibiotic therapy at less than the recommended dose the major contributor [9]. This study aimed to assess the extent of dose-related off-label prescribing of antibiotics to children in primary care and to determine whether in particular the receipt of a prescription for less than the recommended dose might result in a clinically meaningful outcome.
Patients and methods
Computerized data collected by electronic questionnaire from 158 Scottish General Practices (representing the age/gender mixture of the Scottish population), using GPASS (General Practice Administration System for Scotland) software, was used to assess paediatric antibiotic prescribing during the period 1 November 1999 to 31 October 2000.
All prescriptions for antibiotics during the study year were identified for the three age bands 0–4, 5–11 and 12–16 years. Using the antibiotic dosage recommendations contained within the SPC 1999–2000 [10], children were categorized as having been prescribed doses that were lower than, higher than or within the recommended dose range for each antibiotic.
To determine possible clinical effects of antibiotic prescribing at less than recommended dose, further antibiotic prescribing in the month and year following the initial low-dose prescription was assessed. A nonparametric (χ2) test was used to identify any difference in returns for further antibiotic prescriptions issued during the following month to children in each age band prescribed either lower than or recommended doses of amoxycillin, erythromycin and phenoxymethylpenicillin (0–4-year-olds n = 1810, 5–11-year-olds n = 2390, 12–16-year-olds n = 1540). A nonparametric Mann–Whitney U-test was used to assess any differences between the total number of antibiotic courses prescribed during the study year for children in each age band prescribed either lower than or recommended dose of antibiotic in the first month of the study year. Children on repeat prescriptions for lower than recommended dose antibiotics were excluded from this analysis. Significance was taken at the 5% level.
Results
Of the 168 396 children registered with the 158 General Practices, a total of 23 911 (14.2%) were prescribed at least one systemic antibiotic during the study year. Of these children 4582 (19.2%) were prescribed a lower than recommended dose and 373 children (2.2%) a higher than recommended dose (Table 1).
Table 1.
Use of antibiotics per age group and per total child population in each age group.
| Low dose | High dose | ||||||
|---|---|---|---|---|---|---|---|
| Age band (years) | No. | % | Rate | No. | % | Rate | Total no. of antibiotics |
| 0–4 | 1154 | 11.8 | 25.7 | 243 | 2.5 | 5.4 | 9767 |
| 5–11 | 1604 | 19.9 | 22.5 | 111 | 1.4 | 1.6 | 8055 |
| 12–16 | 1827 | 30.0 | 34.9 | 19 | 0.3 | 0.4 | 6089 |
| Total | 4582 | 19.2 | 27.1 | 373 | 1.6 | 2.2 | 23911 |
Percent total number children prescribed an antibiotic. Rate/1000 registered children.
Phenoxymethylpenicillin and erythromycin were most commonly prescribed at less than the recommended dose. In children aged 0–4 years erythromycin alone accounted for the majority of antibiotic prescriptions at less than the recommended dose (57%), while in the older age groups phenoxymethylpenicillin and erythromycin together accounted for 57% of lower than recommended dose prescribing in 5–11-year-olds and 88% in 12–16-year-olds (Table 2). Co-amoxiclav, flucloxacillin, cefaclor, cephalexin and clarithromycin were not commonly prescribed to children aged 0–4 and 12–16 years of age and so their contribution to overall lower than recommended dose prescribing was relatively low (Table 2), accounting for 10.7% and 7.7%, respectively. However, in 5–11-year-olds, these antibiotics accounted for 24.1% of lower than recommended dose prescribing with high individual rates of underdosing per 1000 5–11-year-olds prescribed. Amoxycillin was the most commonly prescribed antibiotic, and although the rate of lower than recommended dose prescribing was small, the absolute numbers of children prescribed low-dose amoxycillin was high (Table 2).
Table 2.
