Abstract
Aims
To determine the pharmacokinetics, pharmacodynamics and tolerability of omapatrilat, a vasopeptidase inhibitor, in healthy subjects.
Methods
The effects of oral omapatrilat were evaluated in healthy men in two double-blind, placebo-controlled, dose-escalation trials. In a single-dose study, subjects received omapatrilat in doses of 2.5, 7.5, 25, 50, 125, 250, or 500 mg. In a multiple-dose study, subjects received doses of 10, 25, 50, 75, or 125 mg daily for 10 days.
Results
In the multiple-dose study, peak plasma concentrations (Cmax = 10–895 ng ml−1; tmax = 0.5–2 h) of omapatrilat were attained rapidly. Omapatrilat exhibited a long effective half-life (14–19 h), attaining steady state in 3–4 days. In the single-dose study, Cmax (1–1009 ng ml−1) and AUC(0,t) (0.4–1891 ng ml–1 h) were linear but not dose proportional. In the multiple-dose study, based on weighted least-squares linear regression analyses vs dose, Cmax but not AUC(0,t) was linear at the lower doses on day 10. The lowest dose of omapatrilat (2.5 mg) almost completely inhibited (> 97%) serum angiotensin converting enzyme activity at 2 h after dosing. In the multiple dose study, angiotensin converting enzyme activity was inhibited by more than 80% 24 h after all doses of omapatrilat. Inhibition of neutral endopeptidase activity was shown by increases in the daily urinary excretion of atrial natriuretic peptide and cyclic guanosine monophosphate at doses of more than 7.5 and 25 mg, respectively. In the single dose study, omapatrilat increased the daily urinary excretion of atrial natriuretic peptide dose-dependently from 10.8 ± 4.1 (± SD) ng 24 h−1 in the placebo group to 60.0 ± 18.2 ng 24 h−1 in the 500 mg group. Omapatrilat did not affect sodium and potassium excretion or urinary volume. Compared with placebo, omapatrilat produced a decrease in mean arterial pressure at 3 h after all doses in both the single- and multiple-dose studies.
Conclusions
Omapatrilat was generally well tolerated. The pharmacokinetic and pharmacodynamic effects of omapatrilat are consistent with once-daily dosing.
Keywords: angiotensin converting enzyme, blood pressure, neutral endopeptidase, omapatrilat, pharmacodynamics, pharmacokinetics, vasopeptidase inhibitor
Introduction
Omapatrilat is the most studied member of a new class of cardiovascular compounds called vasopeptidase inhibitors (VPIs) [1]. VPIs are single molecules that simultaneously inhibit neutral endopeptidase (NEP) and angiotensin-converting enzyme (ACE), resulting in enhanced amounts of peptides with vasodilatory and cardiovascular protective properties and inhibition of production of the vasoconstrictor angiotensin II. NEP is widely distributed in the body and has many possible substrates, such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), C-type natriuretic peptide, bradykinin, enkephalins, substance P, and neurotensin [2]. Many of these substrates play integral roles in the regulation of the cardiovascular system, affecting multiple activation pathways. Omapatrilat inhibits both NEP and ACE with nearly identical potency in vitro (Ki for NEP = 8.9 nm and Ki for ACE = 6 nm) [3]. By inhibiting both NEP and ACE, omapatrilat can potentially provide benefits in the treatment of hypertension, heart failure, and other cardiac and vascular diseases. Omapatrilat has shown antihypertensive action in low-, normal-, and high-renin models of experimental hypertension [3]. In addition, omapatrilat was well tolerated and produced persistent reductions in ambulatory systolic blood pressure (SBP) and diastolic blood pressure (DBP) in patients with mild to moderate hypertension [4–6].
Two double-blind, placebo-controlled, phase I, dose-escalation studies of omapatrilat are described. The first study examined the effects of seven single oral doses of omapatrilat ranging from 2.5 to 500 mg. The second, a multiple-dose study, evaluated five doses ranging from 10 to 125 mg given daily for 10 days. The purpose of these studies was to characterize the pharmacokinetic and pharmacodynamic profiles and evaluate the range of tolerability of omapatrilat in healthy adults.
Methods
These studies were approved by the Institutional Review Board at The Medical Center at Princeton and performed in accordance with the principles of the Declaration of Helsinki and its amendments. All subjects signed informed consent forms prior to participation in the study.
Single-dose study
Sixty-three healthy male subjects were randomly assigned to omapatrilat or placebo, and all completed the study according to the protocol. Subjects had a mean (± SD) body weight of 79.1 ± 9.0 kg (range 62.0–99.3 kg) and a mean age of 32 ± 8 years (range 19–45 years).
The study consisted of a 5-day lead-in period and a 1-day treatment period. Subjects were enrolled in the lead-in period on the evening of day −5 for baseline urine and blood collections. Subjects began a controlled daily diet on day −4 that continued for the entire study period. It consisted of fixed protein (90 g), fixed salt (6 g [260 mEq] sodium, 4 g [100 mEq] potassium) and ≥ 2 l of water. Subjects were not permitted to smoke or to consume alcohol or caffeine containing substances for the duration of the study. An evening fast began on day −2. Subjects received a lead-in dose of placebo on day −1 and continued to have urine and blood collected and baseline vital signs recorded. On study day 1, subjects were randomly assigned to receive a single oral dose of placebo or a 2.5, 7.5, 25, 50, 125, 250, or 500 mg dose of omapatrilat in double-blind manner (six on active drug and three on placebo per ascending dose panel) and fasted for an additional 4 h after dosing.
Multiple-dose study
Of 46 healthy male subjects randomized on day 1, 42 completed the study (one subject discontinued for personal reasons and was replaced). Subjects had a mean (± SD) body weight of 78.1 ± 9.0 kg (range 57.0–100.0 kg) and a mean age of 31 ± 7 years (range 22–49 years).
This study was identical to that of the single-dose study with respect to baseline urine and blood sampling, the initiation and composition of the controlled diet, and the overnight fast. On days −4 to −1, subjects received single-blind, lead-in doses of placebo. Baseline vital signs (including orthostatic pulse rate and blood pressure [BP]) were recorded on days −4 and −1, and samples for baseline pharmacodynamic assessments were collected on day −1. On day 1, subjects were randomly assigned in a double-blind manner to once-daily dosing of placebo or to 10, 25, 50, 75, or 125 mg of omapatrilat (six on active drug and three on placebo per ascending dose panel). On days 1 and 10, subjects fasted for an additional 4 h after dosing. Subjects were discharged on day 12.
Sample collection and processing
For assessment of pharmacokinetic parameters, venous blood samples (7 ml) were obtained predose and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 h postdose on day 1 for the single-dose study and at the same time points on days 1 and 10 for the multiple-dose study. Additional blood samples were drawn at 36, 48, 60, and 72 h postdose in the single-dose study and immediately predose on days 4, 6, 8, and 9 in the multiple-dose study. Samples were collected in Vacutainer® tubes (BD, Franklin Lakes, NJ) using K3EDTA as an anticoagulant and containing a premeasured amount of methyl acrylate (10 µl ml−1 blood), which is used as a blocking agent to prevent omapatrilat from forming disulphide derivatives ex vivo.
