Abstract
Aims
The influence of ageing on the pharmacokinetics of zolpidem, an extensively prescribed hypnotic medication, was evaluated in healthy human volunteers.
Methods
A series of 16 elderly (age: 61–85 years) and 24 young (age: 22–42 years) volunteers received single 5 mg oral doses of zolpidem tartrate. Serum zolpidem concentrations were determined by HPLC with fluorescence detection in samples drawn during 8 h after dosage. The effect of testosterone on zolpidem biotransformation was evaluated in vitro using human liver microsomes. Possible induction of CYP3A protein expression and function was studied in cultured human hepatocytes.
Results
Among men, apparent oral clearance of zolpidem was decreased in elderly compared to young subjects (3.8 vs 11.0 ml min−1 kg−1, P < 0.01), Cmax was increased (93 vs 40 ng ml−1, P < 0.01), and half-life increased (2.7 vs 1.5 h, P < 0.03). Among women, zolpidem oral clearance was decreased in the elderly (3.0 vs 5.8 ml min−1 kg−1, P < 0.02), Cmax increased (108 vs 60 ng ml−1, P < 0.001), with no difference in t1/2 (2.3 vs 2.4 h). Among male subjects, free serum testosterone concentrations were lower in the elderly (10.5 vs 19.0 pg ml−1, P < 0.01), and were significantly correlated with zolpidem clearance (r2 = 0.46, P < 0.001). Multiple regression analysis indicated a greater relative contribution of serum testosterone than age to the oral clearance of zolpidem among men. In human liver microsomes, co-incubation of zolpidem (10 µm) with varying concentrations of testosterone produced activation of biotransformation of zolpidem to its principal hydroxylated metabolite. Maximum activation was achieved at equimolar concentrations of testosterone (10 µm). However, testosterone did not induce immunoactive CYP3A4 expression or catalytic function in cultured human hepatocytes.
Conclusions
The increased Cmax and lower oral clearance of zolpidem in the elderly are consistent with recommendations of lower clinical doses of zolpidem in the elderly. Our clinical and in vitro data both suggest that reduced free serum testosterone may have a modulatory role in age-dependent changes in zolpidem pharmacokinetics in men.
Keywords: ageing, pharmacokinetics, testosterone, zolpidem
Introduction
The proportion of the elderly in the US and Europe is increasing and is expected to increase further within the next few decades [1]. Sleep disorders requiring the use of hypnotics are also quite common in elderly individuals [2, 3], as is multiple drug use with the attendant possibility of drug interactions, adverse drug reactions, cognitive impairment, and injuries [4–8]. As such, a better understanding of the pharmacokinetics of commonly used drugs in the elderly is of considerable importance. Older persons appear to be more susceptible to adverse drug reactions compared to patients of younger age. This is particularly important with respect to drugs that influence the central nervous system. Drug action and response in the elderly may differ from that in the younger population as a result of changes in disease characteristics associated with old age, age-related intrinsic changes in drug sensitivity [9], or pharmacokinetic alterations related to the ageing process [9–15].
Zolpidem is a nonbenzodiazepine imidazopyridine hypnotic, which is a full agonist for the benzodiazepine (BZ) component of the GABAA receptor complex, and has relative selectivity for the BZ-1 (omega-1) subtype and lower affinity for the other receptor subtypes [16–20]. Since its introduction to clinical use in the United States in the early nineties, zolpidem has become one of the most commonly prescribed hypnotics in the elderly population [18]. Because of its short elimination-half-life, zolpidem appears to have a low likelihood of producing residual sedative effects, with the probability of a reduced risk of falls and fractures in the elderly. Zolpidem also is reported to have a lower risk of causing rebound insomnia upon discontinuation [21].
In vitro studies using human liver microsomes and heterologously expressed individual human cytochromes P450 (CYPs) have demonstrated that zolpidem is biotransformed to three pharmacologically inactive hydroxylated metabolites [22, 23] by a series of CYP enzymes including CYP3A4, CYP2C9, CYP1A2, CYP2D6 and CYP2C19, in decreasing order of importance [22]. CYP3A4 is the principal enzyme responsible for zolpidem metabolism, accounting for approximately 60% of net CYP-mediated hepatic clearance. Three distinct hydroxylated metabolites result from zolpidem biotransformation. One of these, termed the M-3 metabolite, accounts for 83% of net metabolite formation, and 58% of net M-3 formation is attributed to CYP3A4. Absolute bioavailability of orally administered zolpidem in healthy young male volunteers averages approximately 70%, suggesting that presystemic extraction is not extensive [24]. The effect of age and gender on zolpidem bioavailability is not established. There is no available evidence to suggest that zolpidem is a substrate for transport by enteric P-glycoprotein or other transporter systems. Zolpidem itself is not a significant inhibitor of human CYP isoforms [25].
Age-related changes in the pharmacokinetic properties of zolpidem are described in summary reports and in labelling information [16, 26]. Because many (but not all) clinical studies of CYP3A substrates show reduced clearance in the elderly [11–15], age-related changes in zolpidem kinetics require further investigation. Among other factors, the contributory importance of age-dependent changes in testosterone homeostasis in elderly men has also not been evaluated. Although testosterone is mainly a substrate for CYP3A4, some in vitro studies suggest that it is also a modulator of CYP3A4 activity. During simultaneous incubation of some CYP3A4 substrates with testosterone, substrate clearance was activated, possibly through conformational changes in the enzymatic active site [27–35].
