Abstract
Helicobacter pylori is the principal cause of peptic ulcer disease and an important risk factor for the development of gastric cancer. The efficacy of 1 week triple therapies, which often have eradication rates of > 90%, is undermined by poor patient compliance and bacterial antimicrobial resistance. The development of new anti-H. pylori therapies presents enormous challenges to clinical pharmacologists, not only in the identification of novel targets, but also in ensuring adequate drug delivery to the unique gastric mucus niche of H. pylori. Animal models of H. pylori infection have been developed but their clinical validity has yet to be established. Vaccination, to prevent or treat infection, has been demonstrated in animal models, but human studies have not been so encouraging.
Keywords: drug development, drug treatment, Helicobacter pylori
Introduction
Helicobacter pylori is a small, curved highly motile gram negative bacillus which only infects the mucus layer of the human stomach [1, 2]. Since the discovery in 1984 that this bacteria was associated with gastritis and peptic ulcer, it is recognized as being highly prevalent but this varies with age and socio-economic status [3, 4]. Infection is mainly acquired in childhood and is usually asymptomatic [5]. However in about 15–20% of subjects long-term infection can lead to peptic ulcer or gastric cancer [6, 7]. The outcome of infection depends mainly on the severity and topography of histological gastritis: infection in infancy leads to pan-gastritis, whilst later acquisition leads to an antral predominant gastritis. With the latter an undamaged gastric corpus secretes a high acid load, which on reaching the duodenum causes duodenal gastric metaplasia. This is then colonized by H. pylori leading to duodenitis and duodenal ulcer. In contrast, pangastritis, with an inflamed corpus, is associated with the loss of acid secretion which leads to atrophic gastritis and an increased risk of gastric ulcer or cancer [8, 9]. The interaction between specific microbial virulence factors, such as the cag pathogenicity island [10] which encodes for a Type II secretory system, and host genetic factors [11, 12], such as pro-inflammatory genotypes (e.g. isoforms of interleukin–1β, interleukin–1β receptor antagonist), are the main determinants of the pattern and severity of gastritis [13–15].
Current status of drug treatment: who to treat
Peptic ulcer disease (PUD)
Eradication of H. pylori in patients with duodenal or gastric ulcer disease cures the disease and prevents relapse [16–20]. Since 1994 the National Institutes of Health and other national specialist organizations have recommended treatment for all infected patients with ulcers [6]. Consequently the prevalence of H. pylori associated PUD appears to be falling (at least in developed countries), which, since PUD is the main indication for current licensed treatments, may impair the recruitment of patients with PUD into Phase II clinical trials. Despite considerable research efforts, it remains unclear whether H. pylori eradication should also be undertaken in infected patients found to have PUD whilst taking nonsteroidal anti-inflammatory drugs (NSAIDs) or aspirin [21–24]. However NSAIDs and aspirin are widely and increasingly being prescribed, and are also the commonest cause of upper gastrointestinal bleeding (often from ulcers). Thus if eradicating coexisting H. pylori was conclusively shown to reduce the risk of bleeding ulcers in patients taking NSAIDs, a sizeable proportion of the adult population would need eradication therapy.
Nonulcer dyspepsia (NUD)
Attempts to study the role of H. pylori in NUD have been plagued by the clinical heterogeneity of patients' symptoms, patterns of specialist referral and the large placebo response [25, 26]. Previously many studies were poorly designed with inadequate blinding of patients and investigators, small sample size and lack of appropriate control groups. However several recent meta-analyses have suggested that there is a small benefit, with a numbers needed to treat (NNT) of 15, but which may be due to eradication of infection in patients with an underlying ulcer diathesis [27–29].
Patients with MALTOMA
The immune response to H. pylori can become deregulated and may rarely lead to the development of MALTOMA, a type of low grade B cell lymphoma. However eradication of H. pylori may reverse the changes with complete resolution of the lymphocytic infiltration [30, 31].
Patients at risk of gastric cancer
The IARC have classified H. pylori as a Type 1 carcinogen [7]. Cure of histological gastritis, the immunological- inflammatory response to H. pylori, only occurs following successful eradication.
However, H. pylori is not the absolute or only cause of gastric cancer, which makes it essential to know the size of the attributable risk (or benefit gained), from eradication, which will vary in different populations. In addition whether gastric atrophy is a valid surrogate end point and able to resolve after eradication of H. pylori [32, 33], together with the age at which eradication is undertaken and the rate of re-infection after eradication are all key issues for any interventional study. Large multicentre double-blind controlled trials (DBRCTs) in developed countries, where the prevalence of H. pylori infection is low and the incidence of gastric cancer is falling, are very expensive due to the size of the study and length of follow up required [34, 35]. In the one study reported to date, the effects of eradication of H. pylori were similar in size to the dietary interventions and no additive effect was seen when interventions were combined [36].
In developed countries the threshold for treating H. pylori in primary care is falling, thus diminishing the population remaining infected who may then no longer be representative of the general population at risk. By contrast in developing countries (in which cancer rates are likely to be high in years to come) high levels of antimicrobial resistance and significant rates of re-infection, following initially successful eradication, will present additional logistical problems for clinical trials.
Current status of drug treatment: what to use
Previously, treatment of H. pylori required bismuth and two antibiotics for 1 month or 2 weeks of high dose proton pump inhibitors (PPIs) and a single antibiotic [37–41]. These regimes were of variable efficacy and often poorly tolerated due to numerous side-effects and poor compliance [42–45]. In 1993 Bazzoli reported a 97%H. pylori eradication rate with a low dose, 1-week regimen of omeprazole, clarithromycin and tinidazole [46]. Numerous other studies have now confirmed the high efficacy of these 1-week regimens that has revolutionized the eradication and management of H. pylori infection [47–54]. The rationale behind these regimens is based on lowering the antimicrobials' mean inhibitory concentration (MIC) by increasing intragastric pH, improving patient compliance by only twice daily dosing for 1 week and minimizing side-effects by using low doses of antimicrobials.
Clarithromycin and nitro-imidazoles are used because of their low MIC for H. pylori, and the acid stability of clarithromycin. With nitro-imidazoles as the second antimicrobial, eradication rates are often > 90% but have the problems of nitro-imidazole resistance, taste disturbance and need for patients to abstain from alcohol during treatment.
Clarithromycin can also be combined with amoxycillin [55, 56], which has none of the drawbacks associated with nitro-imidazoles, and thus, with a PPI, may potentially be a better regimen. However, the dose of clarithromycin used is higher (500 mg twice daily) than when combined with a nitro-imidazole and fewer studies have consistently reported eradication rates > 90%. A PPI with amoxycillin and metronidazole is an alternative regime, but in the only comparative multicentre, randomized study this regimen had the lowest eradication rate [50]. It is also important to note that, because of the high eradication rates achieved by all these regimes, these studies were only powered to demonstrate equivalence (rather than superiority) between the different treatment arms.