Urine was analysed for ANP and cyclic guanosine monophosphate (cGMP), which are both markers for NEP inhibition; aldosterone, creatinine, and electrolyte (sodium and potassium) determinations were also performed. Urine samples were collected at the following intervals relative to dosing on day 1 in both studies: −96 to −72 h, −72 to −48 h, and −48 to −24 h; samples were also obtained on days −1 and 1, at 0–4 h, 4–8 h, 8–12 h, and 12–24 h. Additional urine samples were collected during the single-dose study at 24–48 h postdose and during the multiple-dose study on days 3 and 10 at the same time intervals as on days −1 and 1. Urine samples were stored frozen at −70 °C until assayed. At this temperature, ANP and cGMP are stable in urine for at least 6 months.
Additional pharmacodynamic parameters included plasma renin activity (PRA), plasma ANP and cGMP, and serum ACE activity. For the single-dose study, venous blood samples for assay of PRA, ANP, and cGMP were obtained at 0 (predose, relative to the anticipated dosing time on day 1) and 0.5 h on days −1 and at 0 (predose), 0.5 h, and 24 h postdose on day 1. Samples for assay of ACE activity were obtained predose (0 h) and at 2 and 24 h postdose. For the multiple-dose study, samples for PRA were obtained at 0 and 4 h on days −1, 1, and 10 and at 24 h on days 1 and 10. Samples for ANP and cGMP were obtained at 0, 2, 4, and 8 h on days −1, 1, and 10, at 24 h on days 1 and 10, and before discharge on day 12. Samples for ACE activity were obtained predose on days 1, 5, and 10, at 2 h on days 1 and 10, at 24 h on day 1, and before discharge on day 12. Serum ACE activity was measured on the day of sample collection without freezing the samples. Venous blood for PRA was collected into prechilled tubes containing K3EDTA, and plasma was stored frozen at −70 °C until assayed. Venous blood for ANP and cGMP was collected into prechilled tubes containing K3EDTA and aprotinin (10 TIU ml−1), and plasma was stored frozen at −70 °C until assayed. At this temperature, ANP, cGMP, and PRA are stable in plasma for at least 6 months.
Assay methods
Omapatrilat concentrations in plasma were quantified by liquid chromatography (LC) coupled with positive ion electrospray mass spectrometry (MS). For quantification, the [M + NH4]+ ions of the analyte and internal standard were measured in the single ion monitoring mode using a validated LC/MS method for human plasma [7]. The intraday and interday precision was ± 4% or better [7]. Mean results are presented for each dose group and were calculated using a value equal to 0 (zero) for plasma concentrations below 0.5 ng ml−1 and 0.1 ng ml−1, the lower limit of quantification (LLQ) in the single and multiple dose studies, respectively.
Urinary and plasma ANP were measured by radioimmunoassay after solid phase extraction, as previously described [8]. Assay sensitivity was 1.3 and 1.7 pg ml−1 for ANP in urine and plasma, respectively. Urodilatin, with a structure similar to that of ANP, another natriuretic peptide isolated only from human urine, shows cross-reactivity in the immunoreactive ANP measurements in urine [9]. The ratio between the concentration of ANP and urodilatin has been reported at 1.3 : 1.0 in normal human urine [10]. Urodilatin is not a substrate of NEP; thus, its urinary excretion is not expected to increase following NEP inhibition by omapatrilat [11]. Urinary and plasma cGMP were measured by radioimmunoassay using a kit from DuPont Medical Products (Boston, MA) after dilution and ethanol extraction, respectively. Following acid hydrolysis, urinary aldosterone was measured by radioimmunoassay using the Aldosterone Coat-A-Count® Kit (Diagnostic Products Corporation, Los Angeles, CA). PRA was measured using the Clinical AssaysTM PRA RIA kit (Incstar Corporation, Stillwater, MN). Serum ACE activity was measured using a radioenzyme assay adapted from a kit previously available from HYCOR Biomedical Inc. (Irvine, CA); this method uses the synthetic substrate Hip-Gly-Gly. Electrolytes were measured on the Hitachi® 704 Chemistry Autoanalyser (Tarrytown, NY) using ion selective electrodes.
The intra-assay coefficients of variation for the hormonal and enzymatic assays were as follows: urinary ANP, 4% to 7%; plasma ANP, 3% to 9%; urinary cGMP, 5% to 16%; plasma cGMP, 2% to 6%; aldosterone, 4% to 18%; PRA, 3% to 8%; and ACE activity, 3% to 6%. The corresponding inter-assay coefficients of variation were as follows: urinary ANP, 3% to 5%; plasma ANP, 7% to 10%; urinary cGMP, 8% to 11%; plasma cGMP, 9% to 14%; aldosterone, 4% to 14%; PRA, 8% to 19%; and ACE activity, 1% to 5%.
Blood pressure measurements
BP measurements were made using a Dinamap® system (GE Medical Systems, Milwaukee, WI), with the cuff placed on the same (dominant) arm for each measurement. For the single-dose study, supine SBP and DBP were measured over a 12-h period on day −1 (before rising, 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, and 12 h) and over a 72-h period commencing on day 1 (before rising, 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 36, 48, 60, and 72 h). For the multiple-dose study, supine SBP and DBP were taken on days −4 and −1, and on days 1, 4, 8, and 10 before rising and at 0.5, 1.5, 2.5, 16, and 21 h postdose.
Other measurements
At screening, medical history was recorded and electrocardiograms (ECGs) and physical examinations performed. On day 1, ECGs and physical examinations were performed, and blood and urine samples were collected for clinical laboratory tests predose and at 3.5 and 24 h postdose. For the multiple-dose study, additional ECGs and physical examinations were performed at 3.5 h postdose on days 4, 8, and 10. Temperature and respiratory rate were recorded before dose administration on day 1 and at discharge.
Adverse events (AEs) included any illness, sign, symptom, or clinically significant laboratory abnormality that appeared or worsened during the course of the clinical trial, regardless of the causal relationship to the study drug. A serious AE was defined as an AE that met any of the following criteria: fatal, life-threatening, permanently disabling, causing inpatient hospitalization or prolonged hospitalization, or overdose.
Pharmacokinetic analysis
The following pharmacokinetic parameters were estimated using noncompartmental analysis:
Cmax (ng ml−1) = maximum observed plasma concentration of omapatrilat after dosing.
tmax (h) = time to reach Cmax.
Cmin (ng ml−1) = minimum plasma concentration measured predose on days 2, 4, 6, 8, 9, and 10 and on day 11.
AUC(0,t) (ng ml−1h) = area under the plasma concentration-time curve from 0 to last measurable concentration. The concentrations in the terminal phase below LLQ were not used in the calculation.
t1/2,eff (h) = effective half-life =−0.693 × t/ln(1–1/accumulation ratio) where t is the dosing interval (24 h) and the accumulation ratio (AI) = AUCss/AUC1 where AUCss = AUC(0,t) at steady state and AUC1 = AUC(0,t) after the first dose. Fieller's theorem was used to estimate the lower and upper confidence limits of this population accumulation ratio [12].
Statistical analysis
All statistical analyses were carried out using SAS/STAT® Version 6.07 (SAS Institute, Cary, NC). Tests of comparison were carried out at the two-sided 5% significance level. Interval estimates were calculated using 95% confidence intervals (CIs). Correlation with dose was explored via a scatter plot of each pharmacokinetic parameter vs dose for each day. If a dose dependent response was evident, weighted least-squares linear regression was used first to evaluate the relationship. The weights were the reciprocals of the variances within each of the dose groups, since increasing variance with increasing dose is an expected result. Departure from dose-linearity was tested using the ‘lack-of-fit’ procedure as described by Littell et al. (SAS/STAT® Version 6.07). A statistically significant lack-of-fit would imply nonlinear dependence on dose. Otherwise, a linear regression of the response on dose was performed. Dose proportionality of a linear relationship was assessed by the intercept of the regression plot. An intercept significantly different from zero would imply the linear relationship was not dose proportional. Comparisons of AUC on days 1 and 10 were performed with an analysis of variance model using subject, day (days 1 and 10), dose, and dose-by-day interaction as factors. Dose was tested using subject as the error term. To test for failure to achieve steady state, analysis of variance was performed on Cmin using group, subject within group, day, and group-by-day interaction. Significant differences in Cmin means between days would indicate that a steady state had not been achieved.