This study was carried out to assess the pharmacokinetic properties of zolpidem in normal elderly compared with young adult subjects and to assess the possible modulatory role of free testosterone in elderly and young males. We also evaluated the effect of testosterone on zolpidem biotransformation using human liver microsomes in vitro, and on CYP3A expression and activity in cultured human hepatocytes.
Methods
Subjects
The study protocol was reviewed by the local Institutional Review Board and informed consent was obtained from all subjects. Forty Caucasian volunteers participated in the study. They were divided into two groups of those aged 21–42 years (n = 24), and those aged 66–85 years (n = 16) (Table 1). All subjects were healthy, ambulatory and medically stable. Among the elderly, two subjects were on digoxin, one on aspirin, and one each on perindopril and phenprocoumon. None of these drugs is known to induce or inhibit CYP3A4. Eight of the 16 young female subjects were taking oral contraceptive preparations; a previous study demonstrated that oral contraceptives have no significant influence on zolpidem clearance [36].
Table 1.
Demographic characteristics (mean (± SD)) of the subject population and the relevant laboratory values.
| Young male | Elderly male | Kruskal–Wallis test | Young female | Elderly female | Kruskal–Wallis test | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Number | 8 | 8 | 16 | 8 | ||
| Age (years) | 23.4 (±5.5) | 73.1 (±8.5) | P < 0.001 | 27.8 (±5.3) | 74.3 (±5.9) | P < 0.001 |
| Weight (kg) | 76.4 (±5.9) | 74.0 (±4.0) | NS | 66.3 (±6.6) | 72.1 (±11.0) | NS |
| Height (m) | 1.84 (±0.11) | 1.72 (±0.04) | P < 0.05 | 1.73 (±0.07) | 1.61 (±0.05) | P < 0.001 |
| Body mass index (kg m−2) | 22.6 (±1.8) | 25.0 (±0.9) | P < 0.01 | 22.3 (±2.3) | 27.9 (±4.0) | P < 0.001 |
| Laboratory values | ||||||
| Serum albumin (g 100 ml−1) | 6.4 (±0.2) | 5.8 (±0.5) | P < 0.05 | 6.0 (±0.3) | 6.0 (±0.03) | NS |
| Serum creatinine (mg 100 ml−1) | 0.9 (±0.1) | 1.1 (±0.3) | P < 0.1 | 0.9 (±0.1) | 0.9 (±0.2) | NS |
| Free testosterone (pg ml−1) | 19.0 (±6.4) | 10.5 (±3.7) | P < 0.01 | 1.8 (±3.5) | 1.4 (±1.0) | NS |
| Growth hormone (ng ml−1) | 0.53 (±0.66) | 0.38 (±0.29) | NS | 2.83 (±4.56) | 0.8 (±0.6) | P < 0.1 |
Procedure
Subjects fasted overnight prior to the study. After a baseline blood sample was obtained (time 0 h), 5 mg of zolpidem tartrate (containing 4.2 mg of zolpidem base) was given with water by mouth. Subjects had a small breakfast (excluding grapefruit juice) 2 h after the oral dose. Venous blood samples were drawn at time 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6 and 8 h after zolpidem dosage. The serum was separated by centrifugation and samples were frozen at −80 °C until assayed.
Analysis of samples
Zolpidem concentrations in serum were determined by high-performance liquid chromatography (HPLC) using fluorescence detection, as described in detail previously [37]. After addition of an internal standard, samples were extracted with hexane. The organic extract was separated, evaporated to dryness, and reconstituted for HPLC analysis. The HPLC system used in the analysis was from Waters Associates (Milford, MA). The instruments and reagents included a reverse-phase C-18 microBondapak column (30 cm by 3.9 mm), a mobile phase consisting of acetonitrile : 50 mm potassium dihydrogen phosphate (50 : 50) at pH 7.4 and a flow rate of 1.8 ml min−1. The effluent from the column was monitored by a Waters (Milford, MA) fluorescence spectrophotometer at excitation and emission wavelengths of 254 and 390 nm, respectively, with the system operating at room temperature. The assay sensitivity limit was 1 ng ml−1, and the within- and between-day coefficients of variance did not exceed 10%. Across 24 analytical runs, the coefficient of variation (CV) for a quality control sample analysed with each set of unknowns was 9.2%. The between day CV in the slope of the calibration curve was 10.6%.
Free testosterone concentrations in single serum samples were determined by standard radioimmunoassay (RIA) and human growth hormone by the double antibody RIA technique.
The slope (λz) of the terminal log-linear phase of each zolpidem serum concentration-time curve was determined by linear regression analysis, and was then used to calculate the apparent elimination half-life. The area under the serum concentration-time curve from time 0 h to the last concentration was determined by the linear trapezoidal method. The residual area extrapolated to infinity (the final concentration divided by λz) was then added to give the total area under the serum concentration-time curve (AUC). Apparent oral clearance was calculated as the dose of zolpidem base divided by the total AUC. The peak plasma concentration (Cmax) and the time to achieve Cmax (tmax), representing the rate of drug appearance in the systemic circulation, were determined for each subject.