In vitro PPIs inhibit the growth of H. pylori, inhibit urease and ATP-ase activity and have MICs which compare favourably with many other antimicrobials [57, 58], However the clinical significance of these observations is obscure since data from animal studies suggest that urease inhibition does not enhance antimicrobial efficacy [59]. The major effect of PPIs on the treatment of H. pylori is due to the beneficial effect of higher pH on the MIC of clarithromycin or amoxycillin (the MIC of nitro-imidazoles does not vary with pH).
Problems with current treatment regimens for H. pylori
Despite the enormous potential market for new and more effective antimicrobials active against H. pylori there is as yet no ‘magic bullet’. This is in part due to problems associated with the drug delivery (vide infra), licensed indications for treatment and the high efficacy of current regimes. Whilst the 1-week low dose PPI based regimes are very effective current treatment regimes have three major drawbacks.
Patient compliance
Even with twice daily dosing, the current regimes require patients to take more than 42 tablets for 7 days, and blister pack dispensing for the three components is, with one exception, not available. The need to avoid alcohol whilst taking nitro-imidazoles is another factor effecting patient compliance [42, 60].
Side-effects
The current regimes are associated with side-effects, especially taste disturbance, diarrhoea and skin rashes. Whilst these are rarely serious or sufficient to impair compliance, they can be troublesome for patients and their physicians [43, 45].
Antimicrobial resistance
The efficacy of the all regimes is undermined by the development of antimicrobial resistance by H. pylori [44, 61, 62]. This is a particular problem given the ability of horizontal transfer of DNA between strains of H. pylori and the widespread use of both nitroimidazoles and macrolides for treating other infections [63, 64]. To date, few studies have formally assessed antimicrobial susceptibility pre- and post-treatment, and with eradication rates of > 90%, the number of treatment failures is small and the data limited.
Methods used in evaluation and development of new drugs for H. pylori
Laboratory based methods
In vitro H. pylori is sensitive to a wide range of antimicrobials and this can be readily assessed using standard techniques such as determining MIC and minimum bacteriocidal concentration (MBCs). For rapid high throughput screening of new compounds, new methods (e.g. flow cytometry) for assessing antibacterial activity is preferred because large numbers of compounds can be assessed and compared simultaneously without the problems of overgrowth, contamination or suboptimal inoculates. The major hurdle is in ensuring adequate drug delivery for both the animal model and phase 1 studies.
Antimicrobial delivery to H. pylori in vivo
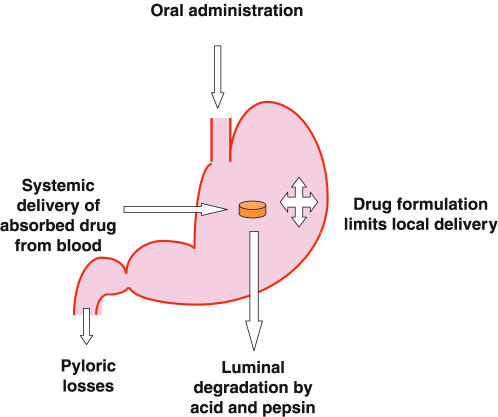
H. pylori inhabits a unique niche within its host. The majority of the burden of infection lies between the gastric epithelial surface and the secreted mucus layer, but organisms can be found free in the gastric juice. This environment presents numerous problems for successful antimicrobial delivery and activity, as to be effective against H. pylori it must reach and be active in all compartments of this niche [65]. In light of this, it is unsurprising that many antimicrobials (e.g. penicillins) which seem highly effective against H. pylori in culture have little or no activity in vivo [66]. Understanding of how antimicrobials are delivered to H. pylori is therefore critical for the development and selection of new drugs for treating this infection (Figure 1).
Figure 1.
Factors influencing intragastric drug delivery.
Antimicrobials can reach H. pylori by two routes, from the lumen of the stomach across the gastric mucus layer (luminal delivery) or after systemic absorption and across the gastric mucosa from the blood (systemic delivery). Some antimicrobials appear to have predominantly luminal delivery such as bismuth preparations and penicillins, but most probably have a combined delivery.
Luminal delivery
The three main barriers to luminal delivery are gastric emptying, gastric acidity and the epithelial mucus layer.
Historically most antimicrobials were developed with the aim of maximizing systemic delivery and therefore were formulated to minimize gastric exposure and/or subsequent degradation. Tablet formulations deliver little drug to the gastric body, which can therefore be a ‘sanctuary site’ during antimicrobial treatment, allowing recrudescence of infection after treatment [67]. Suspensions achieve a more widespread delivery although may have a shorter intragastric residence time and are more prone to degradation [68]. Food and proton pump inhibitors delay gastric emptying and may increase luminal delivery [69] (Figure 1).
Proton pump inhibitors also reduce degradation of acid-labile antibiotics by increasing pH and reducing the effect of pepsin. This effect is modest for most antibiotics but is probably important for macrolides. Proton pump inhibitors substantially reduce gastric volume which increases antimicrobial concentration [70].
The mucus layer prevents diffusion of many antibiotics to H. pylori from the gastric lumen although formulation can dramatically influence diffusion of drug [71]. Proton pump inhibitors probably damage (either directly or indirectly by increasing pH) this layer promoting diffusion to the juxta-epithelial space [72]. Mucolytics reduce the mucus layer thickness and alter the physio-chemical properties of mucus, but also increase gastric inflammation. This increases gastric delivery of some antimicrobials, but may reduce systemic delivery of others.
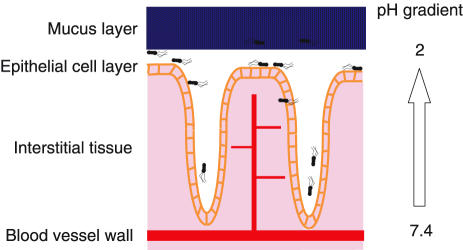
Systemic delivery
Many drugs have been detected in gastric juice following intravenous infusion or intramuscular injection (Figure 2).
Figure 2.
Barriers to drug delivery across gastric mucosa.
The physicochemical properties of antimicrobials are the main factors influencing systemic drug delivery. Most appear to diffuse into gastric juice but some are actively secreted. Penicillins can be detected in low concentrations compared to plasma following intravenous dosing [73]. These agents diffuse poorly across the gastric epithelium and diffusion is limited by protein binding and mucosal oedema [74]. Nitroimidazoles diffuse readily across the gastric mucosa, achieving plasma concentrations rapidly in the luminal compartment. Metronidazole, being a weak base of pKa 2.5, exhibits ionic trapping at low intragastric pH and may achieve higher concentrations in gastric juice than plasma [70, 75]. Macrolides also achieve higher intragastric levels than plasma, but there is plentiful evidence that this is due to active secretion rather than plain diffusion [70, 76, 77]. This active secretion may explain why clarithromycin is the most effective antimicrobial against H. pylori in vivo.