Results
Pharmacokinetics
Single-dose study
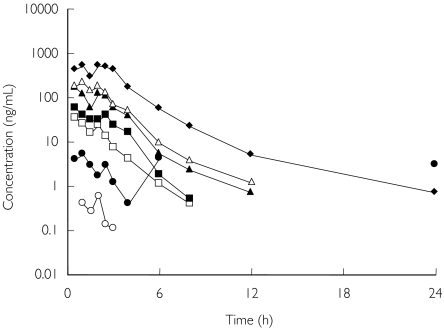
Mean pharmacokinetic parameters are shown in Table 1, and the time course of the plasma concentration is shown in Figure 1. The tmax for omapatrilat was ≤ 2.25 h for all doses.
Table 1.
Pharmacokinetic parameters following single dose administration of omapatrilat.
| Omapatrilat dose | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | 2.5 mg | 7.5 mg | 25 mg | 50 mg | 125 mg | 250 mg | 500 mg |
| Cmax (ng ml−1) | 1.28 | 9.6 | 51.09 | 89.29 | 286.35 | 491.61 | 1008.92 |
| (0.44) | (7.96) | (23.43) | (49.15) | (162.01) | (303.76) | (645.88) | |
| tmax (h)* | 2.0 | 2.0 | 1.5 | 1.5 | 2.25 | 1.25 | 1.5 |
| (1.0, 2.0) | (1.0, 6.0) | (0.5, 4.0) | (0.5, 4.0) | (0.5, 3.0) | (05, 3.0) | (0.5, 4.0) | |
| AUC(0,t) (ng ml−1h) | 0.43 | 12.52 | 69.19 | 149.6 | 406.52 | 741.39 | 1890.76 |
| (0.4) | (17.34) | (12.86) | (48.89) | (85.52) | (309.32) | (803) | |
Results are presented as mean (SD).
Median (minimum, maximum).
Figure 1.
Time course of plasma omapatrilat concentration after single doses of the drug. The tmax for omapatrilat was ≤ 2.25 h for all doses. Mean results are presented for each dose group. Note that the relatively high mean plasma concentration at 6 and 24 h after the 7.5 mg dose is due to one single outlier subject (data points: 6 h: 25.3 ng ml−1; 24 h: 18.0 ng ml−1). 2.5 mg (○); 7.5 mg (•); 25 mg (□); 50 mg (▪); 125 mg (▴); 250 mg (▵); and 500 mg (♦).
There was a linear but not a proportional relationship between Cmax and dose up to 500 mg. The estimated linear regression (predicted Cmax = −3.80 + 2.03 × dose; r2 = 0.742) and the 95% CI for the intercept (−4.8478, −2.7422) indicated that the latter was significantly different from 0. Single-dose AUC(0,t) was linear but not proportional to dose. The estimated linear regression (predicted AUC(0,t) = −7.50 + 3.17 × dose; r2 = 0.9233) and the 95% CI (−8.3242, −6.6695) indicated that the intercept was significantly different from 0.
Multiple-dose study
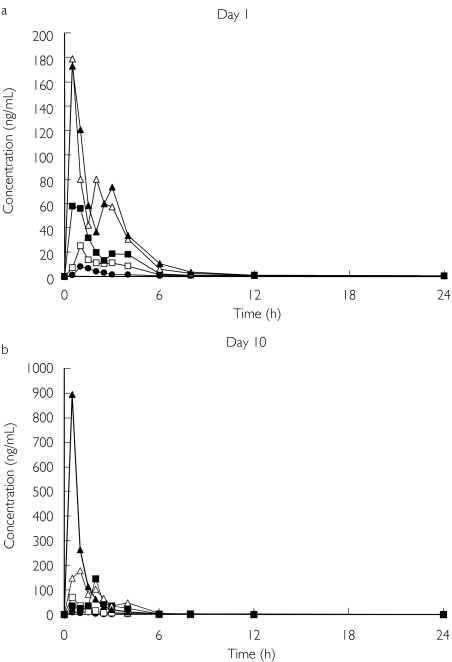
In this study mean t1/2,eff was estimated to be between 14.3 and 19.3 h (Table 2). The time course of the plasma concentration for all doses on days 1 and 10 is shown in Figure 2.
Table 2.
Pharmacokinetic parameters on days 1 and 10 following multiple dose administration of omapatrilat.
| Omapatrilat dose (n = 6/group) | Cmax (ng ml−1) | tmax* (h) | AUC(0,t) (ng ml−1h) | t1/2,eff (h) |
|---|---|---|---|---|
| Day 1 | ||||
| 10 mg | 9.6 | 1.5 | 19.5 | NA |
| (5.5) | (1.0, 4.0) | (6.8) | ||
| 25 mg | 26.4 | 1.0 | 63.8 | NA |
| (8.9) | (1.0, 2.5) | (16.9) | ||
| 50 mg | 93.5 | 0.8 | 147.9 | NA |
| (58.5) | (0.5, 4.0) | (32.2) | ||
| 75 mg | 181.7 | 0.5 | 341.6 | NA |
| (98.7) | (0.5, 3.0) | (100.7) | ||
| 125 mg | 203.3 | 0.5 | 369.4 | NA |
| (112.5) | (0.5, 3.0) | (124.0) | ||
| Day 10 | ||||
| 10 mg | 14.4 | 0.8 | 30.1 | 19.3 |
| (6.1) | (0.5, 3.0) | (6.3) | (17.1) | |
| 25 mg | 80.7 | 0.5 | 92.4 | 17.4 |
| (46.9) | (0.5, 2.0) | (20.1) | (9.7) | |
| 50 mg | 175.9 | 2.0 | 226.9 | 17.1 |
| (105.6) | (0.5, 4.0) | (43.0) | (11.6) | |
| 75 mg | 297.1 | 1.5 | 420.9 | 14.3 |
| (144.2) | (0.5, 4.0) | (55.4) | (8.9) | |
| 125 mg† | 895.3 | 0.5 | 745.2 | NA |
| (284.2) | (0.5, 0.5) | (125.3) |
Results are presented as mean (SD)
Median (minimum, maximum); NA = not available
n = 2
Figure 2.
Time course of plasma omapatrilat concentration on days 1 (a) and 10 (b) after multiple doses of the drug. The tmax for omapatrilat was 0.5–2 h. Mean results are presented for each dose group. 10 mg (•); 25 mg (□); 50 mg (▪); 75 mg (▵); and 125 mg (▴).
The multiple-dose study showed that Cmax on day 1 was proportional to dose. The AUC(0,t) showed no significant departure from linearity (P = 0.07), but the intercept was significantly different from 0, indicating that the AUC is linear but not proportional to dose. On day 10, Cmax showed no significant departure from linearity (P = 0.35). However, the intercept was significantly different from 0, indicating Cmax was linear, not proportional to dose. There was significant departure from linearity (P = 0.037) for AUC(0,t), indicating it was not linear with dose. An analysis of variance found no statistically significant differences in mean Cmin values between the days, suggesting that steady state was achieved in 3–4 days.
