Abstract
Aims
To investigate pharmacokinetics of the enantiomers of citalopram (CT) and its metabolites desmethylcitalopram (DCT) and didesmethylcitalopram (DDCT) in Swedish healthy volunteers in relation to CYP2C19 and CYP2D6 geno- and phenotypes.
Methods
Racemic CT was given for seven days to panels with different genotypes and the following mephenytoin (Me) and debrisoquine (De) hydroxylation phenotypes: EMDe/EMMe, PMDe/EMMe, EMDe/PMMe (n = 6 in all groups), and one PMDe/PMMe subject. Blood sampling was carried out during day 7, and all urine was collected for 12 h after the last dose of CT.
Results
The AUC of S-CT was significantly higher in the EMDe/PMMe panel compared to the EMDe/EMMe and PMDe/EMMe panels (P < 0.05), whereas the AUC of R-CT did not differ between the panels. Similar differences, although they did not reach statistical significance, were noted for S-DCT and R-DCT. The enantiomers of DDCT were not quantifiable in PMDe, and there was no difference in DDCT enantiomer concentrations between the other two panels. A PMDe/PMMe subject stopped taking CT after five days due to severe adverse effects. Based on two time points, this subject had a very long CT half-life of 95 h. The value of 1.0 for the S/R ratio of the CT trough in this subject was similar to the mean S/R CT trough ratio of the EMDe/PMMe panel, but higher than the S/R CT ratio of the EMDe/EMMe panel (0.56; 95% CI 0.49–0.63) and the PMDe/EMMe panel (0.44; 95% CI 0.31–0.57). Thus the latter two phenotypes eliminated S-CT more rapidly via CYP2C19. An adverse effect described as an ‘alcohol hangover’ feeling was reported by one subject from each of the three panels. These individuals had the highest concentrations of both CT enantiomers.
Conclusions
The AUC of S-, but not R-(CT) was found to be significantly higher in PM of mephenytoin compared to EMs, PMs may need a lower dosage of CT.
Keywords: adverse effect, antidepressant, citalopram, CYP2C19, CYP2D6, enantiomers, genotype, mania, mephenytoin, metabolism, metabolic ratio, metabolites, pharmacokinetics, phenotype, stereoisomerism, Swedes, yawning
Introduction
Citalopram (CT) is a selective inhibitor of neuronal serotonin reuptake causing increased serotonin neurotransmission, but with little effect on norepinephrine and dopamine reuptake [1]. CT is used for treatment of depression and dysthymia [2], and also to treat panic disorder [3], obsessive compulsive disorder [4], substance abuse [5], and dementia-related behavioural disturbances [6].
CT is a bicyclic phtalane derivate and is marketed as a racemate. There is no inversion betwen the S and R enantiomers and the S-enantiomer appears to mediate the major antidepressant effect [7]. Thus, it has been suggested that therapeutic monitoring of the S-enantiomer of CT is more useful for the establishment of a concentration/response relationship [8].
CT is eliminated from the body by N-demethylation to DCT, which is catalysed by CYP2C19 [9]. Further N-demethylation of DCT by CYP2D6 to DDCT occurs [10, 11]. The metabolites DCT and DDCT are not considered to have clinically relevant antidepressive effects [7]. CT is only a weak inhibitor of CYP2C19 both in vitro[12] and in vivo[9], and of CYP2D6 in vitro[13]. Some investigators have also suggested a role for CYP3A4 in the metabolism of CT [14].
Some studies have investigated the nonclinical metabolism of CT in panels of different CYP genotypes with measurement of total (i.e. nonchiral) CT concentrations [9]. Other studies have investigated the enantiomeric disposition of CT [8, 15, 16] but only in patients or healthy subjects with EM (extensive metaboliser) or unknown genotype. To our knowledge, no protocol has involved the metabolism of the enantiomers of CT in different CYP2C19 and CYP2D6 genotypes and phenotypes.
The aim of this study was to characterize the relationship between CYP2C19 and CYP2D6 genotype/phenotype and the pharmacokinetics of CT and its metabolites and their enantiomers, in white healthy volunteers.
Materials and Methods
Subjects and protocol
Nineteen unrelated white Swedes with previously determined CYP2C19 and CYP2D6 genotypes and phenotypes participated in the study. The genotypes were determined by PCR identification of the CYP2C19*2 and *3 and CYP2D6*3, *4 and *5 alleles. One of the subjects was a smoker and three used nicotine snuff. The subjects were divided into the following phenotypes groups: EMDe/EMMe = EM of both debrisoquine (De, the marker used for CYP2D6 activity) and mephenytoin (Me, the marker used for CYP2C19 activity) metabolism (n = 6); PMDe/EMMe = PM of debrisoquine and EM of mephenytoin (n = 6); EMDe/PMMe = EM of debrisoquine and PM of mephenytoin (n = 6). All EMs were homoygous for the wild-type allele. In addition, a single individual was a PM of both substrates, PMDe/PMMe. There were no significant differences between the panels regarding age, body weight, gender (Table 1), nicotine habits or daily caffeine intake. There was a complete concordance between geno- and phenotypes.
