Abstract
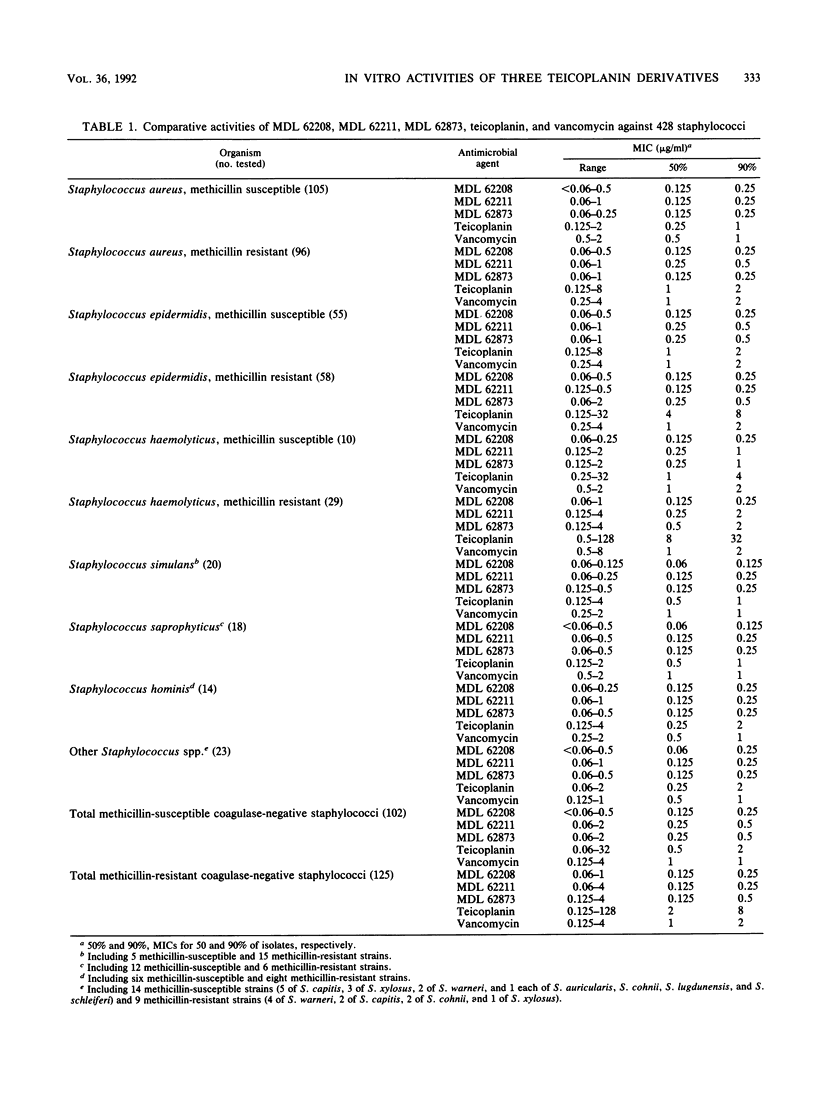
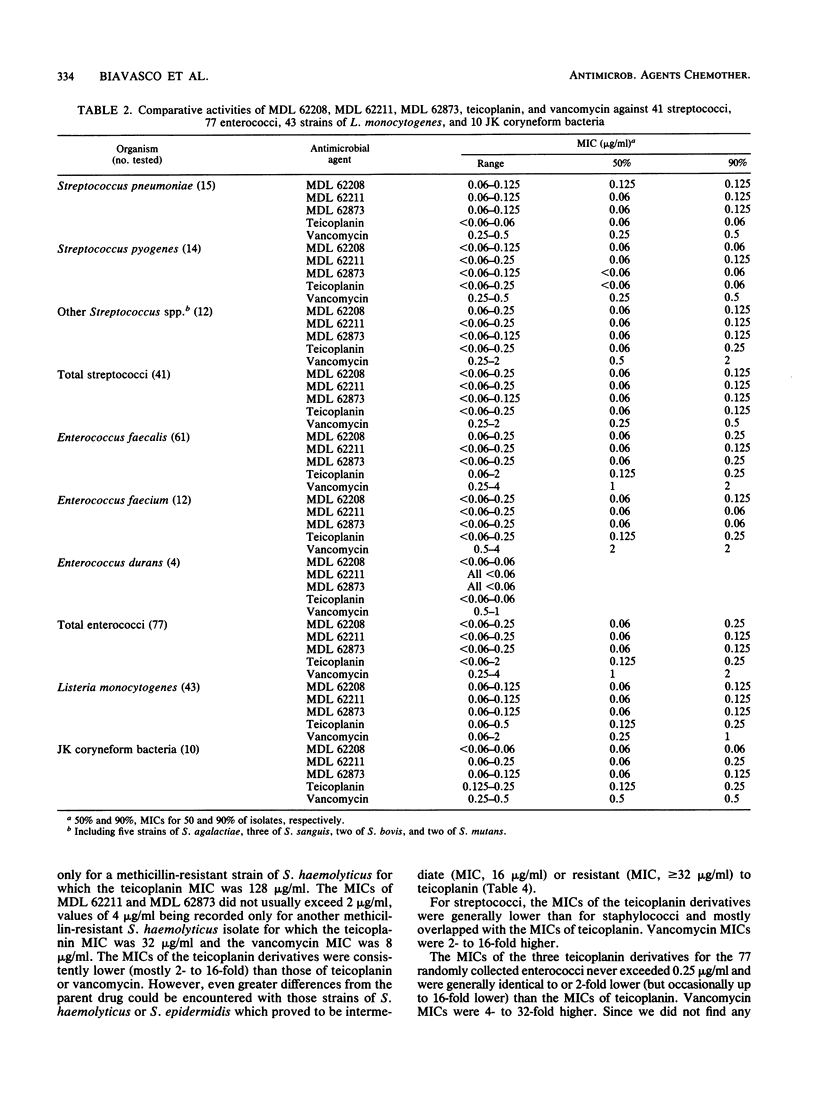
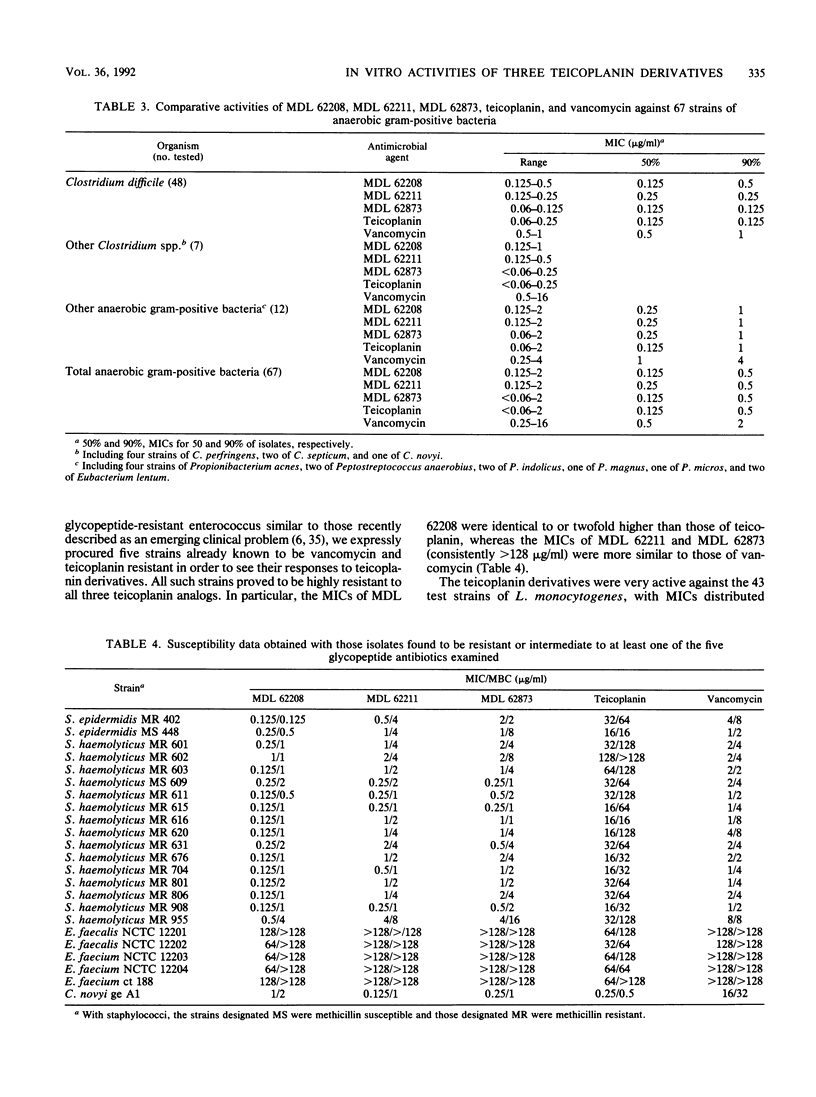
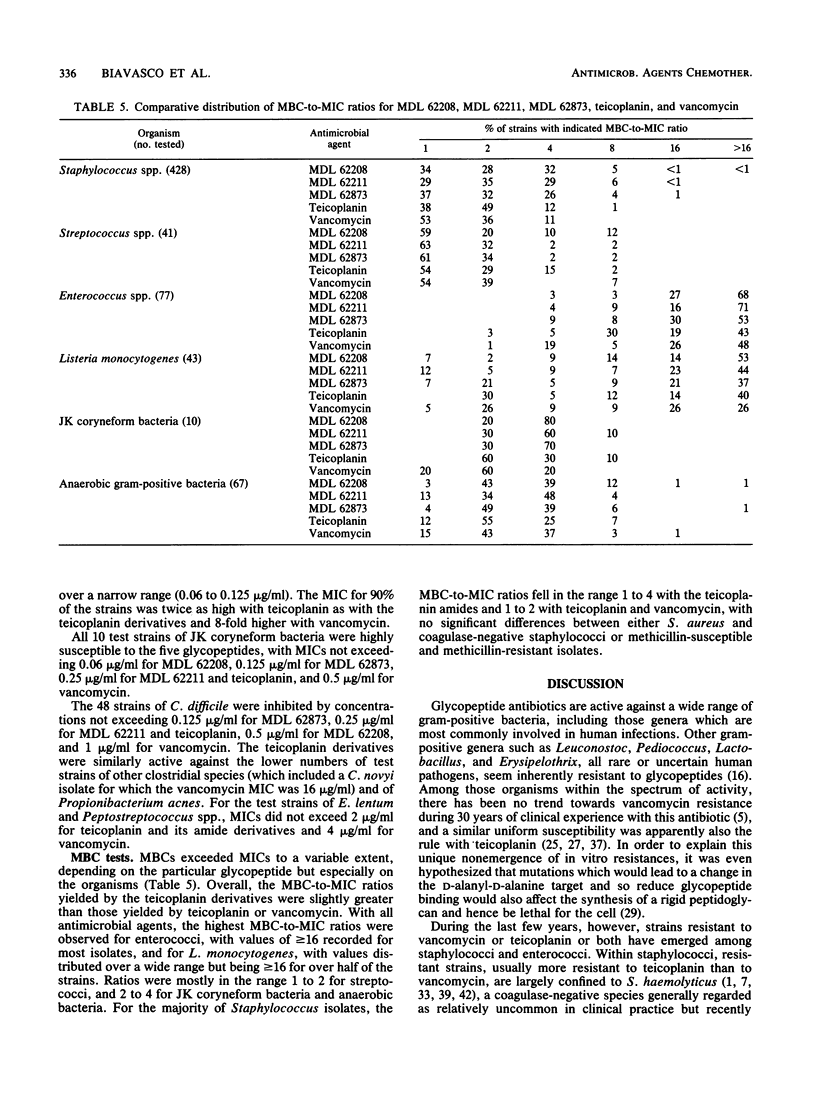
MDL 62208, MDL 62211, and MDL 62873 are three semisynthetic amide derivatives of teicoplanin (MDL 62208 is an amide of teicoplanin aglycone, MDL 62211 is an amide of the teicoplanin A2 complex, and MDL 62873 is the corresponding derivative of peak A2-2 of the complex). The three semisynthetic glycopeptides were evaluated for in vitro antibacterial activity in comparison with the parent drug (teicoplanin) and vancomycin. A variety of gram-positive bacteria of clinical origin, whose species were carefully determined and that included 428 staphylococci (207 methicillin susceptible and 221 methicillin resistant), 41 streptococci, 82 enterococci, 43 strains of Listeria monocytogenes, 10 JK coryneform bacteria, and 67 anaerobes belonging to the genera Clostridium, Propionibacterium, Peptostreptococcus, and Eubacterium, were tested. The only resistances to MDL 62208, MDL 62211, and MDL 62873 were encountered with vancomycin- and teicoplanin-resistant enterococci. All of the other test strains, including some teicoplanin-resistant coagulase-negative staphylococci of the species Staphylococcus haemolyticus and Staphylococcus epidermidis, were highly susceptible to the three teicoplanin amides. Only minor differences in activity were observed among MDL 62208, MDL 62211, and MDL 62873, whereas the three experimental compounds were usually found to be more potent than teicoplanin or vancomycin (especially against staphylococci, with differences mostly ranging from 2- to 16-fold). The MBC-to-MIC ratios varied depending on the organisms, with the highest ratios usually observed for enterococci and listeriae. Overall, the MBC-to-MIC ratios yielded by the teicoplanin analogs were slightly greater than those yielded by teicoplanin or vancomycin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arioli V., Pallanza R. Teicoplanin-resistant coagulase-negative staphylococci. Lancet. 1987 Jan 3;1(8523):39–39. doi: 10.1016/s0140-6736(87)90724-0. [DOI] [PubMed] [Google Scholar]