Accumulation of omapatrilat was assessed by statistical analysis of the accumulation index (AI) for day 10 vs day 1. AI did not depend on dose and ranged from 1.4 to 2.5. Analysis of variance for AI showed that dose and day effects were statistically significant (P < 0.0001 for both) but that dose–by–day interactions were not (P = 0.49). For all dose groups except for the 75 mg group, the lower limit of the 95% CI around the mean ratio was larger than 1, indicating statistically significant drug accumulation.
Pharmacodynamics
Urinary ANP excretion
In both the single- and multiple-dose studies of omapatrilat, urinary ANP excretion increased on day 1 compared with the placebo group and baseline values (Table 3). Peak excretion occurred within 8 h after dosing and increased in a dose-dependent manner. The magnitude of the peak excretion within each dose group was not different after multiple days of dosing. At doses ≥ 25 mg, urinary ANP excretion remained elevated over baseline values for 24 h.
Table 3.
Daily urinary excretion of ANP following a) single and b) multiple doses of omapatrilat.
| a) Omapatrilat dose (Single-dose study) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | 2.5 mg | 7.5 mg | 25 mg | 50 mg | 125 mg | 250 mg | 500 mg | |
| Urinary ANP | 10.8 | 12.2 | 15.9 | 26.2 | 34.1 | 45.9 | 55.0 | 60.0 |
| (ng 24 h−1) | (4.1) | (6.0) | (1.7) | (3.8) | (13.8) | (9.4) | (14.0) | (18.2) |
| b) Omapatrilat dose (Multiple-dose study) | |||||||
|---|---|---|---|---|---|---|---|
| Day | Placebo | 10 mg | 25 mg | 50 mg | 75 mg | 125 mg | |
| Urinary ANP | Pre-dose | 9.4 | 11.3 | 10.5 | 12.5 | 10.6 | 9.3 |
| (ng 24 h−1) | (2.8) | (4.0) | (3.9) | (4.7) | (2.7) | (2.7) | |
| Urinary ANP | Day 1 | 10.7 | 16.3 | 32.2 | 37.0 | 31.9 | 47.2 |
| (ng 24 h−1) | (3.5) | (5.3) | (11.0) | (8.7) | (8.2) | (10.4) | |
| Urinary ANP | Day 3 | 9.3 | NS | 29.2 | 37.2 | 41.6 | 42.1 |
| (ng 24 h−1) | (2.9) | (11.0) | (14.0) | (7.8) | (15.3) | ||
| Urinary ANP | Day 10 | 9.7 | 16.9 | 29.4 | 30.3 | 33.3 | 33.8* |
| (ng 24 h−1) | (2.8) | (3.4) | (8.6) | (8.7) | (7.2) | (13.2) | |
Results are presented as mean (SD); NS: No samples collected
n = 2
Plasma ANP concentration
Baseline values were 31.6 ± 10.6 pg ml−1 in the placebo group in the single dose study and 27.5 ± 8.8 pg ml−1 in the multiple dose study. Small increases of less than 40% from baseline plasma ANP occurred in both the single- and multiple-dose studies (data not shown).
Urinary cGMP excretion
In both the single- and multiple-dose studies, increases in urinary cGMP were consistent at omapatrilat doses ≥ 25 mg, at which dose the mean peak urinary cGMP excretion on day 1 approached twice the mean baseline excretion ratio of 0.35 ± 0.14 nmol cGMP mg−1 creatinine in the placebo group for the single dose study and 0.37 ± 0.08 nmol cGMP mg−1 creatinine in the placebo group for the multiple dose study.
Plasma cGMP concentrations
At omapatrilat doses of 10 mg or more, the plasma cGMP concentration increased by approximately 1.25- to 1.80-fold (data not shown). Peak cGMP values were reached at 4–8 h postdose and were greater on day 1 than on day 10. Baseline plasma cGMP concentrations were 7.3 ± 1.3 nmol l−1 in the placebo group during the multiple dose study.
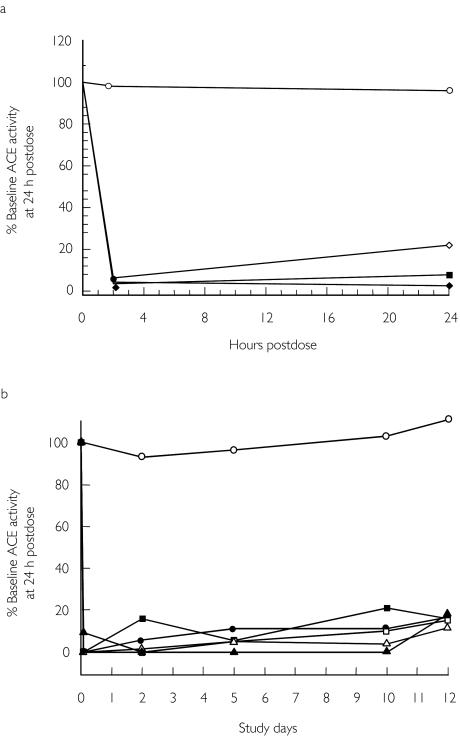
Inhibition of ACE
ACE activity was nearly completely inhibited by all single and multiple doses of omapatrilat at both 2 and 24 h postdose. This extensive inhibition was sustained from day 1–10 in the multiple-dose study, and marked ACE inhibition persisted on day 12, 48 h after the last dose (Figure 3).
Figure 3.
Mean ACE activity after administration of omapatrilat and placebo over 24 h for the single-dose study (a) and over 12 days for the multiple-dose study. Placebo (○); 2.5 mg (⋄); 7.5 mg (•); 25 mg (□); 50 mg (▪); 125 mg (▴); 250 mg (▵); and 500 mg (♦). (b). Note that in the multiple-dose study, the initial reading for omapatrilat dose groups is at 2 h on day 1. Placebo (○); 10 mg (•); 25 mg (□); 50 mg (▪); 75 mg (▵); and 125 mg (▴).
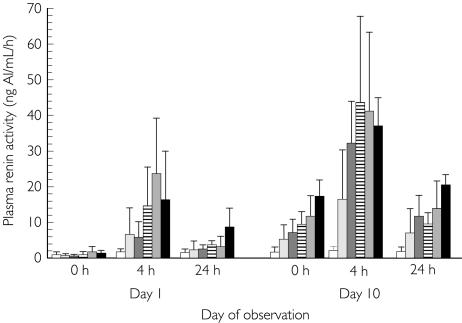
Plasma renin activity
For each dose group in the multiple-dose study, mean plasma renin activities were higher (up to 50-fold elevation at the 25 mg dose and above after 10 daily doses) at 4 h postdose than at predose (0 h) and appeared to be dose dependent (Figure 4). At all omapatrilat doses, plasma rennin activities remained elevated for up to 24 h postdose on day 1. There was also a sustained elevation in plasma rennin activity at 24 h postdose after 10 days of dosing.
Figure 4.
Mean PRA before and after administration of omapatrilat in the multiple-dose study. Concentrations were higher at 4 than at 0 h and appeared to be dose-dependent. At all doses, PRA was elevated for less than 24 h postdose on day 1. Placebo (□); 10 mg ( ); 25 mg (
); 25 mg ( ); 50 mg (
); 50 mg ( ); 75 mg (
); 75 mg ( ); and 125 mg (▪).
); and 125 mg (▪).
