Abstract
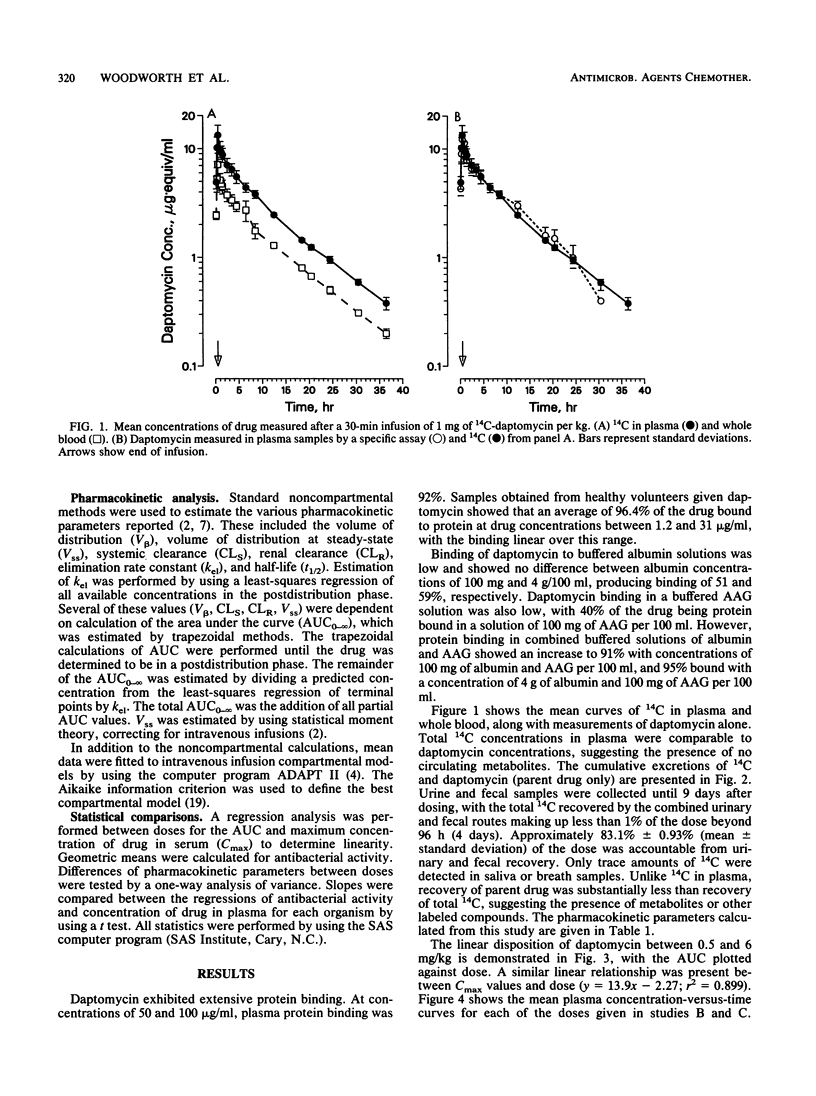
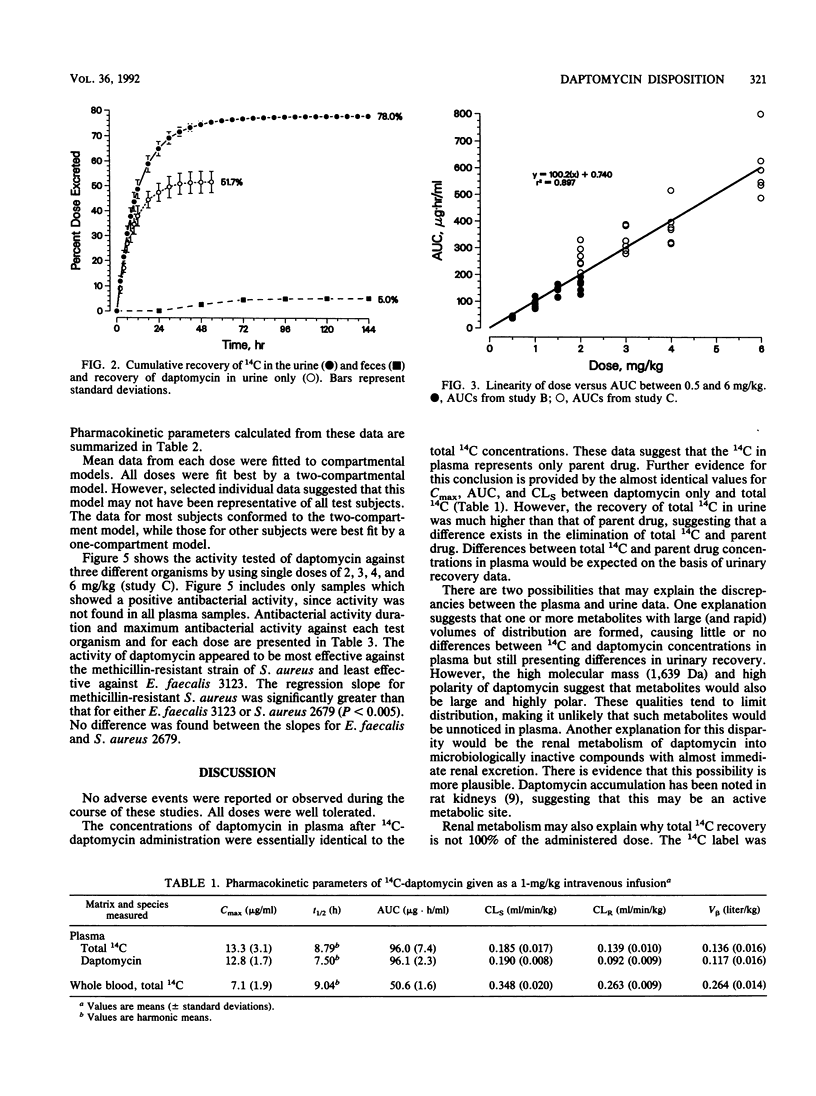
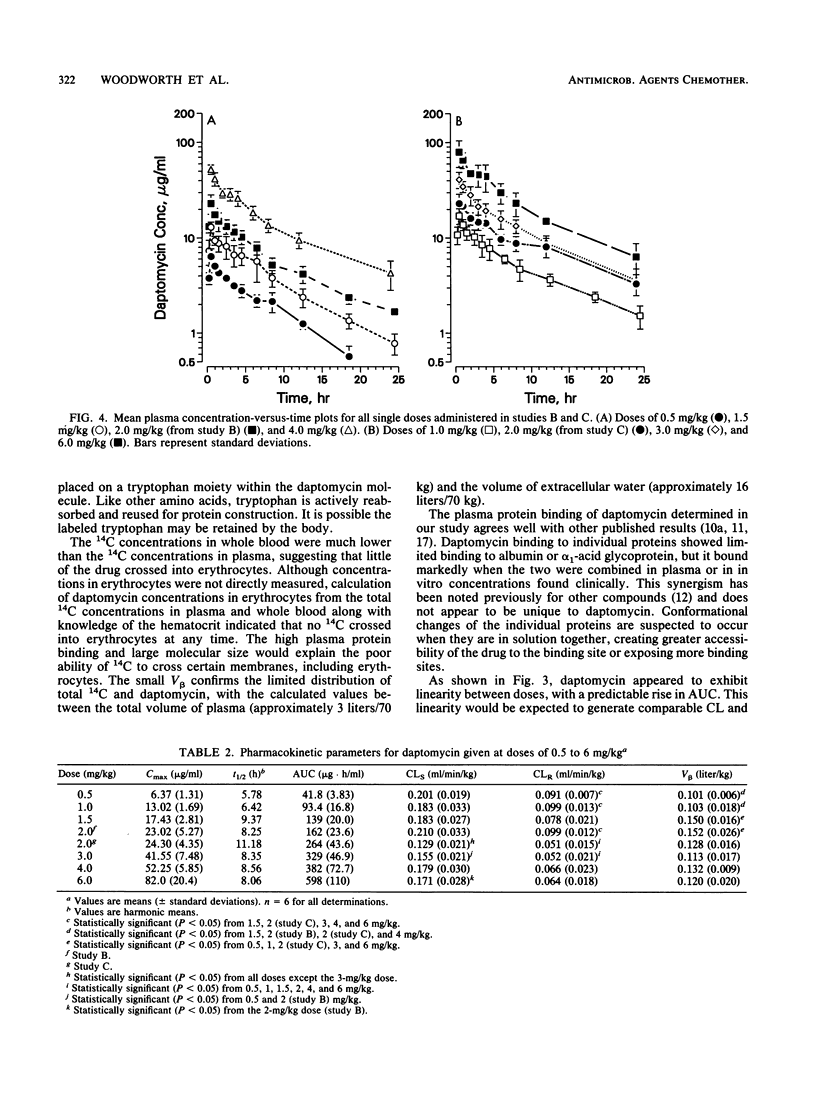
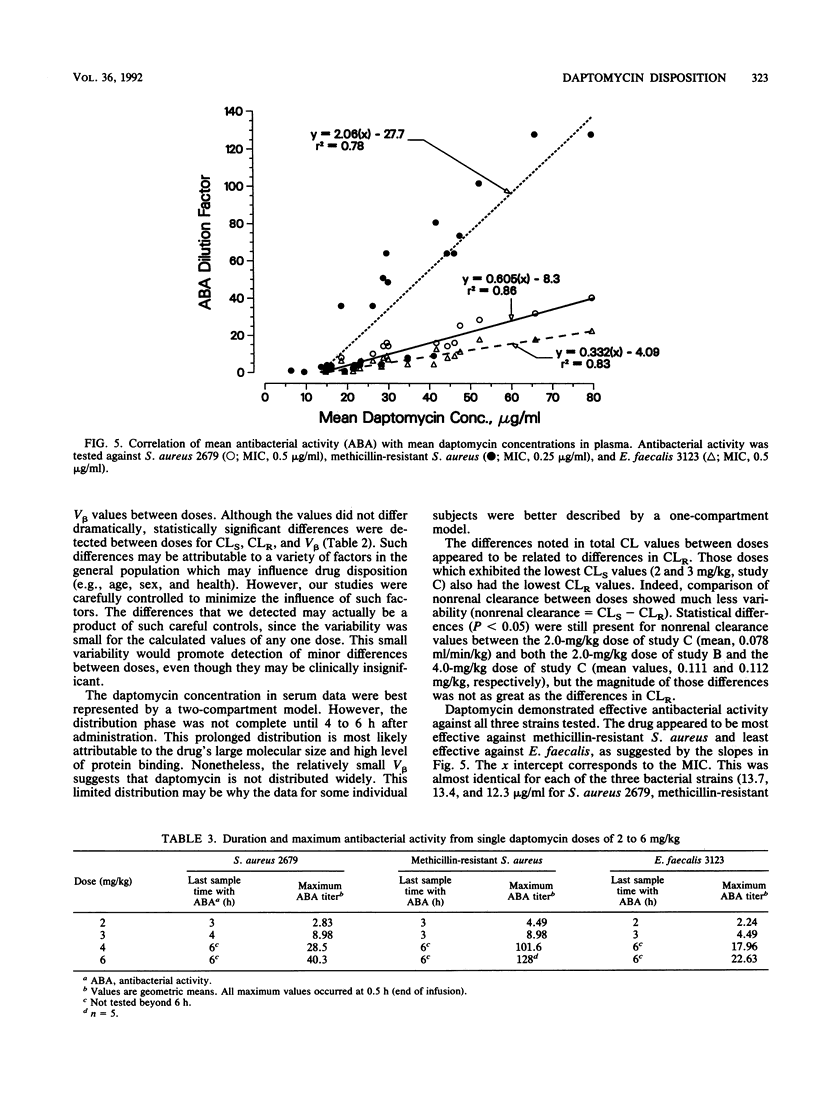
Three separate single-dose studies were performed to define the disposition and pharmacokinetics of daptomycin in healthy volunteers. Daptomycin was administered as a single 14C-labeled dose (1.0 mg/kg of body weight) and as single doses between 0.5 and 6.0 mg/kg. All doses were intravenous. Antibacterial activity was determined from doses of 2.0, 3.0, 4.0, and 6.0 mg/kg against two strains of Staphylococcus aureus (one methicillin resistant) and one Enterococcus strain. After administration of 14C-labeled daptomycin, recovery of 14C in urine and feces accounted for 83% of the administered dose, with the greatest fraction (78%) appearing in the urine. Specific analysis for daptomycin in both urine and plasma indicated that metabolic products were present in urine, but total 14C in plasma consisted of daptomycin only. Doses between 0.5 and 6 mg/kg were linear, with a limited total body clearance (0.13 to 0.21 ml/min/kg) and a small volume of distribution (0.10 to 0.15 liter/kg). The small volume of distribution may be a factor of the high plasma protein binding (90 to 95%). Renal clearance made up 34 to 54% of total body clearance. Daptomycin demonstrated in vivo antibacterial