Br J Clin Pharmacol 2003; 56 (1); 46–56.
A population pharmacokinetic model for paclitaxel in the presence of a novel P-gp modulator, Zosuquidar (LY335979)
S. Callies, D. P de Alwis, A. Harris, P. Vasey, J. H. Beijnen, J. H. Schellens, M. Burgess & L. Aarons
P. 52. The publisher regrets that Table 3 was printed incorrectly. The correct Table is printed below.
Table 3.
Paclitaxel pharmacokinetic parameters from the basic and covariate (categorical and continous) population pharmacokinetic models.
| Basic model | Categorical relationship (final model) | Continuous relationship | |
|---|---|---|---|
| OBJF | 8727.003 | 8703.350 | 8680.895 |
| Parameters (value (SE%)) | |||
| CL changing with time* | |||
| Slope (θ2) (lh−2) | 9.35 (7.32) | 10.0 (7.92) | 9.66 (6.95) |
| Min CL (θ1) (lh−1) | 7.64 (12.2) | 8.48 (12.9) | 8.59 (29.9) |
| t50 (θ4) (h) | 8.76 (16.4) | 9.36 (28.2) | 7.27 (102) |
| γ1 (θ3) | 2.94 (23.5) | 2.68 (30.2) | 2.12 (95.3) |
| Effect of zosuquidar on paclitaxel CL | |||
| Decrease with LY Cmax>350(µgl−1) (%) | – | 25.2 (12.4) | – |
| Emax (lh−1) | – | – | 5.49 (43.5) |
| LY Cmax50 (µgl−1) | – | – | 328 (15.4) |
| γ2 | – | – | 9.18 (129) |
| V1 (l) | 7.93 (14.0) | 7.95 (13.8) | 8.38 (13.0) |
| V2 (l) | 198 (7.78) | 196 (7.81) | 194 (16.0) |
| Q2 (lh−1) | 11.1 (7.37) | 10.8 (9.35) | 11.2 (11.3) |
| Q3 (lh−1) | 6.57 (15.8) | 6.76 (16.4) | 6.35 (39.4) |
| V3(l) | 7.00 (15.4) | 7.51 (18.9) | 10.2 (164) |
| ω CL (%) | 27.2 (33.2) | 25.9 (29.7) | 24.8 (34.3) |
| ω CL-Q2 (%) | 32.6 (23.4) | 30.5 (23.2) | 29.6 (24.7) |
| ω Q2 (%) | 44.5 (28.0) | 43.7 (26.1) | 43.5 (37.0) |
| ω CL-V2 (%) | 29.3 (30.7) | 26.1 (38.4) | 24.2 (39.5) |
| ω Q2-V2 (%) | 40.7 (29.9) | 39.6 (29.2) | 37.5 (35.1) |
| ωV2 (%) | 43.7 (26.6) | 42.8 (26.0) | 40.9 (31.4) |
| ωV1 (%) | 38.5 (58.6) | 40.0 (52.4) | 41.7 (58.6) |
| ω IOV CL (%) | 20.9 (33.2) | 15.2 (53.9) | 16.1 (56.2) |
| ω IOV V1 (%) | 57.5 (39.6) | 54.5 (42.8) | 46.6 (47.9) |
| Residual variance (%) | 22.7 (7.75) | 22.9 (7.77) | 22.5 (8.00) |
During the infusion *postinfusion 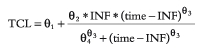 with INF the length of the infusion and time the time from the start of the infusion.
with INF the length of the infusion and time the time from the start of the infusion.