Urinary excretion of aldosterone
At omapatrilat doses of 75 mg and higher, there was a decrease from baseline (8.7 ± 3.9 µg 24 h−1, single dose study; 8.8 ± 4.5 µg 24 h−1, multiple dose study) of 16% to 42% in the mean 24-h urinary excretion of aldosterone (data not shown). Because of the relatively small numbers of subjects in each treatment group, this decrease could not be related to dose.
Urinary electrolytes and volume
No significant differences were observed in daily urinary excretion of sodium and potassium between subjects receiving omapatrilat and placebo in either study. Furthermore, no dose-dependent changes were apparent from baseline in daily urinary volume (Table 4). Additionally, in the single-dose study, omapatrilat did not produce acute (0–4 h) natriuresis or diuresis at doses up to 500 mg.
Table 4.
Urinary sodium and potassium excretion and volume following administration of omapatrilat.
| Sodium (mEq day−1) | Potassium (mEq day−1) | Urinary volume (l day−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Day −1 | Day 1 | Day 3 | Day 10 | Day −1 | Day 1 | Day 3 | Day 10 | Day −1 | Day 1 | Day 3 | Day 10 |
| Placebo | 175 | 160 | 166 | 134 | 71 | 97 | 87 | 99 | 2.38 | 2.03 | 2.26 | 1.96 |
| (n = 15) | (38) | (59) | (31) | (39) | (14) | (25) | (20) | (34) | (0.64) | (0.57) | (0.34) | (0.44) |
| 10 mg | 200 | 174 | NS | 132 | 79 | 78 | NS | 95 | 2.60 | 2.24 | NS | 2.13 |
| (38) | (44) | (38) | (16) | (13) | (13) | (0.35) | (0.54) | (0.56) | ||||
| 25 mg | 216 | 195 | 192 | 158 | 61 | 80 | 78 | 86 | 2.39 | 2.15 | 2.32 | 1.91 |
| (36) | (42) | (32) | (23) | (15) | (23) | (3) | (15) | (0.45) | (0.55) | (0.43) | (0.26) | |
| 50 mg | 222 | 188 | 215 | 143 | 70 | 84 | 107 | 89 | 2.85 | 2.56 | 2.68 | 1.97 |
| (37) | (20) | (33) | (42) | (20) | (14) | (16) | (20) | (0.54) | (0.38) | (0.41) | (0.43) | |
| 75 mg | 187 | 125 | 203 | 160 | 73 | 71 | 83 | 87 | 2.62 | 1.73 | 2.51 | 1.96 |
| (48) | (17) | (26) | (28) | (12) | (16) | (10) | (8) | (0.29) | (0.33) | (0.21) | (0.17) | |
| 125 mg | 176 | 189 | 181 | 182* | 69 | 80 | 82† | 105* | 2.26 | 2.46 | 2.68 | 2.24* |
| (25) | (32) | (40) | (37) | (17) | (20) | (10) | (11) | (0.33) | (0.38) | (0.52) | (0.38) | |
Results are presented as mean (SD); NS: no sample collected for subjects in 10 mg dose group
n = 2
n = 4
Blood pressure
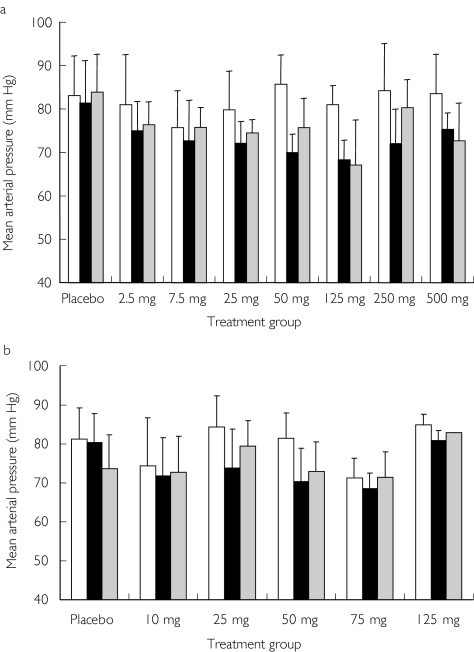
In the single-dose study, decreases in supine SBP, DBP, and mean arterial pressure (MAP) were seen 3 h after administration of omapatrilat compared with predose values. No changes in MAP were seen in the placebo group over the same time period (Figure 5a). SBP decreases of approximately 10 mmHg were observed in all omapatrilat treatment groups (2.5–500 mg), and DBP decreases of approximately 7 mmHg were observed in subjects receiving more than 2.5 mg. Statistical comparisons were not performed due to small sample sizes in these dose groups. There was a trend towards a decrease in MAP in the multiple dose study at 3 h after all the doses. Results from the 10th consecutive day of dosing are shown in Figure 5b. There was also a trend towards a decrease in predose trough supine SBP and DBP on days 8, 10, and 11 (24 h after the day 10 dose) after all omapatrilat doses, whereas no changes were seen over time in the placebo group. No significant changes were observed between placebo and omapatrilat treated groups in orthostatic blood pressure or heart rate.
Figure 5.
Comparison of mean arterial blood pressure at predose (□) and at 3 (▪) and 24 h ( ) after the dose in the single-dose study (a) and on day 10 in the multiple-dose study (b). Results are presented as mean ± SD.
) after the dose in the single-dose study (a) and on day 10 in the multiple-dose study (b). Results are presented as mean ± SD.
Tolerability of omapatrilat
The most frequent adverse events (AEs) in both the single- and multiple-dose tolerance studies are shown in Table 5. In the single-dose study, omapatrilat was generally well tolerated at doses between 2.5 mg and 500 mg, with no serious AEs. No subject discontinued active treatment because of an AE. In the multiple-dose tolerance study, omapatrilat was well tolerated at doses between 10 mg and 75 mg. A number of mild to moderate adverse events were reported, including one case of oedema of the lip (75 mg dose). The most frequently reported AE was orthostatic tachycardia (defined as a greater than 20 beats min−1 difference between supine and standing heart rate), which affected 11 subjects treated with omapatrilat and one subject treated with placebo. An orthostatic tachycardia event was reported at least once in each dose group, but there was no relationship between dose and magnitude of the response. These events usually occurred during the middle of the drug treatment, although some happened between day 1 to day 10. No significant changes in orthostatic events were seen as result of chronic omapatrilat dosing in normal subjects. The mean orthostatic heart rate change in the placebo treated subjects was + 18 beats min−1 and that after omapatrilat treatment (all doses) was + 23 beats min−1. The next most commonly reported AE was headache, reported for nine subjects (one after the 50 mg dose, two after placebo and the 75 mg dose and four reports after the 125 mg dose). Dizziness was reported for six subjects (one after the 50 mg dose, two after the 75 mg dose and three after the 125 mg dose). At the highest dose (125 mg), dose-limiting side-effects such as lethargy, headache, and dizziness were noted. Four subjects taking this dose withdrew from the study because of these AEs.
Table 5.