Number, percentage and rate of children prescribed different antibiotics at less than the recommended dose.
| Age (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–4 | 5–11 | 12–16 | |||||||
| Drug name | No. | % | Rate | No. | % | Rate | No. | % | Rate |
| Amoxycillin | 324 | 27.5 | 46.4 | 332 | 18.8 | 79.0 | 89 | 4.8 | 43.9 |
| Co-amoxiclav | 47 | 4.0 | 90.9 | 132 | 7.5 | 275.6 | 30 | 1.6 | 92.3 |
| Flucloxacillin | 41 | 3.5 | 42.7 | 90 | 5.1 | 66.7 | 72 | 3.9 | 55.2 |
| Phenoxymethylpenicillin | 52 | 4.4 | 65.5 | 577 | 32.7 | 388.8 | 1272 | 68.6 | 741.3 |
| Cefaclor | 2 | 0.1 | 9.6 | 46 | 2.6 | 380.2 | 3 | 0.1 | 42.9 |
| Cephalexin | 35 | 3.0 | 101.2 | 124 | 7.0 | 406.6 | 33 | 1.8 | 165.0 |
| Clarithromycin | 1 | 0.1 | 5.75 | 33 | 1.9 | 240.9 | 5 | 0.3 | 47.6 |
| Erythromycin | 676 | 57.4 | 456.8 | 430 | 24.4 | 432.2 | 351 | 18.9 | 299.5 |
Percent total number children prescribed an antibiotic at less than the recommended dose. Rate/1000 children prescribed that antibiotic.
Age trends for lower than recommended dose prescribing were apparent for each of the three antibiotic classes: penicillins, cephalosporins and macrolides (Figures 1, 2 and 3), with the highest number of prescriptions for less than the recommended dose occurring at those ages at which the SPC recommended an increase in dosage.
Figure 1.
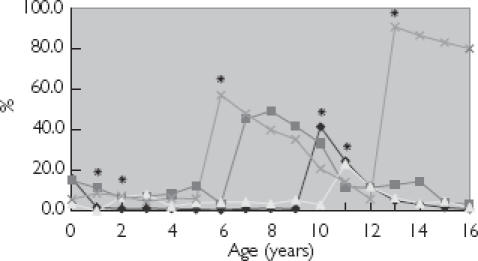
Age trends for penicillin prescribing at less than recommended dose. *Summary of Product Characteristics recommended age change in dosage. Co-amoxiclav is dosed as mg kg−1 day−1. Amoxycillin (♦); co-amoxiclav flucloxacillin (▪); and phenoxymethyl penicillin (×).
Figure 2.
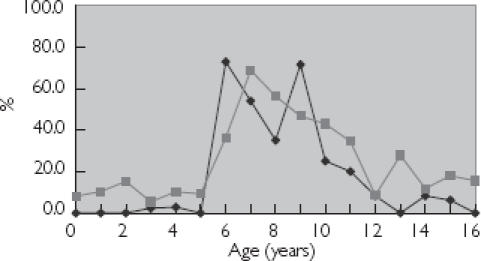
Age trends for cephalosporin prescribing at less than recommended dose. Cefaclor (♦); and cephalexin (▪) are dosed as mg kg−1 day−1.
Figure 3.
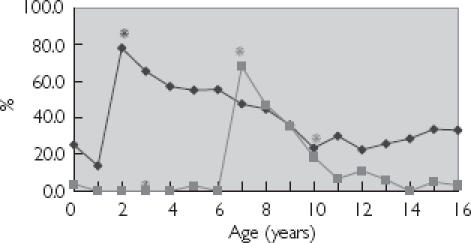
Age trends for macrolide prescribing at less than recommended dose. *Summary of Product Characteristics recommended age change in dosage. Erythromycin (♦); and clarithromycin (▪).