The results were analysed by both linear and nonlinear regression. Since the distributions under study were vulnerable to the effects of outlying values and heterogeneous variance, nonparametric Kruskal–Wallis tests were used to evaluate statistical differences between mean values.
In vitro study of human liver microsomes
Liver samples from four individual human donors with no known liver disease were provided by the International Institute for the Advancement of Medicine, Exton, PA, the Liver Tissue Procurement and Distribution System, University of Minnesota, Minneapolis, MN, or the National Disease Research Interchange, Philadelphia, PA. Acquisition of de-indentified tissue samples from these sources was reviewed by the Human Investigation Review Committee (the Institutional Review Board) serving Tufts University School of Medicine and Tufts-New England Medical Center, and designated as exempt. All samples were of the CYP2D6 and CYP2C19 extensive metabolizer phenotype based on prior in vitro studies.
Human liver microsomes were prepared by ultracentrifugation, and microsomal pellets were suspended in 0.1 m potassium phosphate buffer containing 20% glycerol and stored at −80 °C until use [22]. Samples of zolpidem and its metabolites were provided by Synthélabo Recherche, Bagneux, France. Other chemical reagents and drug entities were purchased from commercial sources or kindly provided by their manufacturers.
Zolpidem (10 µm) and testosterone (0–50 µm) were added to the incubation tubes in a small volume of organic solvent, which was evaporated to dryness. Phosphate buffer 50 mm, 5 mm Mg++, 0.5 mm NADP+, and an isocitrate/isocitric dehydrogenase regenerating system were then added and the mixture was brought to 37 °C. Reactions were initiated by addition of microsomal protein (0.25 mg ml−1). After 20 min of incubation, reactions were stopped by addition of acetonitrile and cooling on ice. Propranolol (5 µg) was added as the internal standard. The samples were centrifuged, and the supernatant was transferred to an autosampling vial for HPLC analysis.
The HPLC system consisted of a Waters C-18 micro-BondaPak column (30 cm length, 10 µ particle size) kept at room temperature, and detection was by UV at 242 nm. The mobile phase was 50 mm KH2PO4 : acetonitrile : methanol (60 : 30 : 10). Assay of incubations containing zolpidem, microsomes and cofactors yielded a major chromatographic peak corresponding to the principal hydroxylated metabolite (designated as M3) [22]. Other metabolite peaks either were not large enough for quantitation, or co-eluted with a metabolite of testosterone. M3 metabolite formation rates with coaddition of testosterone were expressed as ratios versus the reaction velocity in the control condition without testosterone.
In vitro study of cultured human hepatocytes
Fresh liver tissue was obtained from a single human donor. The hepatocytes were isolated and cultured in 24-well plates with plating media for a 24 h attachment period at 37 °C in an atmosphere of 5% CO2, and then were cultured with incubation media [38–40]. Testosterone 10 µm or rifampicin 20 µm in 0.5% or vehicle control (0.5% DMSO) in DMSO, were added and incubated with hepatocytes for 48 h at 37 °C in an atmosphere of 5% CO2.
The probe substrate for CYP3A4 activity was triazolam 250 µm prepared in media containing 0.5% methanol. Cells were incubated with substrate for 1.5 h at 37 °C in an atmosphere of 5% CO2. Reactions were stopped by the addition of 200 µl of acetonitrile. Plates containing media were frozen at −80 °C until HPLC analysis.
The media was thawed and the internal standard phenacetin (50 µl of 4.5 mg 100 ml−1 methanol solution) was added directly to the plate wells and mixed by pipetting. Media containing internal standard was transferred directly to autosampling vials for HPLC analysis of α-hydroxytriazolam. The HPLC mobile phase consisted of 710 ml of 10 mm potassium phosphate buffer, 200 ml acetonitrile, and 100 ml methanol. A reverse phase Nova-Pak C18 column (Waters Associates, Milford, MA) was used with a flow rate of 1.5 ml min−1 and a detection wavelength of 220 nm [53, 54]. Peak height ratios of α-hydroxytriazolam and internal standard phenacetin were determined.
For the analysis of CYP3A4 immunoactive protein, the media was aspirated from the cells, and the latter were lysed by addition of a lysis buffer. The contents of each well were sonicated, centrifuged, and stored at −80 °C. CYP3A4 immunoactive protein was quantitated by Western blot analysis as described previously [55–57].
Results
Clinical study
Demographic features of the subject groups and pharmacokinetic parameters for zolpidem are shown in Tables 1 and 2. Overall, apparent oral clearance values ranged from 66 to 1595 ml min−1 and the overall mean (± SD) value was 411 (± 345) ml min−1.
Table 2.