Most frequently reported adverse events* in a) the single-dose study and b) the multiple dose study.
| a) Single-dose study (2.5–500 mg omapatrilat) | |||
|---|---|---|---|
| Adverse event | Placebo (n = 21) | Omapatrilat 2.5–75 mg (n = 24) | Omapatrilat 125–500 mg (n = 18) |
| Tachycardia | 1 | 0 | 2 |
| Fatigue (Lethargy) | 0 | 0 | 2 |
| Headache | 2 | 4 | 4 |
| Dizziness | 0 | 0 | 4 |
| Flushing | 0 | 0 | 7 |
| Sensation of warmth | 0 | 1 | 4 |
| b) Multiple-dose study (10–125 mg omapatrilat). | |||
|---|---|---|---|
| Adverse event | Placebo (n = 21) | Omapatrilat 10–75 mg (n = 36) | Omapatrilat 125 mg (n = 6) |
| Orthostatic tachycardia | 1 | 8 | 3 |
| Fatigue (Lethargy) | 0 | 1 | 3 |
| Headache | 2 | 3 | 4 |
| Dizziness | 0 | 3 | 3 |
| Flushing | 0 | 0 | 1 |
| Sensation of warmth | 0 | 1 | 0 |
| Rash | 0 | 0 | 1 |
Number of events: a single subject may have reported more than one event.
Discussion
These studies describe the pharmacokinetics and pharmacodynamics of the VPI omapatrilat in healthy adults. By definition, VPI molecules inhibit the enzymes NEP and ACE and regulate local and circulating concentrations of a number of important vasoactive peptides, including angiotensin II, bradykinin, substance P, adrenomedullin, ANP, and BNP [13, 14]. These peptides can directly influence volume status, vascular resistance, cell growth, and the secretion of other vasoactive mediators such as nitric oxide and prostaglandins [13, 14].
Many of the peptides influenced by NEP and ACE inhibition can interact with one another. For instance, bradykinin and adrenomedullin induce nitric oxide release from endothelial cells, which causes smooth muscle relaxation [15, 16]. Angiotensin II, on the other hand, in addition to directly inducing smooth muscle constriction, stimulates the formation of superoxide anions [17] that can inactivate nitric oxide. Another example is the interaction between angiotensin II and the natriuretic peptides. Angiotensin II normally enhances sodium reabsorption in the kidney and the natriuretic peptides inhibit this effect to promote natriuresis and volume homeostasis [18]. However, under certain circumstances angiotensin II has been shown to down-regulate the ANP receptor, decreasing the number of ANP binding sites [19, 20], an effect which would be expected to attenuate the vasodilator and natriuretic effects of ANP and BNP. In the presence of a VPI, the concentration and effects of the vasoconstrictor peptide angiotensin II would be decreased and the concentrations and effects of the vasodilator peptides would be increased.
Because of the complex interactions among these peptides, the overall response to a VPI is determined by the specific enzyme inhibitory profile of the drug. Unlike molecules that have only one target, each VPI will have a unique profile of activity depending on the magnitude and duration of increases and/or decreases in the vasoactive peptides resulting from inhibition of NEP and ACE. Omapatrilat, for example, inhibits both NEP and ACE with nearly identical inhibitory constants in vitro (Ki for NEP = 8.9 nm and Ki for ACE = 6 nm) [3]. Other VPI molecules are likely to have unique inhibitory and pharmacodynamic profiles [21–24].
The steady state pharmacokinetic profile of omapatrilat indicates rapid systemic availability and a sufficiently long half-life for once-daily dosing. Steady-state plasma concentrations were reached in 3–4 days. In the multiple-dose study, Cmax on day 1 was linear and proportional to dose. On day 10 in the multiple-dose study, Cmax was linear, but not proportional to dose. Comparison of the mean values for Cmax and AUC(0,t) on day 1 vs day 10 indicated accumulation of omapatrilat after multiple daily dosing.
Compared with placebo, omapatrilat lowered SBP, DBP, and MAP in all subjects. The peak BP-lowering effect was observed at 3–6 h after dosing. There was also a trend toward a dose-dependent, sustained lowering of BP, as indicated by trough values. In contrast, ACE inhibitors such as captopril [25] and cilazapril [26] show little effect on BP in healthy volunteers. Similarly, pure NEP inhibitors, such as candoxatril [27] and SCH34826 [28], also produce no changes in BP in normotensive volunteers.
Unlike ACE, NEP is not present in large quantities in plasma, and assays for NEP activity in plasma or serum are not widely available [29]. High concentrations of NEP are found in tissues associated with renal and intestinal brush borders, pulmonary endothelium, and cardiac tissue [30–32]. Inhibition of NEP results in an increase in ANP in plasma or urine. ANP in turn activates its guanylyl cyclase–linked receptor, causing an increase in intracellular and extracellular cGMP concentrations. This relationship provides the rationale for using ANP and cGMP as indirect indicators of NEP inhibition. The ability of omapatrilat to produce acute and chronic NEP inhibition is supported by marked elevations in urinary excretion of ANP and plasma concentrations of cGMP [33].
Peak increases in urinary ANP, plasma cGMP, and urinary cGMP occurred within 12 h of dosing (i.e. at or a few hours after the time of maximal omapatrilat plasma concentrations). Compared with placebo, omapatrilat increased 24-h urinary excretion of ANP at doses of 10 mg and higher on days 1, 3, and 10 in the multiple-dose study and at doses of 7.5 mg and higher in the single-dose study. The increase in ANP observed on day 1 (1.4- to 5.1-fold higher than baseline values) produced by 10–125 mg of omapatrilat was greater than the increase (up to 2.2-fold) produced by 200 mg of the NEP inhibitor candoxatril [34]. Urinary cGMP concentrations increased in a similar manner (1.25- to 1.5-fold higher than baseline values). The concomitant rise in plasma cGMP concentrations (1.25–1.80 times baseline) may imply a systemic effect of plasma ANP and/or other vasoactive peptides, such as bradykinin, whose actions are also potentiated by NEP inhibitors [35]. Although the present study failed to show a clear increase in plasma ANP concentrations, another larger study demonstrated that 40 mg of omapatrilat increased plasma concentrations of ANP by 50–107% at 2 h after dosing [36].
Potent ACE inhibition (> 90%) was seen at omapatrilat doses as low as 2.5 mg and as early as 2 h postdose. The inhibitory effect on ACE activity was sustained over 24 h following a single dose and after multiple daily doses. Concomitantly with the inhibition of ACE activity, PRA increased at 4–24 h after dosing. Importantly, there was a sustained elevation in PRA at 24 h after 10 days of dosing. Also consistent with the inhibition of ACE, a decrease in 24-h urinary excretion of aldosterone was observed after higher doses of omapatrilat.
ANP infusion characteristically induces natriuresis and diuresis [37, 38], effects observed with NEP inhibitors such as candoxatril [39, 40]. However, ACE inhibitors such as enalapril are known to have no or very mild acute natriuretic or diuretic effects [41]. Interestingly, the natriuresis and diuresis induced by the pure NEP inhibitor candoxatril can be attenuated by coadministration of the ACE inhibitor enalapril [42]. Enalapril may produce this effect as a function of its action to reduce angiotensin II production. Basal angiotensin II normally promotes Na+ reabsorption in the proximal tubule and ANP induces natriuresis by inhibiting this effect of angiotensin II. In the absence of angiotensin II, the natriuretic effect of ANP may be attenuated [42].
In both the single- and multiple-dose studies, the acute (0–4 h) and 24-h data indicated that omapatrilat did not increase urinary excretion of sodium or potassium or alter urinary volume in subjects who were stabilized on fixed diets (6 g sodium and 4 g potassium) before and during the study. In addition, omapatrilat had no diuretic, natriuretic, kaliuretic, or additive effect in salt-sensitive hypertensive patients [5] or when administered concomitantly with frusemide 20 mg to healthy young subjects [43]. The basis underlying the absence of natriuresis, kaliuresis, or diuresis by a VPI deserves further investigation.