- Bartoloni A., Colao M. G., Orsi A., Dei R., Giganti E., Parenti F. In-vitro activity of vancomycin, teicoplanin, daptomycin, ramoplanin, MDL 62873 and other agents against staphylococci, enterococci and Clostridium difficile. J Antimicrob Chemother. 1990 Nov;26(5):627–633. doi: 10.1093/jac/26.5.627. [DOI] [PubMed] [Google Scholar]
- Biavasco F., Giovanetti E., Montanari M. P., Lupidi R., Varaldo P. E. Development of in-vitro resistance to glycopeptide antibiotics: assessment in staphylococci of different species. J Antimicrob Chemother. 1991 Jan;27(1):71–79. doi: 10.1093/jac/27.1.71. [DOI] [PubMed] [Google Scholar]
- Biavasco F., Manso E., Varaldo P. E. In vitro activities of ramoplanin and four glycopeptide antibiotics against clinical isolates of Clostridium difficile. Antimicrob Agents Chemother. 1991 Jan;35(1):195–197. doi: 10.1128/aac.35.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene V. E., John J. F., Jr, Twitty J. A., Lewis J. W. Anti-staphylococcal activity of teicoplanin, vancomycin, and other antimicrobial agents: the significance of methicillin resistance. J Infect Dis. 1986 Aug;154(2):349–352. doi: 10.1093/infdis/154.2.349. [DOI] [PubMed] [Google Scholar]
- Dutka-Malen S., Leclercq R., Coutant V., Duval J., Courvalin P. Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in gram-positive bacteria. Antimicrob Agents Chemother. 1990 Oct;34(10):1875–1879. doi: 10.1128/aac.34.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froggatt J. W., Johnston J. L., Galetto D. W., Archer G. L. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1989 Apr;33(4):460–466. doi: 10.1128/aac.33.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B. P., Selva E., Gastaldo L., Berti M., Pallanza R., Ripamonti F., Ferrari P., Denaro M., Arioli V., Cassani G. A40926, a new glycopeptide antibiotic with anti-Neisseria activity. Antimicrob Agents Chemother. 