activity against all three test strains, with the greatest activity observed against methicillin-resistant S. aureus. The predicted MIC for all three strains was approximately 13 micrograms/ml, corresponding to total (bound plus unbound) drug. On the basis of the drug's pharmacokinetics and antibacterial activity, doses of 4 to 6 mg/kg/day, possibly in divided doses, are predicted to be effective.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E., Hobbs J. N., Alborn W. E., Jr Inhibition of peptidoglycan biosynthesis in gram-positive bacteria by LY146032. Antimicrob Agents Chemother. 1987 Jul;31(7):1093–1099. doi: 10.1128/aac.31.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet L. Z., Galeazzi R. L. Noncompartmental determination of the steady-state volume of distribution. J Pharm Sci. 1979 Aug;68(8):1071–1074. doi: 10.1002/jps.2600680845. [DOI] [PubMed] [Google Scholar]
- Flandrois J. P., Fardel G., Carret G. Early stages of in vitro killing curve of LY146032 and vancomycin for Staphylococcus aureus. Antimicrob Agents Chemother. 1988 Apr;32(4):454–457. doi: 10.1128/aac.32.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison M. W., Vance-Bryan K., Larson T. A., Toscano J. P., Rotschafer J. C. Assessment of effects of protein binding on daptomycin and vancomycin killing of Staphylococcus aureus by using an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1990 Oct;34(10):1925–1931. doi: 10.1128/aac.34.10.