Pharmacokinetic parameters (mean (± SD)).
| Kruskal–Wallis | Kruskal–Wallis | Difference in mean value between young and elderly | ||||||
|---|---|---|---|---|---|---|---|---|
| Young male | Elderly male | test | Young female | Elderly female | test | Men | Women | |
| Cmax (ng ml−1) | 40 (± 16) | 93 (± 45) | P < 0.01 | 60 (± 19) | 108 (± 30) | P < 0.001 | 54 | 48 |
| [26, 53] | [56, 131] | [49, 70] | [83, 133] | [17, 90] | [27, 69] | |||
| tmax (h) | 0.8 (± 0.3) | 1.1 (± 0.4) | NS | 1.2 (± 0.4) | 0.8 (± 0.7) | NS | 0.25 | −0.34 |
| [0.6, 1.2] | [0.7, 1.4] | [0.9, 1.4] | [0.2, 1.4] | [−0.12, 0.62] | [−0.83, 0.14 | |||
| Total AUC (ng ml−1 h) | 110 (± 68) | 400 (± 326) | P < 0.01 | 249 (± 133) | 398 (± 189) | P < 0.05 | 289 | 150 |
| [54, 167] | [127, 672] | [178, 320] | [241, 557] | [37, 542] | [12, 287] | |||
| Clearance | ||||||||
| (ml min−1) | 820 (± 445) | 276 (± 179) | P < 0.01 | 376 (± 271) | 209 (± 122) | P < 0.05 | −545 | −167 |
| [448, 1193] | [126, 425] | [232, 520] | [107, 311] | [−909, −181] | [−377, 44] | |||
| (ml min−1 kg−1) | 11.0 (± 6.4) | 3.8 (± 2.5) | P < 0.01 | 5.8 (± 4.8) | 3.0 (± 1.9) | P < 0.02 | −7.2 | −2.9 |
| [5.6, 16.3] | [1.6, 5.9] | [3.3, 8.4] | [1.4, 4.5] | [−12.4, −2.0] | [−6.5, 0.8] | |||
| Half-life (h) | 1.5 (± 0.5) | 2.7 (± 1.2) | P < 0.03 | 2.4 (± 0.9) | 2.3 (± 0.7) | NS | 1.2 | −0.2 |
| [1.1, 1.8] | [1.7, 3.6] | [1.9, 3.9] | [1.7, 2.9] | [0.2, 2.1] | [−0.9, 0.6] | |||
95% confidence intervals are shown in square brackets.
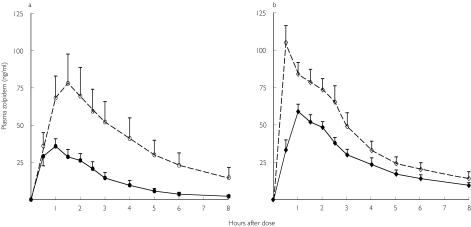
Among men, zolpidem oral clearance (with and without normalization for body weight) was significantly lower in the elderly, AUC and Cmax were significantly higher, and elimination half-life was longer (Table 2, Figure 1). Among women, Cmax and AUC were also significantly higher in the elderly group, and zolpidem oral clearance was lower in the elderly group (Table 2, Figure 1). Elimination half-life was unchanged between young and elderly women.
Figure 1.
Mean (± SEM) serum zolpidem concentrations–time plots for young (•, ♦) and elderly (○, ◊) male (a) and female (b) volunteers.
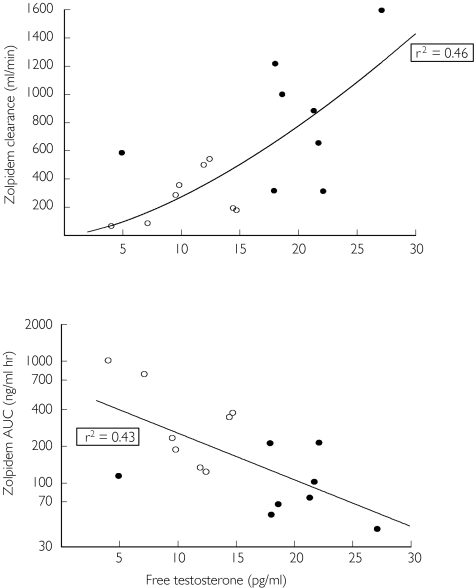
Among male subjects, mean free serum testosterone concentration was significantly lower in the elderly compared with the young subjects (10.5 and 19.0 pg ml−1, respectively, P < 0.01). Free testosterone concentration was significantly correlated with log-transformed zolpidem AUC (r2 = 0.43), and with oral clearance of zolpidem, based on a nonlinear function of the form: γ = bxA (r2 = 0.46, P < 0.001) (Figure 2). Multiple regression analysis indicated that age and free testosterone collectively accounted for a significant proportion of the variance in zolpidem oral clearance (multiple r2 = 0.48, P < 0.02). However, standardized regression coefficients suggested that the contribution of free testosterone was greater than that of age, though the difference was not statistically significant. Free testosterone concentration was low in all females and was unrelated to age or zolpidem clearance. Human growth hormone concentrations, though higher in young women, were not significantly related to age and gender.
Figure 2.
Relationship between free serum testosterone concentration and zolpidem oral clearance (top) and zolpidem AUC (bottom) in male subjects. For zolpidem AUC, linear regression was used after logarithmic transformation of AUC values (r2 = 0.43). For zolpidem clearance, the line represents a function of the form: γ = BxA (r2 = 0.46). Elderly male (○) and young male (•).