1987 Dec;31(12):1961–1966. doi: 10.1128/aac.31.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein F. W., Coutrot A., Sieffer A., Acar J. F. Percentages and distributions of teicoplanin- and vancomycin-resistant strains among coagulase-negative staphylococci. Antimicrob Agents Chemother. 1990 May;34(5):899–900. doi: 10.1128/aac.34.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P. R., George R. C., Griffiths J. W. Mathematical modeling of antimicrobial susceptibility data of Staphylococcus haemolyticus for 11 antimicrobial agents, including three experimental glycopeptides and an experimental lipoglycopeptide. Antimicrob Agents Chemother. 1990 Sep;34(9):1769–1772. doi: 10.1128/aac.34.9.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Uttley A. H., Woodford N., George R. C. Resistance to vancomycin and teicoplanin: an emerging clinical problem. Clin Microbiol Rev. 1990 Jul;3(3):280–291. doi: 10.1128/cmr.3.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Goldstein F. W., Zhou X. Y. Activities of two new teicoplanin amide derivatives (MDL 62211 and MDL 62873) compared with activities of teicoplanin and vancomycin against 800 recent staphylococcal isolates from France and the United States. Antimicrob Agents Chemother. 1991 Mar;35(3):584–586. doi: 10.1128/aac.35.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Redding J. S., Maher L. A. Antibacterial activity of the new glycopeptide antibiotic SKF104662. Antimicrob Agents Chemother. 1989 Apr;33(4):560–561. doi: 10.1128/aac.33.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Gilligan P. H., Facklam R. R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988 Jun;26(6):1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Malabarba A., Trani A., Strazzolini P., Cietto G., Ferrari P., Tarzia G., Pallanza R., Berti M. Synthesis and biological properties of N63-carboxamides of teicoplanin antibiotics. Structure-activity relationships. J Med Chem. 1989 Nov;32(11):2450–2460. doi: 10.1021/jm00131a007. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity of teichomycin compared with those of other antibiotics. Antimicrob Agents Chemother. 1983 Sep;24(3):425–428. doi: 10.1128/aac.24.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Cole C. T., Preston D. A., Schabel A. A., Nagarajan R. Activity of glycopeptides against vancomycin-resistant gram-positive bacteria. Antimicrob Agents Chemother. 1989 Sep;33(9):1477–1481. doi: 10.1128/aac.33.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanza R., Berti M., Goldstein B. P., Mapelli E., Randisi E., Scotti R., Arioli V. Teichomycin: in-vitro and in-vivo evaluation in comparison with other antibiotics. J Antimicrob Chemother. 1983 May;11(5):419–425. doi: 10.1093/jac/11.5.419. [DOI] [PubMed] [Google Scholar]
- Perkins H. R. Vancomycin and related antibiotics. Pharmacol Ther. 1982;16(2):181–197. doi: 10.1016/0163-7258(82)90053-5. [DOI] [PubMed] [Google Scholar]
- Rocourt J., Schrettenbrunner A., Seeliger H. P. Différenciation biochimique des groupes génomiques de Listeria monocytogenes (sensu lato). Ann Microbiol (Paris) 1983 Jan-Feb;134A(1):65–71. [PubMed] [Google Scholar]
- Rolston K. V., Nguyen H., Messer M. In vitro activity of LY264826, a new glycopeptide antibiotic, against gram-positive bacteria isolated from patients with cancer. Antimicrob Agents Chemother. 1990 Nov;34(11):2137–2141. doi: 10.1128/aac.34.11.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbe R. S., Ritz W. J., Verma P. R., Barranco E. A., Gilligan P. H. Selection for vancomycin resistance in clinical isolates of Staphylococcus haemolyticus. J Infect Dis. 1990 Jan;161(1):45–51. doi: 10.1093/infdis/161.1.45. [DOI] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Al-Obeid S., Shlaes J. H., Williamson R. Activity of various glycopeptides against an inducibly vancomycin-resistant strain of Enterococcus faecium (D366). J Infect Dis. 1989 Jun;159(6):1132–1135. doi: 10.1093/infdis/159.6.1132. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Binczewski B. Enterococcal resistance to vancomycin and related cyclic glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):106–110. doi: 10.1007/BF01963634. [DOI] [PubMed] [Google Scholar]