Systemic drug delivery is, unsurprisingly, influenced by drug metabolism. Concurrent use of other drugs may improve systemic delivery of some antimicrobials. PPIs interfere with their own and other drugs’ metabolisms and systemic delivery of clarithromycin is increased when omeprazole is used via this mechanism [77].
Effect of H. pylori infection on intragastric drug disposition
Much of the above data has been obtained from studies in animals not infected with Helicobacters or healthy human volunteers. There is little data on the effect of H. pylori on drug delivery to the intragastric compartment, but some inferences can be drawn.
Gastric inflammation increases systemic delivery of amoxycillin and metronidazole in rats [78], but there is no apparent difference in systemic delivery of these antimicrobials or of clarithromycin in H. pylori infected patients before and after treatment or compared with uninfected controls [79]. H. pylori increases gastric blood flow and mucosal permeability to some drugs, which will result in increased systemic delivery. However, inflammation also results in mucosal oedema (increasing the diffusion barrier for some drugs) and destruction of the mucus layer and thus concentrations of mucus bound drugs such as macrolides [80].
H. pylori can have varying effects on gastric acid secretion. Most duodenal ulcer patients have increased acid secretion, whilst those with pangastritis tend to have reduced acid secretion. It is unlikely that increased acid secretion has much effect on the intragastric pH and drug stability, but reduced acid secretion will lead to increased drug stability [81]. Some anti-Helicobacter drugs also intrinsically delay gastric emptying (e.g. clarithromycin), which increases gastric residency time [70].
Methods for investigating drug delivery to H. pylori
If we accept that drug delivery is crucial to the success of antimicrobial regimens against H. pylori, we require effective ways of studying gastric drug disposition and several have been developed over the past decade. Excluding in vitro drug stability and mucus permeability studies, two broad classes of studies have been used to assess intragastric drug delivery, namely animal and human studies. Neither is ideal and there are many methodological limitations to these studies.
Animal studies
The first studies to address this area used guinea pigs [82], which were sacrificed, at intervals following intramuscular injection of drugs. The concentrations of drugs in plasma and gastric tissue were then compared. Although limited (because of the small amounts of tissue available and low concentrations of drug present), these studies indicated which classes of antibiotics achieved high intragastric concentrations.
An important refinement of these studies was the use of 14C-radiolabelled drugs, which can be measured at much lower concentrations and at the microscopic level. One group has studied the distribution of oral and intravenous 14C-radiolabelled amoxycillin and clarithromycin in rats [83, 84]. These studies have assessed the effects of acid-suppression and other antibiotics on drug distribution within different regions of the rat stomach and also the cellular compartments of the mucosa. The effects of H. pylori infection or inflammation have not been assessed, although this model would be suitable for such studies.
Our own group has used an ex vivo Ussing chamber model [74] and an in vivo isolated stomach model [79] to assess the factors influencing drug transfer across gastric mucosa. The former allows careful control of pH and study of many different agents on drug transfer. However, there are numerous limitations and this model is probably only useful for screening of large numbers of drugs. The latter model is far more relevant to the in vivo situation in man, and allows the effect of H. pylori infection to be assessed.
Human studies
There have been some studies of the distribution of radiolabelled drugs in humans, and these have given insight into the affects of H. pylori infection and acid-suppression on drug disposition within the gastric lumen [69]. They do not, though, allow measurement of concentration within tissue. To achieve this, more invasive studies are needed.
Nasogastric aspiration of gastric juice allows the transfer of an intravenously dosed drug across gastric mucosa to be studied [70, 75]. This allows formal pharmacokinetic modelling and thus controlled comparison of drugs and the effects of either H. pylori infection or acid suppression to be made. Several new pharmacokinetic measures have been derived by these methods including gastric clearance [akin to renal clearance] and gastric transfer fraction. The limitations of this type of study are that regional differences within the stomach cannot be assessed and, perhaps more importantly, orally dosed drugs cannot be studied.
Endoscopic biopsy studies, on the other hand, do allow regional differences to be assessed and also allow orally dosed drugs to be studied. Mucus concentrations as well as gastric mucosal concentrations can be made. Multiple endoscopies, however, are discouraging for volunteers and the large cellular component of biopsies may render some results meaningless. Both endoscopic and nasogastric studies are limited by the limits of detection for drug assays.
Methods used in assessing drug efficacy: clinical studies
Non-invasive tests for detecting H. pylori infection
H. pylori can be detected noninvasively by the nonradioactive 13C-urea breath test (13C-UBT) which has been used extensively in the development of new drug regimes for H. pylori [85–88]. It exploits the abundant urease activity of H. pylori, which rapidly hydrolyses an ingested solution of 13C-urea, to release 13CO2, which is then absorbed and excreted as 13CO2 in the expired breath. The 13C-UBT can be used to assess several important aspects of infection during the early stages of clinical development of new chemical entities active against H. pylori.
Extent of H. pylori infection
There are no ‘gold standards’ for measuring the extent of H. pylori infection, which makes it difficult to prove that the 13C-UBT is a quantitative test of infection. At endoscopy multiple biopsies from throughout the stomach can be taken, but histology may detect nonviable organisms and culture may not be successful or become sufficiently well established to quantify bacterial populations by serial dilutions. Nonetheless several groups have demonstrated a relationship between the extent of 13CO2 excretion and culture or histology [89].
In addition there is no correlation between the specific urease activity of isolated strains in vitro with hydrolysis of 13C-urea in vivo [13C UBT], implying that differences in UBT values are due to differences in the numbers of infecting organisms and thus the extent of colonization [90]. Intra-subject quantitative comparisons are possible and semiquantitative assessments made if necessary.
Suppression
If the 13C-UBT is used to monitor the load of H. pylori, suppression is defined as a >50% fall in the excretion of 13CO2. This not only allows rapid and easy assessments of differences between similar agents, which is of particular value for phase 1 or volunteer studies, but also allows more subtle effects on H. pylori to be monitored [91]. Thus the effect of lowering intragastric pH on H. pylori by omeprazole was first observed with the 13C-UBT that, unlike the antral biopsy, accurately demonstrated suppression, but not clearance of infection [92].
Clearance and recurrence
Clearance of H. pylori is defined as a negative 13C-UBT immediately after finishing treatment [93]. Differences in clearance rates will thus reflect differences in eradication rates or rates of recurrence. When assessing clearance with the 13C-UBT it is important to record the time interval between the last dose and the test. Thus even after 1 month of colloidal bismuth or triple therapy some patients, despite suppression of H. pylori during treatment, will have a positive breath test less than 12 h after the last dose [94–96].
Serial 13C-UBTs at weekly or twice weekly intervals will show the rate of recurrence of H. pylori following clearance of infection at the end of treatment. Drugs with the slowest recurrence rates will be the most effective in eradicating H. pylori.