Human liver microsomal study
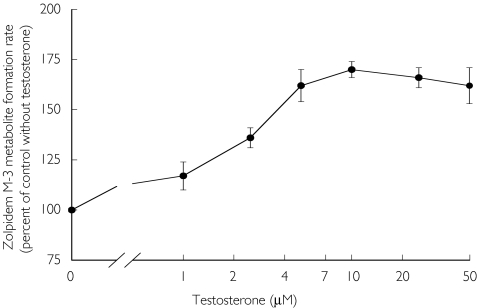
Biotransformation of zolpidem to its principal hydroxylated metabolite by human liver microsomes in vitro was modulated by testosterone. Testosterone (0-10 µm) caused a concentration-dependent increase in the rate of zolpidem biotransformation (Figure 3). At 10 µm testosterone (the same concentration as that of the substrate, zolpidem), zolpidem metabolite formation was increased by a mean factor of 1.7 compared with the control reaction velocity. At higher concentrations of testosterone, no additional increase was seen.
Figure 3.
Effect of testosterone on the rate of formation of the zolpidem M-3 metabolite from zolpidem (10 µm, equivalent to 2.88 µg ml−1) by human liver microsomes. Rates of formation are expressed as a percent of the control velocity with no testosterone present. Each point is the mean (± SEM) of four separate human liver microsomal preparations.
Cultured human hepatocyte study
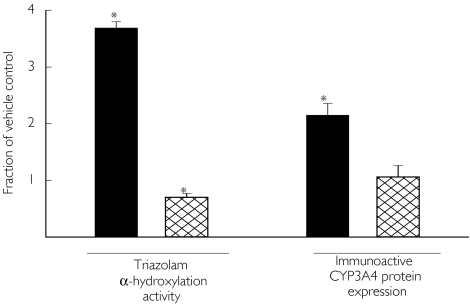
Exposure to rifampicin 20 µm increased the rate of triazolam α-hydroxylation by a factor of 3.7 compared with vehicle control. In contrast, exposure to testosterone 10 µm did not increase activity compared with control (Figure 4). Rifampicin also increased immunoactive CYP3A protein expression by 2.1-fold compared with control, whereas the change attributable to testosterone was negligible (1.1-fold).
Figure 4.
Effect of 48 h of exposure to rifampin (20 µm) ( ) or testosterone (10 µm) (
) or testosterone (10 µm) ( ) on triazolam α-hydroxylation activity and on the expression of immunoactive CYP3A4 protein in human hepatocytes in cell culture. Bars indicate the mean (± SEM) values relative to cells cultured with vehicle control (0.5% DMSO). Asterisk (*) indicates a significant difference from 1.0 based on Student's t-test (n = 4 separate experiments for triazolam α-hydroxylation, n = 2 for immunoactive protein).
) on triazolam α-hydroxylation activity and on the expression of immunoactive CYP3A4 protein in human hepatocytes in cell culture. Bars indicate the mean (± SEM) values relative to cells cultured with vehicle control (0.5% DMSO). Asterisk (*) indicates a significant difference from 1.0 based on Student's t-test (n = 4 separate experiments for triazolam α-hydroxylation, n = 2 for immunoactive protein).
Discussion
During the last decade there has been a trend towards the use of short half-life hypnotics to minimize daytime residual effects and drug accumulation after multiple dosage, both of which are of particular concern for the elderly. Older benzodiazepine hypnotics, such as flurazepam, are eliminated slowly [46], cause residual daytime sedation, and accumulate during multiple dosage, and have been associated with sedation, cognitive impairment and injuries in the elderly [47]. Zolpidem is now prescribed extensively as a hypnotic, particularly because of its shorter half-life. Although reports indicate that the clearance of zolpidem may diminish in the elderly [16, 26], the effect of age on its pharmacokinetics is not fully understood.
Our study shows that the elderly clear zolpidem much more slowly than younger subjects. Oral clearance in elderly men was about one third of that in younger men, and AUC was about four times higher than in younger men. Cmax was increased more than two fold in the elderly and half-life was approximately doubled. A similar difference, though less pronounced, was evident in elderly women compared with the younger group with regard to oral clearance and, to a lesser extent, Cmax. We also observed higher values for oral clearance and lower Cmax concentrations in men compared with women within both age groups. These latter differences were not subjected to separate statistical comparison, but are consistent with previous reports [48].