Receipt of a prescription for one of the three most common antibiotics (amoxycillin, erythromycin, phenoxymethylpenicillin) for less than the recommended dose was not associated with an increased risk of subsequent antibiotic prescriptions during the following month at any age {0–4 years odds ratio [OR] 1.1 [95% confidence interval (CI) 0.8, 1.5], χ2 test P = 0.59; 5–11 years OR 0.8 [95% CI 0.6, 1.1], χ2 test P = 0.29; 12–16 years OR 1.0 [95% CI 0.7, 1.4], χ2 test P = 0.92}.
Similarly an antibiotic prescription for less than the recommended dose was not associated with a significant increase in the number of antibiotic prescriptions over the study year for children aged 0–4 and 12–16 years [0–4 years mean = 1.99 (95% CI 1.73, 2.25) vs. 1.82 (95% CI 1.75, 1.89), Mann–Whitney U-test P = 0.26; and 12–16 years mean = 1.90 (95% CI 1.68, 2.12) vs. 1.60 (95% CI 1.49, 1.70), Mann–Whitney U-test P = 0.10]. However, in the 5–11-year-olds prescribed a lower than recommended dose a significant increase in the number of antibiotic prescriptions during the following year was observed [mean = 2.09 (95% CI 1.79, 2.39) vs. 1.41 (95% CI 1.35, 1.47), Mann–Whitney U-test P < 0.001].
Higher than recommended dose prescribing of antibiotics was not common, accounting for 1.6% of all antibiotic prescribing, occurring mainly in 0–4-year-olds and decreasing steadily with age (Table 1). The most frequently prescribed antibiotic at higher than recommended dose was amoxycillin.
Discussion
Prescription of antibiotics at less than the recommended dose accounted for the majority of off-label prescribing (Table 1), the peak rates of which were closely linked to the antibiotic SPC recommended ages for dose increment. The rate of prescribing for less than the recommended dose increased immediately following a change in dosage recommendation and then fell progressively until the next age-related increment (Figures 1–3). Such a situation might be explained by a lack of awareness of age-related changes in dosage recommendations, failure to consult the relevant formulary, or an understandable confusion created by the use of different age bandings and dosage schedules for each antibiotic. Although antibiotic dose formulations are identical, recommended dose increments occur at different ages and by different amounts [11]. Antibiotics such as coamoxiclav, cefaclor and cephalexin have dosage recommendations calculated according to body weight up until 12 years of age [11]. Such recommendations frequently result in a calculated antibiotic dose which is not a simple multiple of 5 mL, so influencing the doctor to prescribe higher or lower than recommended doses to fit the standard formulations. This conclusion is supported by the observation that the majority of less than recommended dose prescribing for these three particular antibiotics occurred in the 5–11 years age group, where the lowest recommended dose falls between the 125- and 250-mg formulations.
Although it is difficult to assess the clinical effects of prescribing less than recommended doses of an antibiotic, in this study there was no evidence that such prescribing was associated with an increased frequency of antibiotic prescribing during the following month when compared with children prescribed the recommended dose, suggesting that antibiotics at lower than recommended dose appear as effective as recommended doses in controlling symptoms. However, in school-aged children (5–11-year-olds) a lower than recommended dose prescription was associated with a significant increase in the total number of antibiotic prescriptions during the study year. A similar but nonsignificant trend was also observed in 0–4 and 12–16-year-olds. The relevance and reasons for this difference in antibiotic prescribing following a lower than recommended dose prescription are unclear.
Appropriate antibiotic prescribing is recognized as an essential step in minimizing bacterial resistance [7], with recommendation that higher antibiotic doses should be used [12–14]. The results of this study indicate that lower than recommended doses of antibiotic do not appear to affect adversely the health of an individual child as assessed by further prescriptions for antibiotic therapy during the month or year following an initial low-dose prescription. However, the widespread use of less than recommended doses of antibiotics, observed in this study, may affect the health of the population by facilitating the development of antimicrobial resistance. Our observations demonstrate that off-label, lower than recommended dose antibiotic prescribing is common in General Practice. Standardization of dosage recommendations for all antibiotics based on physiological and major developmental stages during childhood would be more appropriate than the current apparently arbitrary age bands. Such an approach would reduce off-label prescribing in children (of which antibiotics account for about 34%[9]) and help to minimize the potential development of antimicrobial resistance.