Omapatrilat was generally well tolerated in both the single and multiple dose studies. No subject discontinued from active treatment because of an AE in the single dose study. However, in the multiple-dose study four subjects withdrew from the highest dose (125 mg) group due to dose-limiting side-effects (lethargy, headache and dizziness). At the lower doses (from 10 mg up to and including 75 mg) omapatrilat was well tolerated. One incident of lip swelling was noted in the 75 mg group. However, this patient did not withdraw from the study. This safety profile is similar to that noted in other clinical studies of omapatrilat [5, 6, 44, 45].
Due to the small sample size of each dose group, there was no attempt to perform any modelling of the pharmacokinetic and the various types of pharmacodynamic data. In addition, later studies of the pharmacokinetics of omapatrilat have demonstrated that omapatrilat is largely circulating in its disulphide forms, which are potentially reducible and may be a reservoir of omapatrilat in plasma and/or tissues [46]. In the current study, only the sulphydryl form of omapatrilat was measured.
In summary omapatrilat was found to be rapidly absorbed with a long effective half-life over a large dose range. The drug exhibited potent ACE and NEP inhibition and did not demonstrate significant natriuretic or diuretic effects. Omapatrilat demonstrated consistent BP lowering in healthy subjects and was generally well tolerated. These data support a once-a-day regimen with omapatrilat for the treatment of hypertension.
Acknowledgments
We acknowledge Floyd Bierle, PhD, and Jeanine Bode, clinical data handling and administrative assistance; Patricia Catanzariti and James Manning, PhD, clinical laboratory tests; Susan Lubin and Jane Blue, Clinical Pharmacology Unit, Nursing and Business Administration; Susan McNulty, RD, nutrition support; Kathy Davis, MS, and Alan Meier, MS, data management.
References
- 1.Burnett JC., Jr Vasopeptidase inhibition: a new concept in blood pressure management. J Hypertens. 1999;17(Suppl 1):S37–S43. [PubMed] [Google Scholar]
- 2.Erdös EG, Skidgel RA. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989;3:145–151. [PubMed] [Google Scholar]
- 3.Trippodo NC, Robl JA, Asaad MM, Fox M, Panchal BC, Schaeffer TR. Effects of omapatrilat in low, normal, and high renin experimental hypertension. Am J Hypertens. 1998;11(3 part 1):363–372. doi: 10.1016/s0895-7061(97)00404-4. [DOI] [PubMed] [Google Scholar]
- 4.Black HR, Chang PI, Reeves RA, Cooper W, Pouleur H. Monotherapy treatment success rate of omapatrilat, a vasopeptidase inhibitor, compared with lisinopril and amlodipine in mild to moderate hypertension [abstract] Am J Hypertens. 1999;12(4 Part 2):26A. [Google Scholar]
- 5.Campese VM, Lasseter KC, Ferrario CM, et al. Omapatrilat versus lisinopril. Efficacy and neurohormonal profile in salt-sensitive hypertensive patients. Hypertension. 2001;38:1342–1348. doi: 10.1161/hy1201.096569. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GF, Izzo JL, Jr, Lacourcière Y, et al. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the Conduit Hemodynamics of Omapatrilat International Research study. Circulation. 2002;105:2955–2961. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
- 7.Jemal M, Hawthorne DJ. Quantitative determination of BMS186716, a thiol compound, in dog plasma by high-performance liquid chromatography–positive ion electrospray mass spectrometry after formation of the methyl acrylate adduct. J Chromatogr B Biomed Sci Appl. 1997;693:109–116. doi: 10.1016/s0378-4347(97)00044-3. [DOI] [PubMed] [Google Scholar]
- 8.Massien C, Azizi M, Guyene T-T, Vesterqvist O, Mangold B, Ménard J. Pharmacodynamic effects of dual neutral endopeptidase–angiotensin-converting enzyme inhibition versus angiotensin-converting enzyme inhibition in humans. Clin Pharmacol Ther. 1999;65:448–459. doi: 10.1016/S0009-9236(99)70140-2. [DOI] [PubMed] [Google Scholar]
- 9.Schulz-Knappe P, Forssmann K, Herbst F, Hock D, Pipkorn R, Forssmann WG. Isolation and structural analysis of ‘urodilatin’, a new peptide of the cardiodilatin-(ANP) -family, extracted from human urine. Klin Wochenschr. 1988;66:752–759. doi: 10.1007/BF01726570. [DOI] [PubMed] [Google Scholar]
- 10.Solc J, Bauer K, Timnik A, et al. Combination of high-performance liquid chromatography and radioimmunoassay for the measurement of urodilatin and α-hANP in the urine of healthy males. Life Sci. 1991;48:2451–2456. doi: 10.1016/0024-3205(91)90380-t. [DOI] [PubMed] [Google Scholar]
- 11.Gagelmann M, Hock D, Forssmann W-G. Urodilatin (CDD/ANP-95–126) is not biologically inactivated by a peptidase from dog kidney cortex membranes in contrast to atrial natriuretic peptide/cardiodilatin (α-hANP/CDD-99–126) FEBS Lett. 1988;233:249–254. doi: 10.1016/0014-5793(88)80436-8. [DOI] [PubMed] [Google Scholar]
- 12.Fieller EC. Some problems in interval estimation. J Royal Statist Soc. 1954;16:175–185. [Google Scholar]
- 13.Corti R, Burnett JC, Jr, Rouleau JL, Ruschitzka F, Lüscher TF. Vasopeptidase inhibitors. A new therapeutic concept in cardiovascular disease? Circulation. 2001;104:1856–1862. doi: 10.1161/hc4001.097191. [DOI] [PubMed] [Google Scholar]
- 14.Nathisuwan S, Talbert RL. A review of vasopeptidase inhibitors: a new modality in the treatment of hypertension and chronic heart failure. Pharmacotherapy. 2002;22:27–42. doi: 10.1592/phco.22.1.27.33502. [DOI] [PubMed] [Google Scholar]
- 15.Mombouli J-V, Vanhoutte PM. Kinins and endothelial control of vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1995;35:679–705. doi: 10.1146/annurev.pa.35.040195.003335. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa H, Hirata Y, Kakoki M, et al. Role of nitric oxide-cGMP pathway in adrenomedullin-induced vasodilation in the rat. Hypertension. 1999;33:689–693. doi: 10.1161/01.hyp.33.2.689. [DOI] [PubMed] [Google Scholar]
- 17.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 18.Melo LG, Pang SC, Ackermann U. Atrial natriuretic peptide: regulator of chronic arterial blood pressure. News Physiol Sci. 2000;15:143–149. doi: 10.1152/physiologyonline.2000.15.3.143. [DOI] [PubMed] [Google Scholar]
- 19.Anand-Srivastava MB. Atrial natriuretic peptide-C receptor and membrane signalling in hypertension. J Hypertens. 1997;15:815–826. doi: 10.1097/00004872-199715080-00004. [DOI] [PubMed] [Google Scholar]
- 20.Gauquelin G, Schiffrin EL, Garcia R. Downregulation of glomerular and vascular atrial natriuretic factor receptor subtypes by angiotensin II. J Hypertens. 1991;9:1151–1160. [PubMed] [Google Scholar]