It is known that elderly men have lower testosterone concentrations than younger men [49–52], as verified in this study. Regression analysis suggested that lower concentrations of testosterone were associated with the reduced clearance of zolpidem in ageing men. Therefore it is possible that the lower plasma concentrations of free testosterone present in the elderly may contribute to lower CYP3A activity. Testosterone, itself a CYP3A4 substrate, has complex effects on hepatic microsomal activity. It does not induce expression of CYP3A protein in humans, which was confirmed in our in vitro study using cultured human hepatocytes. However, exposure to testosterone is known to activate the biotransformation of a number of CYP3A substrates in vitro [27–35], such as the CYP3A-mediated production of both 3-OH diazepam and N-desmethyldiazepam from diazepam [27]. Testosterone activates 4-OH triazolam formation and at the same time inhibits α-OH triazolam production from triazolam [28, 31]. A similar pattern is observed with midazolam [27, 35]. We found that the rate of transformation of zolpidem to its major hydroxylated metabolite in human liver microsomes was increased by an average of 70% by co-incubation with equimolar amounts of testosterone. This study has important limitations, in that the ratio of testosterone relative to zolpidem concentrations is much higher than would be encountered in vivo. Nonetheless, these in vitro data, together with the clinical results, raise the possibility that testosterone can modulate age-related changes in the clearance of zolpidem, and possibly other CYP3A substrates, in men. This hypothesis will require further clinical investigation. It remains unclear why age-dependent differences in zolpidem clearance among women were unrelated to testosterone concentration.
Our study also suggests that human growth hormone (HGH) does not appear to exert any modulatory effect on the metabolism of zolpidem across age groups and gender. This is consistent with a recent study that showed that HGH has no effect on CYP3A4 [53].
In conclusion, we have demonstrated substantially diminished clearance of zolpidem in the elderly, particularly in elderly men. This finding supports the recommendation that zolpidem dosage should be decreased in the elderly, to minimize the likelihood of elevated serum concentrations [54].
Acknowledgments
Supported by Grants AG-17880, MH-01237, DA-05258, DA-13209, MH-58435, DK/AI-58496, and RR-00054 from the Department of Health and Human Services. Dr. Olubodun is supported by the Tufts-Pfizer Fellowship Program in Clinical Pharmacology.
References
- 1.von Moltke LL, Greenblatt DJ, Romach MK, Sellers EM. Cognitive toxicity of drugs used in the elderly. Dialogues Clin Neurosci. 2001;3:181–190. doi: 10.31887/DCNS.2001.3.3/llvonmoltke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment. Prevalence and correlates. Arch Gen Psychiatry. 1985;42:225–232. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- 3.Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6, 800 persons over three years. Sleep. 1999;22(Suppl 2):S366–S372. [PubMed] [Google Scholar]
- 4.Moore AR, O'Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15–28. doi: 10.2165/00002512-199915010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging. 1999;14:141–152. doi: 10.2165/00002512-199914020-00005. [DOI] [PubMed] [Google Scholar]
- 6.Beyth RJ, Shorr RI. Epidemiology of adverse drug reactions in the elderly by drug class. Drugs Aging. 1999;14:231–239. doi: 10.2165/00002512-199914030-00005. [DOI] [PubMed] [Google Scholar]
- 7.Cumming RG. Epidemiology of medication-related falls and fractures in the elderly. Drugs Aging. 1998;12:43–53. doi: 10.2165/00002512-199812010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Hemmelgarn B, Suissa S, Huang A, Boivin JF, Pinard G. Benzodiazepine use and the risk of motor vehicle crash in the elderly. JAMA. 1997;278:27–31. [PubMed] [Google Scholar]
- 9.Barnhill JG, Greenblatt DJ, Miller LG, Gaver A, Harmatz JS, Shader RI. Kinetic and dynamic components of increased benzodiazepine sensitivity in aging animals. J Pharmacol Exp Ther. 1990;253:1153–1161. [PubMed] [Google Scholar]
- 10.Durnas C, Loi C-M, Cusack BJ. Hepatic drug metabolism and aging. Clin Pharmacokinet. 1990;19:359–389. doi: 10.2165/00003088-199019050-00002. [DOI] [PubMed] [Google Scholar]
- 11.Greenblatt DJ, Harmatz JS, Shader RI. Clinical pharmacokinetics of anxiolytics and hypnotics in the elderly: therapeutic considerations. Clin Pharmacokinet. 1991;21:165–177. doi: 10.2165/00003088-199121030-00002. 262–273. [DOI] [PubMed] [Google Scholar]
- 12.von Moltke LL, Abernethy DR, Greenblatt DJ. Kinetics and dynamics of psychotropic drugs in the elderly. In: Salzman C, editor. Clinical Geriatric Psychopharmacology. Baltimore: Williams & Wilkins; 1998. pp. 70–93. [Google Scholar]