Eradication
The 13C-UBT is the best method of following eradication of bacteria in patients [97, 98]. Because eradication of H. pylori is associated with resolution of histological gastritis and prevention of relapse of DU, the 13C-UBT can be used as the sole method of follow-up. Long-term follow up studies of eradication documented by multiple endoscopic and UBT assessments of H. pylori status over a 12-month period showed almost complete concordance, with < 1% of patients having discordant results [99].
H. pylori can also be detected non-invasively by ELISA serology or by stool antigen tests [100, 101]. The role of serology is mainly in the identification of infected individuals who may volunteer for Phase 1 studies, or in selecting infected dyspeptic patients to facilitate the recruitment to Phase II studies. There is some variation in antibody responses to H. pylori and local validation is recommended. False negative antibody tests have been reported in the elderly and titres fall only slowly after successful eradication.
Stool antigen tests detect the presence of H. pylori antigens in faeces using a simple sandwich ELISA [102]. Studies have reported sensitivities and specificities that compare favourably with those seen with the 13C-UBT (> 90%) [103]. They may be also used as an adjunct to the 13C-UBT in assessing suppression or clearance in human volunteers and have been used in animal models.
Invasive tests for detecting H. pylori infection
H. pylori can be readily detected at endoscopy by histology, culture or urease tests, but all biopsy-based methods are liable to sampling error, because infection is patchy [104]. Up to 14% of patients with H. pylori will not have antral infection but will have H. pylori elsewhere in the stomach, especially if there is gastric atrophy, intestinal metaplasia or bile reflux. In addition after partially effective eradication therapy low levels of recurrent infection can be easily missed by biopsy leading to overestimates of the efficacy of eradication therapy. For these reasons consensus guidelines recommend taking multiple biopsies from the antrum and corpus for both histology and one other method (either culture or urease testing) [97, 98].
Although H. pylori may be recognized on H&E stained sections alone, special supplementary stains (e.g. Giemsa, Gimenez) are always needed to detect low levels of infection and show the characteristic morphology of H. pylori [104]. Histology also provides an historical record: sections (or additional sections) can always be (re)-examined and atrophy or intestinal metaplasia assessed. Additional biopsies from other parts of the stomach can be retained in formalin and only processed if antral histology is inconclusive. Microbiological isolation is the theoretical ‘gold standard’ for identifying any bacterial infection, however, for H. pylori culture can be unreliable, with risks of overgrowth or contamination making it the least sensitive method of detection and the least readily available of endoscopic methods. Culture and antibiotic sensitivity testing is also necessary for any clinical trial because of the prevalence of multiple antibiotic resistant strains. Biopsy urease tests are quick and easy tests, which indicate only the presence or absence of H. pylori, but often have a higher sensitivity than other biopsy tests because the entire biopsy specimen is placed in the media thereby avoiding the additional sampling or processing error associated with histology or culture. They also often allow rapid confirmation of infection (within 30–60 min) at the time of endoscopy thereby allowing patients to be invited to enter into clinical studies, although for maximum sensitivity the test should be read 24 h later.
Challenges in designing Phase I-III studies
The development of new drugs for treating H. pylori infection poses many unique challenges to the clinical investigator. For Phase 1 studies the first hurdle is recruiting infected subjects. Since the prevalence of infection in young people from developed countries is already low (∼15–25%) volunteers will require an initial screening test to document the presence of infection. Whilst serology is the easiest and cheapest (and can be done on stored serum banks), there may be false positives; so further confirmatory testing (by 13C-UBT) is still required. If noninvasive tests of new drugs (under clinical trial exemption (CTX)) suggest high therapeutic efficacy, more extensive pharmacokinetic endoscopic studies assessing intragastric distribution will be needed. An additional challenge for Phase 1 studies is determining what to do with subjects who remain infected at the end of the study. Currently it is still unclear if the risks of side-effects from conventional eradication regimes greater than the risks (or benefits) of remaining infected.
For Phase II studies the principal challenges are in the design and organization of the study. Identifying infected patients prior to the trial will depend upon local gastroenterological practice in the management of dyspepsia. Clinicians who adopt a ‘test and scope’ strategy provide the ideal setting for recruitment, since patients will already be known to be infected prior to endoscopy as a result of routine local clinical practice. If test and treat is local practice it may be necessary to conduct the initial recruitment of subjects in primary care.
For most endoscopic studies a two-stage consent process will be required. Patients may need to consent to a screening test for H. pylori prior to endoscopy (by serology or 13C-UBT) or for an initial consent to cover the taking of additional biopsies for trial purposes, followed by a full consent for those patients subsequently found to be infected. The latter process has many advantages and can be readily undertaken if carefully explained to patients.
The other challenge in Phase II studies relates to microbiological assessments of infection, not only to document infection, but also to monitor pre- and post-treatment resistance. These assessments need to be done in a centralized facility. However, successful culture of H. pylori requires rapid processing of samples, which should therefore be undertaken locally [98]. Alternatively biopsy samples for culture need to be stored and transported on dry ice to the central facility, which is costly and logistically cumbersome. As with phase 1 studies, careful consideration needs to be made regarding what to do to subjects who remain infected at the end of the study.
Clinical trials of new eradication therapies
Detailed guidelines on the design and methodology of clinical trials on H. pylori and peptic ulcer have been published by European Helicobacter pylori Study Group. A summary of methods to be used for assessing H. pylori status before and after treatment during clinical trials of new agents or regimes using agents to which H. pylori may develop resistance are given in Table 1[97]. The working party also recommended assessments of re-infection rates at 1 year using the 13C-UBT with repeat endoscopy and biopsy in patients with positive breath tests [98].
Table 1.
Recommended biopsy sampling protocol for assessing H. pylori status.
| Histology | Culture | Urease test | PCR | |
|---|---|---|---|---|
| Pretreatment | ||||
| Antrum | ++ | ++ | ++ | |
| Corpus | xx | |||
| Post-treatment | ||||
| Antrum | ++ | ++ | + | |
| Corpus | ++ | ++ | + | |
+ = one biopsy sample, ++ = two biopsy samples, xx = should be taken if assessing atrophy/intestinal metaplasia.
Licensing issues
Eradication of H. pylori infection alone is not usually accepted as a license indication by regulatory authorities. Previous license indications include PUD and NUD. For NUD the primary endpoint should be cure of H. pylori gastritis and symptom improvement should only be a secondary endpoint because of the difficulties associated with studies in NUD. The falling prevalence of PUD is likely to impact on the rate at which infected subjects can be recruited. In addition, with eradication rates from current regimes of ∼90% studies should be powered to show equivalence rather than superiority to current available treatments.