- Uttley A. H., Collins C. H., Naidoo J., George R. C. Vancomycin-resistant enterococci. Lancet. 1988 Jan 2;1(8575-6):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- Varaldo P. E., Debbia E., Schito G. C. In vitro activity of teichomycin and vancomycin alone and in combination with rifampin. Antimicrob Agents Chemother. 1983 Mar;23(3):402–406. doi: 10.1128/aac.23.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veach L. A., Pfaller M. A., Barrett M., Koontz F. P., Wenzel R. P. Vancomycin resistance in Staphylococcus haemolyticus causing colonization and bloodstream infection. J Clin Microbiol. 1990 Sep;28(9):2064–2068. doi: 10.1128/jcm.28.9.2064-2068.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODLEY D. W., HALL W. H. The treatment of severe staphylococcal infections with vancomycin. Ann Intern Med. 1961 Aug;55:235–249. doi: 10.7326/0003-4819-55-2-235. [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C. In-vitro induction of resistance in coagulase-negative staphylococci to vancomycin and teicoplanin. J Antimicrob Chemother. 1988 Sep;22(3):321–324. doi: 10.1093/jac/22.3.321. [DOI] [PubMed] [Google Scholar]
- Williams A. H., Grüneberg R. N. Teicoplanin revisited. J Antimicrob Chemother. 1988 Oct;22(4):397–401. doi: 10.1093/jac/22.4.397. [DOI] [PubMed] [Google Scholar]
- Wilson A. P., O'Hare M. D., Felmingham D., Grüneberg R. N. Teicoplanin-resistant coagulase-negative staphylococcus. Lancet. 1986 Oct 25;2(8513):973–973. doi: 10.1016/s0140-6736(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Wise R., Donovan I. A., McNulty C. A., Waldron R., Andrews J. M. Teicoplanin, its pharmacokinetics, blister and peritoneal fluid penetration. J Hosp Infect. 1986 Mar;7 (Suppl A):47–55. doi: 10.1016/0195-6701(86)90007-1. [DOI] [PubMed] [Google Scholar]