- 13.von Moltke LL, Greenblatt DJ. Pharmacokinetics of psychotropic drugs in the elderly. Annu Rev Gerontol Geriatr. 1999;19:53–71. [Google Scholar]
- 14.von Moltke LL, Greenblatt DJ, Harmatz JS, Shader RI. Psychotropic drug metabolism in old age: principles and problems of assessment. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 1461–1469. [Google Scholar]
- 15.Pollock BG. Psychotropic drugs and the aging patient. Geriatrics. 1998;53(Suppl 1):S20–S24. [PubMed] [Google Scholar]
- 16.Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem: therapeutic implications. Clin Pharmacokinet. 1995;29:142–153. doi: 10.2165/00003088-199529030-00002. [DOI] [PubMed] [Google Scholar]
- 17.Darcourt G, Pringuey D, Sallière D, Lavoisy J. The safety and tolerability of zolpidem: an update. J Psychopharmacol. 1999;13:81–93. doi: 10.1177/026988119901300109. [DOI] [PubMed] [Google Scholar]
- 18.Rush CR. Behavioral pharmacology of zolpidem relative to benzodiazepines: a review. Pharmacol Biochem Behav. 1998;61:253–269. doi: 10.1016/s0091-3057(98)00102-6. [DOI] [PubMed] [Google Scholar]
- 19.Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs. 2000;59:865–889. doi: 10.2165/00003495-200059040-00014. [DOI] [PubMed] [Google Scholar]
- 20.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278:2170–2176. [PubMed] [Google Scholar]
- 21.Ware JC, Walsh JK, Scharf MB, Roehrs T, Roth T, Vogel GW. Minimal rebound insomnia after treatment with 10-mg zolpidem. Clin Neuropharmacol. 1997;20:116–125. doi: 10.1097/00002826-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 22.von Moltke LL, Greenblatt DJ, Granda BW, et al. Zolpidem metabolism in vitro: responsible cytochromes, chemical inhibitors, and in vitro correlations. Br J Clin Pharmacol. 1999;48:89–97. doi: 10.1046/j.1365-2125.1999.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pichard L, Gillet G, Bonfils C, Domergue J, Thenot J-P, Maurel P. Oxidative metabolism of zolpidem by human liver cytochrome P450s. Drug Metab Dispos. 1995;23:1253–1262. [PubMed] [Google Scholar]
- 24.Patat A, Trocherie S, Thebault JJ, et al. EEG profile of intravenous zolpidem in healthy volunteers. Psychopharmacology. 1994;114:138–146. doi: 10.1007/BF02245455. [DOI] [PubMed] [Google Scholar]
- 25.von Moltke LL, Weemhoff JL, Perloff MD, et al. Effect of zolpidem on human Cytochrome P450 activity, and on transport mediated by P-glycoprotein. Biopharm Drug Dispos. 2002;23:361–367. doi: 10.1002/bdd.329. [DOI] [PubMed] [Google Scholar]
- 26.Bianchetti G, Dubruc C, Thiercelin JF, et al. Clinical pharmacokinetics of zolpidem in various physiological and pathological conditions. In: Sauvanet JP, Langer SZ, Morselli PL, editors. Imidazopyridines in Sleep Disorders: A Novel Experimental and Therapeutic Approach. Vol. 6. New York: Raven Press; 1988. pp. 155–163. [Google Scholar]
- 27.Wang RW, Newton DJ, Liu N, Atkins WM, Lu AY. Human cytochrome P-450 3A4: in vitro drug–drug interaction patterns are substrate-dependent. Drug Metab Dispos. 2000;28:360–366. [PubMed] [Google Scholar]
- 28.Schrag ML, Wienkers LC. Triazolam substrate inhibition: evidence of competition for heme-bound reactive oxygen within the CYP3A4 active site. Drug Metab Dispos. 2001;29:70–75. [PubMed] [Google Scholar]
- 29.Schrag ML, Wienkers LC. Covalent alteration of the CYP3A4 active site: evidence for multiple substrate binding domains. Arch Biochem Biophys. 2001;391:49–55. doi: 10.1006/abbi.2001.2401. [DOI] [PubMed] [Google Scholar]
- 30.Hosea NA, Miller GP, Guengerich FP. Elucidation of distinct ligand binding sites for cytochrome P450 3A4. Biochemistry. 2000;39:5929–5939. doi: 10.1021/bi992765t. [DOI] [PubMed] [Google Scholar]
- 31.Kenworthy KE, Clarke SE, Andrews J, Houston JB. Multisite kinetic models for CYP3A4: simultaneous activation and inhibition of diazepam and testosterone metabolism. Drug Metab Dispos. 2001;29:1644–1651. [PubMed] [Google Scholar]
- 32.Shou M, Dai R, Cui D, Korzekwa KR, Baillie TA, Rushmore TH. A kinetic model for the metabolic interaction of two substrates at the active site of cytochrome P450 3A4. J Biol Chem. 2001;276:2256–2262. doi: 10.1074/jbc.M008799200. [DOI] [PubMed] [Google Scholar]
- 33.Lu P, Lin Y, Rodrigues AD, Rushmore TH, Baillie TA, Shou M. Testosterone. 7-benzyloxyquinoline, and 7-benzyloxy-4-trifluoromethyl-coumarin bind to different domains within the active site of cytochrome P450 3A4. Drug Metab Dispos. 2001;29:1473–1479. [PubMed] [Google Scholar]
- 34.Nakamura H, Nakasa H, Ishii I, et al. Effects of endogenous steroids on CYP3A4-mediated drug metabolism by human liver microsomes. Drug Metab Dispos. 2002;30:534–540. doi: 10.1124/dmd.30.5.534. [DOI] [PubMed] [Google Scholar]