Bio-informatics
The recent sequencing of two bacterial genomes will help in the development of new drugs. Bio-informatic analysis allows the identification of novel targets that might be unique to H. pylori as well as suggesting novel modes of action by identifying essential genes as potential new drug targets. Automated high throughput screening is an essential part of drug development allowing rapid assessment of new compounds to identify potential lead drugs. In-silico techniques and top class medicinal chemists can optimize lead drugs prior to selection for preclinical development. However few of the major pharmaceutical companies currently have an active drug development programme for H. pylori.
New approaches: Prospect for vaccines
Several major research groups are working on developing a vaccine for H. pylori which offers a radically different approach to the management of infection [105, 106]. Protection against infection has been demonstrated in several animal models of Helicobacter infection. In addition therapeutic vaccination to treat established infection (an effect probably mediated by breaking tolerance or up-regulating normal immune responses) has also been demonstrated in animals. However human volunteer studies using enteric vaccines (either therapeutic or protective) have, so far, been disappointing. In addition studies aimed at defining the minimum infectious dose resulted in volunteers developing symptomatic gastritis even though a ‘nonpathogenic’ strain was used as the innoculum. More recently classical systemic vaccination using three key H. pylori antigens (CagA, VacA and NAP) has been undertaken with some encouraging initial results [107, 108].
Conclusions
The discovery of H. pylori and the development of effective treatment for eradicating infection has radically changed the management of dyspepsia and peptic ulcer disease. The efficacy of current treatment regimes may be undermined by the development of multiple antibiotic resistance in H. pylori, especially given the ability of H. pylori to transfer DNA between strains. Although the prevalence of infection is falling in developed countries, the burden of infection in developing countries remains high and, without biomedical intervention, will only fall with an improvement in public health. The clinical pharmacological problems associated with the treatment and development of new anti-H. pylori drugs are complex. Biomedical interventions aimed at either treating infection, or vaccination to prevent infection as part of a national campaign, may reduce the burden of disease associated with H. pylori infection in poor countries. Whether the economics of drug or vaccine development will be sufficient to support such strategies remains to be determined.
References
- 1.Marshall BJ, Warren JR, Goodwin CS. Duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1989;i:846. doi: 10.1016/s0140-6736(89)92287-3. [DOI] [PubMed] [Google Scholar]
- 2.Jones DM, Lessels AM, Eldridge J. Campylobacter like organisms in the gastric mucosa: Culture, histological and serological studies. J Clin Pathol. 1984;37:1002–1006. doi: 10.1136/jcp.37.9.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logan RPH, Walker MM. Epidemiology and diagnosis of Helicobacter pylori infection. Br Med J. 2001;323:920–922. doi: 10.1136/bmj.323.7318.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitaker CJ, Dubiel AJ. Gaplin. Social and geographical risk factors in Helicobacter pylori infection. Epidemiol Infect. 1993;111:63. doi: 10.1017/s0950268800056685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neale KR, Logan RPH. The epidemiology and transmission of Helicobacter pylori infection in children. Aliment Pharmacol. 1995;9(Suppl 2):77–84. [PubMed] [Google Scholar]
- 6.NIH Consensus Development Panel on Helicobacter pylori in peptic ulcer disease. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. Vol. 61. Lyon: IARC; 1994. Schistosomes, liver flukes and Helicobacter pylori; pp. 177–241. [Google Scholar]
- 8.Blaser MJ. Hypothesis on the pathogenesis and natural history of H. pylori inflammation. Gastroenterol. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 9.Baron JH, Logan RPH. Infection by Helicobacter pylori is the major cause of duodenal ulcer. Proc R Coll Physicians Edin. 1994;24:21–36. [Google Scholar]
- 10.Crabtree JE, Wyatt JI, Tredosiewicz LK, et al. Interleukin-8 expression in Helicobacter pylori infected, normal and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillemin K, Salama NR, Tompkins LS, Falkow S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc Natl Acad Sci USA. 2002;99:15136–15141. doi: 10.1073/pnas.182558799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sipponen P, Kekki M, Siurala M. The Sydney System: Epidemiology and natural history of chronic gastritis. J Gastroenterol Hepatol. 1991;6:244–251. doi: 10.1111/j.1440-1746.1991.tb01472.x. [DOI] [PubMed] [Google Scholar]
- 14.El-Omar EM, Carrington M, Chow WH, et al. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99. doi: 10.1038/35083631. [DOI] [PubMed] [Google Scholar]
- 15.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of non-cardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 16.Marshall BJ, Goodwin CS, Warren JR, et al. A prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988;ii:1437–1441. doi: 10.1016/s0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- 17.Coghlan JG, Humphries H, Dooley C, et al. Campylobacter pylori and recurrence of duodenal ulcers – a 12-month follow-up study. Lancet. 1987;ii:1109–1111. doi: 10.1016/s0140-6736(87)91545-5. [DOI] [PubMed] [Google Scholar]
- 18.Rauws EAJ, Tytgat GNJ. Cure of duodenal ulcer with eradication of Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 19.Axon AR. Duodenal ulcer: the villain unmasked? Br Med J. 1991;302:919–921. doi: 10.1136/bmj.302.6782.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson WL. Helicobacter pylori and peptic ulcer disease. N Engl J Med. 1991;324:1048. doi: 10.1056/NEJM199104113241507. [DOI] [PubMed] [Google Scholar]
- 21.Graham DY. NSAIDs, Helicobacter pylori, and Pandora's Box. N Engl J Med. 2002;347:2162–2164. doi: 10.1056/NEJMe020153. [DOI] [PubMed] [Google Scholar]