Acknowledgments
We would like to thank Bob Milne and Alistair Coutts, custodians of the database and the other staff of the GPASS Data Evaluation Project for all their help and support. P.J.H. had the idea for the study and framed the questions with S.E.-D. S.E.-D. extracted and analysed the data and S.E.-D., P.J.H. and J.S.M. wrote the paper with contributions from M.W.T. and C.R.S. M.W.T. is guarantor of the study. P.J.H. has current grants from Glaxo Wellcome, and Merck, Sharp & Dohme. He has also performed consultancies for Merck Sharp & Dohme, Astra Zeneca and Glaxo Wellcome. J.S.M. has current grant support from Glaxo Wellcome, SmithBeecham, Merck Sharp & Dohme, Roche Pharmaceuticals, Hesperion.
References
- 1.Schirm E, Tobi H, De Jong-Van Den Berg LT. Unlicensed and off label drug use by children in the community: cross sectional study. Br Med J. 2002;324:1312–1313. doi: 10.1136/bmj.324.7349.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy S. Developments in relation to unlicensed and off label drug use. Paediatric Perinatal Drug Ther. 1998;2:23–26. [Google Scholar]
- 3.Collier J. Paediatric prescribing: using unlicensed drugs and medicines outside their licensed indications. Br J Clin Pharmacol. 1999;48:5–8. doi: 10.1046/j.1365-2125.1999.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heath PT, Breathnach AS. Treatment of infections due to resistant organisms. Br Med Bull. 2002;61:231–245. doi: 10.1093/bmb/61.1.231. [DOI] [PubMed] [Google Scholar]
- 5.Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343:1917–1924. doi: 10.1056/NEJM200012283432603. [DOI] [PubMed] [Google Scholar]
- 6.Guillemot D, Carbon C, Balkau B, et al. Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA. 1998;279:365–370. doi: 10.1001/jama.279.5.365. [DOI] [PubMed] [Google Scholar]
- 7.CDC. A Public Health Action Plan to Combat Antimicrobial Resistance. Atlanta: Office of Health Communication; 1999. [Google Scholar]
- 8.‘t Jong GW, Eland IA, Sturkenboom MC, van den Anker JN, Stricker BH. Unlicensed and off label prescription of drugs to children: population based cohort study. Br Med J. 2002;324:1313–1314. doi: 10.1136/bmj.324.7349.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McIntyre J, Conroy S, Avery A, Corns H, Choonara I. Unlicensed and off label prescribing of drugs in general practice. Arch Dis Child. 2000;83:498–501. doi: 10.1136/adc.83.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ABPI. Compendium of Data Sheets and Summaries of Product Characteristics. London: Datapharm Publications Limited; 1999. [Google Scholar]
- 11.British Medical Association. British National Formulary. 40. London: BMA; 2000. Royal Pharmaceutical Society of Great Britain. [Google Scholar]
- 12.Craig WA. Antimicrobial resistance issues of the future. Diagn Microbiol Infect Dis. 1996;25:213–217. doi: 10.1016/s0732-8893(96)00162-9. [DOI] [PubMed] [Google Scholar]
- 13.Schrag SJ, Pena C, Fernandez J, et al. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA. 2001;286:49–56. doi: 10.1001/jama.286.1.49. [DOI] [PubMed] [Google Scholar]
- 14.Guillemot D, Carbon C, Vauzelle-Kervroedan F, et al. Inappropriateness and variability of antibiotic prescription among French office-based physicians. J Clin Epidemiol. 1998;51:61–68. doi: 10.1016/s0895-4356(97)00221-7. [DOI] [PubMed] [Google Scholar]