- 35.Mäenpää J, Hall SD, Ring BJ, Strom SC, Wrighton SA. Human cytochrome P4503A (CYP3A) mediated midazolam metabolism: the effect of assay conditions and regioselective stimulation by alpha–naphthoflavone, terfenadine and testosterone. Pharmacogenetics. 1998;8:137–155. [PubMed] [Google Scholar]
- 36.Olubodun JO, Ochs HR, Trüten V, et al. Zolpidem pharmacokinetic properties in young females: influence of smoking and oral contraceptive use. J Clin Pharmacol. 2002;42:1142–1146. doi: 10.1177/009127002401382623. [DOI] [PubMed] [Google Scholar]
- 37.Durol ALB, Greenblatt DJG. Analysis of zolpidem in human plasma by high-performance liquid chromatography with fluorescence detection: application to single-dose pharmacokinetic studies. J Anal Toxicol. 1997;37:437–441. doi: 10.1093/jat/21.5.388. [DOI] [PubMed] [Google Scholar]
- 38.Li AP, Reith MK, Rasmussen A, et al. Primary human hepatocytes as a tool for the evaluation of structure- activity relationship in cytochrome P450 induction potential of xenobiotics: evaluation of rifampin, rifapentine and rifabutin. Chem Biol Interact. 1997;107:17–30. doi: 10.1016/s0009-2797(97)00071-9. [DOI] [PubMed] [Google Scholar]
- 39.Lu C, Li AP. Species comparison in P450 induction: effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague-Dawley rat, minipig, and beagle dog. Chem Biol Interact. 2001;134:271–281. doi: 10.1016/s0009-2797(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 40.Ruegg CE, Silber PM, Mughal RA, et al. Cytochrome-P450 induction and conjugated metabolism in primary human hepatocytes after cryopreservation. In Vitro Toxicol. 1997;10:217–222. [Google Scholar]
- 41.von Moltke LL, Greenblatt DJ, Harmatz JS, et al. Triazolam biotransformation by human liver microsomes in vitro: effects of metabolic inhibitors, and clinical confirmation of a predicted interaction with ketoconazole. J Pharmacol Exp Ther. 1996;276:370–379. [PubMed] [Google Scholar]
- 42.von Moltke LL, Greenblatt DJ, Duan SX, Harmatz JS, Shader RI. Inhibition of triazolam hydroxylation by ketoconazole, itraconazole, hydroxyitraconazole and fluconazole in vitro. Pharm Pharmacol Comm. 1998;4:443–445. [Google Scholar]
- 43.Warrington JS, Poku JW, von Moltke LL, Shader RI, Harmatz JS, Greenblatt DJ. The effects of age on in vitro midazolam biotransformation in male CD-1 mouse liver microsomes. J Pharmacol Exp Ther. 2000;292:1024–1031. [PubMed] [Google Scholar]
- 44.Perloff MD, von Moltke LL, Court MH, Kotegawa T, Shader RI, Greenblatt DJ. Midazolam and triazolam biotransformation in mouse and human liver microsomes: relative contribution of CYP3A and CYP2C isoforms. J Pharmacol Exp Ther. 2000;292:618–628. [PubMed] [Google Scholar]
- 45.Venkatakrishnan K, von Moltke LL, Court MH, Harmatz JS, Crespi CL, Greenblatt DJ. Comparison between cytochrome P450 (CYP) content and relative activity approaches to scaling from cDNA-expressed CYPs to human liver microsomes: ratios of accessory proteins as sources of discrepancies between the approaches. Drug Metab Dispos. 2000;28:1493–1504. [PubMed] [Google Scholar]
- 46.Greenblatt DJ, Divoll M, Harmatz JS, MacLaughlin DS, Shader RI. Kinetics and clinical effects of flurazepam in young and elderly noninsomniacs. Clin Pharmacol Ther. 1981;30:475–486. doi: 10.1038/clpt.1981.191. [DOI] [PubMed] [Google Scholar]
- 47.Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262:3303–3307. [PubMed] [Google Scholar]
- 48.Greenblatt DJ, Harmatz JS, von Moltke LL, et al. Comparative kinetics and response to the benzodiazepine agonists triazolam and zolpidem: evaluation of sex-dependent differences. J Pharmacol Exp Ther. 2000;293:435–443. [PubMed] [Google Scholar]
- 49.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts male aging study. J Clin Endocrinol Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 50.Nahoul K, Roger M. Age-related decline of plasma bioavailable testosterone in adult men. J Steroid Biochem. 1990;35:293–299. doi: 10.1016/0022-4731(90)90287-3. [DOI] [PubMed] [Google Scholar]
- 51.Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 52.Denti L, Pasolini G, Sanfelici L, et al. Aging-related decline of gonadal function in healthy men: correlation with body composition and lipoproteins. J Am Geriatr Soc. 2000;48:51–58. doi: 10.1111/j.1532-5415.2000.tb03028.x. [DOI] [PubMed] [Google Scholar]
- 53.Jürgens G, Lange KHW, Reuther LØ, Rasmussen BB, Brøsen K, Christensen HR. Effect of growth hormone on hepatic cytochrome P450 activity in healthy elderly men. Clin Pharmacol Ther. 2002;71:162–168. doi: 10.1067/mcp.2002.121373. [DOI] [PubMed] [Google Scholar]
- 54.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Zolpidem use and hip fractures in older people. J Am Geriatr Soc. 2001;49:1685–1690. doi: 10.1111/j.1532-5415.2001.49280.x. [DOI] [PubMed] [Google Scholar]