- 22.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 23.Hawkey CJ, Wilson I, Naesdal J, Langstrom G, Swannell AJ, Yeomans ND. Influence of sex and Helicobacter pylori on development and healing of gastroduodenal lesions in non-steroidal anti-inflammatory drug users. Gut. 2002;51:344–350. doi: 10.1136/gut.51.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stack WA, Atherton JC, Hawkey GM, Logan RF, Hawkey CJ. Interactions between Helicobacter pylori and other risk factors for peptic ulcer bleeding. Aliment Pharmacol Ther. 2002;16:497–506. doi: 10.1046/j.1365-2036.2002.01197.x. [DOI] [PubMed] [Google Scholar]
- 25.Kemmer TP, Dominguez-Munoz JE, Klingel H, Zemmler T, Kuhn K, Malfertheiner P. The association between non-ulcer dyspepsia and Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1994;6:571–577. [Google Scholar]
- 26.Veldhuerzen van Zanten S, Malatjalian D, Tanton R, et al. The effect of eradication of Helicobacter pylori (Hp) on symptoms of no-ulcer dyspepsia (NUD): a randomised double blind placebo controlled trial. Gastroenterology. 1995;108:A250. [Google Scholar]
- 27.Moayyedi P, Deeks J, Forman D. Helicobacter pylori eradication therapy for nonulcer dyspepsia. Ann Intern Med. 2002;136:555–556. doi: 10.7326/0003-4819-136-7-200204020-00016. [DOI] [PubMed] [Google Scholar]
- 28.Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2003;1:CD002096. doi: 10.1002/14651858.CD002096. [DOI] [PubMed] [Google Scholar]
- 29.Vaira D, Miglioli M, Mule P, et al. The prevalence of peptic ulcer in Helicobacter pylori positive blood donors. Gut. 1994;35:309–312. doi: 10.1136/gut.35.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wotherspoon AC. A critical review of the effect of Helicobacter pylori eradication on gastric MALT lymphoma. Curr Gastroenterol Report. 2000;6:494–498. doi: 10.1007/s11894-000-0014-z. [DOI] [PubMed] [Google Scholar]
- 31.Isaacson PG, Diss TC, Wotherspoon AC, Barbazza R, De Boni M, Doglioni C. Long-term follow-up of gastric MALT lymphoma treated by eradication of H. pylori with antibodies. Gastroenterology. 1999;117:750–751. doi: 10.1016/s0016-5085(99)70483-x. [DOI] [PubMed] [Google Scholar]
- 32.Sakaki N, Momma K, Egawa N, Yamada Y, Kan T, Ishiwata J. Influence of Helicobacter pylori infection on the progress of gastric mucosal atrophy and the occurrence of gastric cancer. Am J Gastroenterol. 1994;89:A462. [PubMed] [Google Scholar]
- 33.Lamouliatte H, Carles B, Cayla R, Zerbib F, de Mascarel A, Megraud F. Helicobacter pylori eradication can normalise gastric mucosa. Am J Gastroenterol. 1994;89:A295. doi: 10.1097/00042737-200012070-00001. [DOI] [PubMed] [Google Scholar]
- 34.Xia HH, Wong BC, Lam SK. Chemoprevention of gastric cancer: current status. Chin Med J (Engl) 2003;116:5–10. [PubMed] [Google Scholar]
- 35.Correa P. Helicobacter pylori infection and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:238S–41S. [PubMed] [Google Scholar]
- 36.Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 37.Rauws EA, Langenberg W, Houthoff HJ, Zanen HC, Tytgat GNJ. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988;94:33–40. [PubMed] [Google Scholar]
- 38.Heatley RV. Review article. the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1992;6:291–303. doi: 10.1111/j.1365-2036.1992.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 39.Chiba N, Rao BV, Rademaker JW, Hunt RH. Meta-analysis of the efficacy of antibiotic therapy in eradicating Helicobacter pylori. Am J Gastroenterol. 1992;87:1716–1727. [PubMed] [Google Scholar]
- 40.Penston JG. Review article: Helicobacter pylori eradication – understandable caution but no excuse for inertia. Aliment Pharmacol Ther. 1994;8:369–389. doi: 10.1111/j.1365-2036.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 41.Tytgat GNJ. Review article. treatments that impact favourably upon the eradication of Helicobacter pylori and ulcer recurrence. Aliment Pharmacol Ther. 1994;8:359–368. doi: 10.1111/j.1365-2036.1994.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 42.Glupczynski Y, Burette A. Drug therapy for Helicobacter pylori infection: problems and pitfalls. Am J Gastroenterol. 1990;85:1545–1551. [PubMed] [Google Scholar]
- 43.Graham DY, Lew GM, Malaty HM, Evans DG, Evans DJ, Klein PD. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology. 1992;102:493–496. doi: 10.1016/0016-5085(92)90095-g. [DOI] [PubMed] [Google Scholar]
- 44.Rautelin H, Seppala K, Renkonen OV, Vainio U, Kosunen TU. Role of metronidazole resistance in therapy of Helicobacter pylori infections. Antimicrob Agents Chemother. 1992;36:163–166. doi: 10.1128/aac.36.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cutler AF, Schubert TT. Patient factors affecting Helicobacter pylori eradication with triple therapy. Am J Gastroenterol. 1993;88:505–509. [PubMed] [Google Scholar]
- 46.Bazzoli F, Zagari RM, Fossi S, et al. Short-term low-dose triple therapy for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1994;6:773–777. [Google Scholar]
- 47.Labenz J, Stolte M, Ruhl GH, et al. One week low dose triple therapy for cure of Helicobacter pylori infection. Eur J Gastroenterol. 1995;7:9–11. [PubMed] [Google Scholar]
- 48.Yousfi MM, El-Zimaity HMT, Al-Assi MT, Cole RA, Genta RM, Graham DY. Metronidazole, omeprazole and clarithromycin: an effective combination therapy for Helicobacter pylori infection. Aliment Pharmacol Ther. 1995;9:209–212. doi: 10.1111/j.1365-2036.1995.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 49.Adamek RJ, Szymanski C, Pfaffenbach B, Opferkuch W, Ricken D, Wegener M. Short term triple treatment of Helicobacter pylori infection with pantoprazole, clarithromycin and metronidazole. Dtsch Med Wschr. 1995;120:358–360. doi: 10.1055/s-2008-1055353. [DOI] [PubMed] [Google Scholar]
- 50.Lind T, Veldhuyzen van Zanten S, Unge P, et al. Eradication of Helicobacter pylori using 1-week triple therapies combining omeprazole with two antimicrobials: the MACH I study. Helicobacter. 1996;1:138–143. doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 51.Calabrese C, Di Febo G, Areni A, et al. Pantoprazole, azithromycin and tinidazole: short duration triple therapy for eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:1613–1617. doi: 10.1046/j.1365-2036.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- 52.Lind T, Megraud F, Unge P, et al. The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1-week triple therapies. Gastroenterology. 1999;116:248–253. doi: 10.1016/s0016-5085(99)70119-8. [DOI] [PubMed] [Google Scholar]