- 21.Anastasopoulos F, Leung R, Kladis A, et al. Marked difference between angiotensin-converting enzyme and neutral endopeptidase inhibition in vivo by a dual inhibitor of both enzymes. J Pharmacol Exp Ther. 1998;284:799–805. [PubMed] [Google Scholar]
- 22.Chatelain RE, Ghai RD, Trapani AJ, et al. Antihypertensive and natriuretic effects of CGS 30440, a dual inhibitor of angiotensin-converting enzyme and neutral endopeptidase 24.11. J Pharmacol Exp Ther. 1998;284:974–982. [PubMed] [Google Scholar]
- 23.French JF, Flynn GA, Giroux EL, et al. Characterization of a dual inhibitor of angiotensin I-converting enzyme and neutral endopeptidase. J Pharmacol Exp Ther. 1994;268:180–186. [PubMed] [Google Scholar]
- 24.Hubner RA, Kubota E, Casley DJ, Johnston CI, Burrell LM. In-vitro and in-vivo inhibition of rat neutral endopeptidase and angiotensin converting enzyme with the vasopeptidase inhibitor gemopatrilat. J Hypertens. 2001;19:941–946. doi: 10.1097/00004872-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Kähönen M, Ylitalo R, Kööbi T, Turjanmaa V, Ylitalo P. Influence of captopril, propranolol, and verapamil on arterial pulse wave velocity and other cardiovascular parameters in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36:483–489. [PubMed] [Google Scholar]
- 26.Erb KA, Essig J, Breithaupt K, Belz GG. Clinical pharmacodynamic studies with cilazapril and a combination of cilazapril and propranolol. Drugs. 1991;41(Suppl 1):11–17. doi: 10.2165/00003495-199100411-00004. [DOI] [PubMed] [Google Scholar]
- 27.Richards M, Espiner E, Frampton C, et al. Inhibition of endopeptidase EC 24.11 in humans. Renal and endocrine effects. Hypertension. 1990;16:269–276. doi: 10.1161/01.hyp.16.3.269. [DOI] [PubMed] [Google Scholar]
- 28.Burnier M, Ganslmayer M, Perret F, et al. Effects of SCH 34826, an orally active inhibitor of atrial natriuretic peptide degradation, in healthy volunteers. Clin Pharmacol Ther. 1991;50:181–191. doi: 10.1038/clpt.1991.123. [DOI] [PubMed] [Google Scholar]
- 29.Tamburini PP, Koehn JA, Gilligan JP, et al. Rat vascular tissue contains a neutral endopeptidase capable of degrading atrial natriuretic peptide. J Pharmacol Exp Ther. 1989;251:956–961. [PubMed] [Google Scholar]
- 30.Gafford JT, Skidgel RA, Erdös EG, Hersh LB. Human kidney ‘enkephalinase’, a neutral metalloendopeptidase that cleaves active peptides. Biochemistry. 1983;22:3265–3271. doi: 10.1021/bi00282a035. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez W, Soleilhac JM, Fournie-Zaluski MC, Roques BP, Michel JB. Characterization of neutral endopeptidase in vascular cells, modulation of vasoactive peptide levels. Eur J Pharmacol. 1998;345:323–331. doi: 10.1016/s0014-2999(98)00038-7. [DOI] [PubMed] [Google Scholar]
- 32.Raut R, Rouleau J-L, Blais CJ, et al. Bradykinin metabolism in the postinfarcted rat heart: role of ACE and neutral endopeptidase 24.11. Am J Physiol. 1999;276(5 Part 2):H1769–H1779. doi: 10.1152/ajpheart.1999.276.5.H1769. [DOI] [PubMed] [Google Scholar]
- 33.Abassi ZA, Tate J, Hunsberger S, Klein H, Trachewsky D, Keiser HR. Pharmacokinetics of ANF and urodilatin during cANF receptor blockade and neutral endopeptidase inhibition. Am J Physiol. 1992;263:E870–E876. doi: 10.1152/ajpendo.1992.263.5.E870. [DOI] [PubMed] [Google Scholar]
- 34.Richards AM, Wittert GA, Crozier IG, et al. Chronic inhibition of endopeptidase 24.11 in essential hypertension: evidence for enhanced atrial natriuretic peptide and angiotensin II. J Hypertens. 1993;11:407–416. doi: 10.1097/00004872-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Seymour AA, Sheldon JH, Smith PL, Asaad M, Rogers WL. Potentiation of the renal responses to bradykinin by inhibition of neutral endopeptidase 3.4.24.11 and angiotensin-converting enzyme in anesthetized dogs. J Pharmacol Exp Ther. 1994;269:263–270. [PubMed] [Google Scholar]
- 36.Liao W, Vesterqvist O, Manning J, Jr, et al. Effects of age and gender on the pharmacodynamics of omapatrilat in healthy volunteers. Am J Geriatr Cardiol. 2001;10:50–54. doi: 10.1111/j.1076-7460.2001.90856.x. [DOI] [PubMed] [Google Scholar]
- 37.Morice A, Pepke-Zaba J, Loysen E, et al. Low dose infusion of atrial natriuretic peptide causes salt and water excretion in normal man. Clin Sci. 1988;74:359–363. doi: 10.1042/cs0740359. [DOI] [PubMed] [Google Scholar]
- 38.Solomon LR, Atherton JC, Bobinski H, Hillier V, Green R. Effect of low dose infusion of atrial natriuretic peptide on renal function in man. Clin Sci. 1988;75:403–410. doi: 10.1042/cs0750403. [DOI] [PubMed] [Google Scholar]
- 39.Singer DR, Markandu ND, Buckley MG, Miller MA, Sagnella GA, MacGregor GA. Dietary sodium and inhibition of neutral endopeptidase 24.11 in essential hypertension. Hypertension. 1991;18:798–804. doi: 10.1161/01.hyp.18.6.798. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt F, Martinez F, Ikeni A, et al. Acute renal effects of neutral endopeptidase inhibition in humans. Am J Physiol. 1994;267(1 Part 2):F20–F27. doi: 10.1152/ajprenal.1994.267.1.F20. [DOI] [PubMed] [Google Scholar]
- 41.Hodsman GP, Zabludowski JR, Zoccali C, et al. Enalapril (MK421) and its lysine analogue (MK521): a comparison of acute and chronic effects on blood pressure, renin-angiotensin system and sodium excretion in normal man. Br J Clin Pharmacol. 1984;17:233–241. doi: 10.1111/j.1365-2125.1984.tb02337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motwani JG, Lang CC, Cramb G, Struthers AD. Natriuretic response to neutral endopeptidase inhibition is blunted by enalapril in healthy men. Hypertension. 1995;25(4 Part 1):637–642. doi: 10.1161/01.hyp.25.4.637. [DOI] [PubMed] [Google Scholar]
- 43.Uderman H, Vesterqvist O, Manning J, Ferreira I, Delaney C, Liao WC. Omapatrilat: neurohormonal and pharmacodynamic profile when administered with furosemide. J Clin Pharmacol. 2001;41:1291–1300. doi: 10.1177/00912700122012878. [DOI] [PubMed] [Google Scholar]
- 44.Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure. The omapatrilat versus enalapril randomized trial of utility in reducing events (OVERTURE) Circulation. 2002;106:920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 45.Coats A. Omapatrilat—the story of Overture and Octave. Int J Cardiol. 2002;86:1. doi: 10.1016/s0167-5273(02)00389-3. [DOI] [PubMed] [Google Scholar]
- 46.O'Grady P, Vesterqvist O, Malhotra B, et al. Omapatrilat in patients with hepatic cirrhosis. Pharmacodynamics and pharmacokinetics. Eur J Clin Pharmacol. 2001;57:249–257. doi: 10.1007/s002280100291. [DOI] [PubMed] [Google Scholar]