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Antimicrobial activity and spectrum of LY146032, a lipopeptide antibiotic, including susceptibility testing recommendations. Antimicrob Agents Chemother. 1987 Apr;31(4):625–629. doi: 10.1128/aac.31.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft B., de Wit C., Krech R., Marre R., Schulz E., Sack K. Experimental studies on nephrotoxicity and pharmacokinetics of LY 146032 (daptomycin) in rats. J Antimicrob Chemother. 1990 Apr;25(4):635–643. doi: 10.1093/jac/25.4.635. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Weber M., Duval J., Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989 Jan;33(1):10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. L., Sachdeva M., Chambers H. F. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob Agents Chemother. 1991 Dec;35(12):2505–2508. doi: 10.1128/aac.35.12.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens S. M., Mayersohn M., Woodworth J. R. Phencyclidine blood protein binding: influence of protein, pH and species. J Pharmacol Exp Ther. 1983 Sep;226(3):656–660. [PubMed] [Google Scholar]
- Pryka R. D., Novak R. M., Wagner D. K., Rodvold K. A. Clinical pharmacokinetics of daptomycin. DICP. 1990 Mar;24(3):255–256. doi: 10.1177/106002809002400309. [DOI] [PubMed] [Google Scholar]
- Reller L. B., Stratton C. W. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977 Aug;136(2):196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- Stratton C. W., Liu C., Ratner H. B., Weeks L. S. Bactericidal activity of deptomycin (LY146032) compared with those of ciprofloxacin, vancomycin, and ampicillin against enterococci as determined by kill-kinetic studies. Antimicrob Agents Chemother. 1987 Jul;31(7):1014–1016. doi: 10.1128/aac.31.7.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W., Reller L. B. Serum dilution test for bactericidal activity. I. Selection of a physiologic diluent. J Infect Dis. 1977 Aug;136(2):187–195. doi: 10.1093/infdis/136.2.187. [DOI] [PubMed] [Google Scholar]
- Stratton C. W., Weeks L. S. Effect of human serum on the bactericidal activity of daptomycin and vancomycin against staphylococcal and enterococcal isolates as determined by time-kill kinetic studies. Diagn Microbiol Infect Dis. 1990 May-Jun;13(3):245–252. doi: 10.1016/0732-8893(90)90067-6. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P. Ex vivo study of serum bactericidal titers and killing rates of daptomycin (LY146032) combined or not combined with amikacin compared with those of vancomycin. Antimicrob Agents Chemother. 1989 Oct;33(10):1783–1790. doi: 10.1128/aac.33.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]