- 53.Bazzoli F, Zagari RM, Pozzato P, et al. Low-dose lansoprazole and clarithromycin plus metronidazole vs full-dose lansoprazole and clarithromycin plus amoxicillin for eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16:153–158. doi: 10.1046/j.1365-2036.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- 54.Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection – The Maastricht 2–2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 55.Huang JQ, Hunt RH. The importance of clarithromycin dose in the management of Helicobacter pylori infection: a meta-analysis of triple therapies with a proton pump inhibitor, clarithromycin and amoxycillin or metronidazole. Aliment Pharmacol Ther. 1999;13:719–729. doi: 10.1046/j.1365-2036.1999.00530.x. [DOI] [PubMed] [Google Scholar]
- 56.Gisbert JP, Gonzalez L, Calvet X, et al. Proton pump inhibitor, clarithromycin and either amoxycillin or nitroimidazole: a meta-analysis of eradication of Helicobacter pylori. Aliment Pharmacol Ther. 2000;14:1319–1328. doi: 10.1046/j.1365-2036.2000.00844.x. [DOI] [PubMed] [Google Scholar]
- 57.Iwahi T, Satoh H, Nakao M, et al. Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against Helicobacter pylori. Antimicrobial Agents Chemother. 1991;35:490–496. doi: 10.1128/aac.35.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Figura N, Armellini D, Bugnoli M, Bayeli PF, Gennari C, Crabtree JE. Activity of omeprazole on H. pylori and relation to toxicity of strains. J Clin Pathol. 1994;47:440–442. doi: 10.1136/jcp.47.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McColm AA, Bagshaw JA, O'Malley CFO. Developmeny of a 14C-urea breath test in ferrets colonised with H. mustelae. Gut. 1993;34:181–186. doi: 10.1136/gut.34.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moayyedi P, Axon ATR. Patient factors that predict failure of omeprazole, clarithromycin and tinidazole (OCT) to eradicate Helicobacter pylori (H. pylori) Gut. 1995;37(Suppl 1):A46. doi: 10.1007/BF01213292. [DOI] [PubMed] [Google Scholar]
- 61.Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115:1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 62.Toracchio S, Cellini L, Di Camli E, et al. Role of antibiotic susceptibility testing on efficacy of triple therapy in Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:639–643. doi: 10.1046/j.1365-2036.2000.00870.x. [DOI] [PubMed] [Google Scholar]
- 63.Glupczynski Y, Megraud F, Lopez-Brea M, et al. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Microbiol Infect Dis. 2001;20:820–823. doi: 10.1007/s100960100611. [DOI] [PubMed] [Google Scholar]
- 64.Van der Wouden EJ, Thijs JC, van Zwet AA, et al. The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta-analysis. Am J Gastroenterol. 1999;94:1751–1759. doi: 10.1111/j.1572-0241.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 65.Goddard AF. Factors influencing antibiotic transfer across gastric mucosa. Aliment Pharmacol Ther. 1998;12:1175–1184. doi: 10.1046/j.1365-2036.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- 66.Back S, Carling L, Ekström P, et al. Eradication of Helicobacter pylori with either phenoxymethylpenicillin, benzylpenicillin or amoxycillin in combination with omeprazole: a Swedish multi-centre study. Gastroenterology. 1995;108:A532 (Abstract). [Google Scholar]
- 67.Atherton JC, Cockayne A, Balsitis M, et al. Detection of the intragastric sites at which Helicobacter pylori evades treatment with amoxycillin and cimetidine. Gut. 1995;36:670–674. doi: 10.1136/gut.36.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cooreman MP, Krausgrill P, Hengels KJ. Local gastric and serum amoxycillin concentrations after different oral application forms. Antimicrob Agents Chemother. 1995;39:2084–2087. doi: 10.1128/aac.37.7.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atherton JC, Washington N, Bracewell MA, et al. Scintigraphic assessment of the intragastric distribution and gastric emptying of an encapsulated drug: the effect of feeding and of a proton pump inhibitor. Aliment Pharmacol Ther. 1994;8:489–494. doi: 10.1111/j.1365-2036.1994.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 70.Goddard AF, Jessa MJ, Barrett DA, et al. Effect of omeprazole on the distribution of metronidazole, clarithromycin and amoxycillin in human gastric juice. Gastroenterology. 1996;111:358–367. doi: 10.1053/gast.1996.v111.pm8690200. [DOI] [PubMed] [Google Scholar]
- 71.Grübel P, Cave D. Factors affecting the penetration of clarithromycin through gastric mucus. Aliment Pharmacol Ther. 1998;12:569–576. doi: 10.1046/j.1365-2036.1998.00329.x. [DOI] [PubMed] [Google Scholar]
- 72.Goddard AF, Spiller RC. The effect of omeprazole on gastric juice viscosity, pH and bacterial counts. Aliment Pharmacol Ther. 1996;10:105–109. doi: 10.1111/j.1365-2036.1996.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 73.Lozniewski A, de Korwin JD, Muhale F, Jehl F. Gastric diffusion of antibiotics used against H. pylori. Int J Antimicrob Agent. 1998;9:181–193. doi: 10.1016/s0924-8579(97)00049-6. [DOI] [PubMed] [Google Scholar]
- 74.Goddard AF, Erah PO, Barrett DA, et al. The effect of protein binding and lipophilicity of penicillins on their in vitro flux across gastric mucosa. J Antimicrob Chemother. 1998;41:231–236. doi: 10.1093/jac/41.2.231. [DOI] [PubMed] [Google Scholar]
- 75.Calafatti SA, dos Santos A, da Silva CMF, et al. Transfer of metronidazole to gastric juice: impact of Helicoabacter pylori infection and omeprazole. Scand J Gastroenterol. 2000;35:699–704. doi: 10.1080/003655200750023354. [DOI] [PubMed] [Google Scholar]
- 76.Pedrazzoli J, Calafatti SA, Ortiz RAM, et al. Transfer of clarithromycin to gastric juice is enhanced by omeprazole in Helicobacter pylori-infected individuals. Scand J Gastroenterol. 2001;36:1248–1253. doi: 10.1080/003655201317097074. [DOI] [PubMed] [Google Scholar]
- 77.Gustavson LE, Kaiser JF, Edmonds AL, et al. Effect of omeprazole on concentrations of clarithromycin in plasma and gastric tissue at steady state. Antimicrob Agents Chemother. 1995;39:2078–2083. doi: 10.1128/aac.39.9.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goddard AF, Erah PO, Atherton JC, et al. Effect of H. pylori, aspirin and anoxia on antibiotic permeability in the rat stomach. Gastroenterology. 1997;112:A128. (Abstract) [Google Scholar]
- 79.Sherwood PV, Wibawa JID, Barrett DA, et al. Effect of Helicobacter gastritis on antibiotic secretion in man. Gut. 2001;48:A31. (Abstract) [Google Scholar]
- 80.Sherwood PV, Wibawa JID, Jordan N, et al. Gastric secretion of amoxycillin and metronidazole is increased by pronase a luminal mucolytic. Gut. 2001;48:A15. (Abstract) [Google Scholar]
- 81.Erah PO, Goddard AF, Barrett DA, et al. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother. 1997;39:5–12. doi: 10.1093/jac/39.1.5. [DOI] [PubMed] [Google Scholar]
- 82.Westblum TU, Duriex DE. Antibiotic concentrations in the gastric mucosa: The guinea pig model. In: Menge H, et al., editors. Helicobacter pylori 1990. Berlin: Springer-Verlag; 1991. pp. 157–161. [Google Scholar]
- 83.Endo H, Yoshida H, Ohmi N, et al. Localisation of (14C) clarithromycin in rat gastric tissue when administered with lansoprazole and amoxicillin. J Antimicrob Chemother. 2002;50:285–288. doi: 10.1093/jac/dkf097. [DOI] [PubMed] [Google Scholar]
- 84.Endo H, Yoshida H, Ohmi N, et al. Effects of lansoprazole, clarithromycin and pH gradient on uptake of (14C) amoxycillin into rat gastric mucosa. J Antimicrob Chemother. 2001;47:405–410. doi: 10.1093/jac/47.4.405. [DOI] [PubMed] [Google Scholar]
- 85.Logan RPH, Dill S, Bauer FE, et al. The European 13C-Urea Breath Test for the detection of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1991;3:915–921. [Google Scholar]
- 86.Logan RPH. The 13C-UBT for the detection of Helicobacter pylori. In: Rathbone BJ, Heatley RV, editors. Helicobacter pylori and gastroduodenal disease. 2. London: Blackwell; 1992. pp. 88–107. [Google Scholar]
- 87.Logan RPH. Breath tests to detect Helicobacter pylori. In: Goodwin S, Worsley B, editors. Helicobacter pylori: Biology and Clinical Practice. Florida: CRC Press Inc; 1993. pp. 307–327. [Google Scholar]
- 88.Klein PD, Graham DY. Minimum analysis requirements for the detection of Helicobacter pylori infection by the 13C-urea breath test. Am J Gastroenterol. 1993;88:1865–1868. [PubMed] [Google Scholar]
- 89.Bazzoli F, Zagari RM, Pozzato P, et al. 13C-urea breath test to quantify H. pylori colonisation of gastric mucosa and association with severity of inflammation. Gastroenterology. 1994;105:A180. [Google Scholar]
- 90.Cacoullis F, Batten JJ, Logan RPH, Karim QN, Walker MM, Baron JH. Quantifying the extent of Helicobacter pylori with the 13C-urea breath test. Gut. 1991;32:A565. [Google Scholar]
- 91.Prewett EJ, Luk YW, Fraser AG, Lam WM, Pounder RE. Comparison of one day oral dosing with three bismuth compounds for the suppression of Helicobacter pylori assessed by the 13C-urea breath test. Aliment Pharmacol Ther. 1992;6:97–102. doi: 10.1111/j.1365-2036.1992.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 92.Logan RPH, Walker MM, Misiewicz JJ, Gummett PA, Baron JH. Changes in the intragastric distribution of H. pylori during treatment with omeprazole. Gut. 1995;36:12–16. doi: 10.1136/gut.36.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Logan RPH, Gummett PA, Misiewicz JJ, Walker MM, Jameson J, Polson RJ, Karim QN, Duggan AE. The efficacy of ranitidine bismuth citrate (GR122311X) in the treatment of Helicobacter pylori infection. Gastroenterology. 1992;102:A114. [Google Scholar]
- 94.Logan RPH, Hurlimann S, Gummett PA, Walker MM, Karim QN, Baron JH, Misiewicz JJ. How quickly does Helicobacter pylori (H. pylori) recur after treatment. Gut. 1992;33(Suppl 1):S3. (Abstract) [Google Scholar]
- 95.Logan RPH, Polson RJ, Baron JH, Misiewicz JJ. Follow-up after anti-Helicobacter pylori treatment. Lancet. 1991;337:562–563. doi: 10.1016/0140-6736(91)91358-2. [DOI] [PubMed] [Google Scholar]
- 96.Logan RPH, Gummett PA, Polson RJ, Baron JH, Misiewicz JJ. What length of treatment with tripotassium dicitrato-bismuthate (TDB) for Helicobacter pylori? Gut. 1990;31:A1178. [Google Scholar]
- 97.Axon A, Deltentre M, Eriksson S, et al. Guidelines for clinical trials in Helicobacter pylori infection. Gut. 1997;41(Suppl 2):1–23. [PubMed] [Google Scholar]
- 98.Megraud F, Burette A, Glupczynski Y, et al. Comparison of tests for the assessment of Helicobacter pylori eradication: results of a multicentre study using centralised facility testing. Eur J Gastroenterol Hepatol. 2000;12:629–633. doi: 10.1097/00042737-200012060-00009. [DOI] [PubMed] [Google Scholar]
- 99.Johnson PG, Duggan AE, Olson C. How do two diagnostic tests for H. pylori compare to two 13C-urea breath tests (UBT) in assessment of eradication? Gut. 1996;39(Suppl 2):A118. [Google Scholar]
- 100.Newell DG, Stacey AR. The serology of Helicobacter pylori. In: Rathbone BJ, Heatley RV, editors. Helicobacter pylori and gastroduodenal disease. 2. London: Blackwell; 1992. pp. 74–82. [Google Scholar]
- 101.Hirschl AM, Rathbone BJ, Wyatt JI, Berger J, Rotter ML. Comparison of ELISA antigen preparations alone or in combination for serodiagnosing Helicobacter pylori infections. J Clin Pathol. 1990;43:511–513. doi: 10.1136/jcp.43.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Braden B, Posselt HG, Dietrich CF, Caspary WF, Lembcke B. Comparison of new faecal antigen test with 13C-urea breath test for detecting Helicobacter pylori infection and monitoring eradication treatment: prospective clinical evaluation. Br Med J. 2000;320:148. doi: 10.1136/bmj.320.7228.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gisbert JP, Pajares JM. Diagnosis of Helicobacter pylori infection stool antigen determination: a systematic review. Am J Gastroenterol. 2001;96:2829–2838. doi: 10.1111/j.1572-0241.2001.04235.x. [DOI] [PubMed] [Google Scholar]
- 104.Morris A, Ali MR, Brown P, Lane M, Patton K. Campylobacter pylori infection in biopsy specimens of gastric antrum: laboratory diagnosis and estimation of sampling error. J Clin Pathol. 1989;42:727–732. doi: 10.1136/jcp.42.7.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McMullen L, Walker MM, Bain LA, Karim QN, Baron JH. Histological identification of Campylobacter using Gimenez technique in gastric antral mucosa. J Clin Pathol. 1987;40:464–465. doi: 10.1136/jcp.40.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Czinn SJ, Nedrun JG. Oral immunisation against H. pylori. Infect Immun. 1991;59:2359–2363. doi: 10.1128/iai.59.7.2359-2363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doidge C, Lee A, Buck F, Hazell S, Manne U, Gust I. Therapeutic immunisation against Helicobacter infection. Am J Gastroenterol. 1994;89:A339. doi: 10.1016/s0140-6736(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 108.Del Giudice G, Covacci A, Telford JL, Montecucco C, Rappuoli R. The design of vaccines against Helicobacter pylori and their development. Annu Rev Immunol. 2001;19:523–563. doi: 10.1146/annurev.immunol.19.1.523. [DOI] [PubMed] [Google Scholar]
- 109.Ruggiero P, Peppoloni S, Berti D, Rappuoli R, Giudice GD. New strategies for the prevention and treatment of Helicobacter pylori infection. Expert Opin Invest Drugs. 2002;11:1127–1138. doi: 10.1517/13543784.11.8.1127. [DOI] [PubMed] [Google Scholar]