Table 1.
Characteristics (mean and range) of healthy Swedish volunteers divided into three panels, and a single PMDe/PMMe subject, according to debrisoquine and mephenytoin phenotypes*.
| EMDe/EMMe n = 6 | PMDe/EMMe n = 6 | EMDe/PMMe n = 6 | PMDe/PMMe n = 1 | |
|---|---|---|---|---|
| S/R mephenytoin | 0.24 (0.10–0.48) | 0.16 (0.06-.31) | 1.08 (1.04–1.15) | 1.09 |
| Debrisoquine MR | 0.55 (0.21–3.46) | 76 (55–110) | 0.30 (0.14–0.65) | 63 |
| Age, years | 27 (24–35) | 34 (24–44) | 31 (26–41) | 26 |
| Body weight, kg | 73 (55–98) | 73 (62–97) | 71 (57–81) | 89 |
| Gender, F/M | 3/3 | 2/4 | 2/4 | M |
There was complete concordance between CYP2C19 phenotype and CYP2D6 phenotype.
Before inclusion, a physician performed a complete physical examination including medical history, routine physical examination (heart, blood pressure, lungs, abdomen, lymph nodes, basic neurology, and ECG), blood chemistry (haemoglobin, SR, serum creatinine, transaminases, HIV, hepatitis B-C and pregnancy test) and urine screening for illegal drugs. The protocol stipulated that alcohol, drugs, grapefruit, and grapefruit juice should not be ingested for the duration of the study and the previous week. All volunteers gave informed written consent before their health check-up on the basis of verbal and written information. The study protocol was approved by the Human Ethics Committee at Karolinska Institutet, Huddinge University Hospital in Stockholm, Sweden.
Most subjects took 10 mg CT (Cipramil; Lundbeck, Valby, Copenhagen, Denmark) twice daily for seven days, until steady state was reached for. The single PMDe/PMMe subject took 10 mg CT daily for seven days.
The subjects attended the Human Laboratory, Division of Clinical Pharmacology, Huddinge University Hospital, on day 7, after taking CT for six days and then fasting overnight. After emptying the bladder, the subjects had a venous cannula inserted. A predose venous blood sample was taken and the last daily CT dose was given at 08.00 hours. The subjects drank a glass of water after swallowing the tablet. Standardized food was served during the day at 2, 6 and 8 h after the dose. Blood samples for pharmacokinetic analysis were drawn at 0, 2, 4, 6, 8, 10 and 12 h after the dose, into heparinized vacuum tubes (Vacutainer®, Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA; or Venoject®, Terumo Europe N.V., Leuven, Belgium). All urine was collected within 12 h after the dose, the volume was measured and 10-ml aliquots were retained. The blood samples were centrifuged and the plasma was separated. Plasma and urine samples were stored frozen at −20 °C until assayed.
Monitoring of adverse effects
Subjects filled out a diary at home during the study, noting the time of each dose, and any perceived side-effects. On day 7, the research nurse asked the volunteers standardized questions about adverse events.
Assay of total (S plus R) citalopram and metabolites
CT, DCT, DDCT and the S- and R-enantiomers of the three compounds were kindly provided by Lundbeck, Denmark. The internal standard ((–)-S)-bromo-N-[(1-n-propyl-2-pyrrolinidinyl)-methyl]-2,6,dimethoxybenzamide (FLA-913) was provided by AstraZeneca (Södertälje, Sweden). The compounds were dissolved in 0.01 m HCl and stored at 4 °C.
The total, achiral concentrations of CT, DCT and DDCT were determined by reversed phase HPLC and UV detection. Plasma or urine (1 ml), after addition of 50 µl 2 µm internal standard and 0.5 ml 1 m sodium hydroxide, was extracted with 3 ml diisopropylether for 10 min. After centrifugation for 5 min at 3000 g, the organic phase was transferred to a clean test tube containing 200 µl 25 mm acetic acid, and extracted for 5 min followed by centrifugation. The organic phase was removed and the remaining acid was washed with 0.5 ml n-heptane, and a 15-µl aliquot was injected onto the HPLC. The compounds were separated on a Zorbax SB C-18-column (75 × 4.6 mm, particle size 3.5 µm) [17]. The mobile phase consisted of acetonitrile and ammonium acetate 50 mm pH 6.5, in the proportion 29 : 71 (v/v). The flow rate was 1.0 ml min−1 and the detection wavelength was 239 nm. The retention times for the internal standard, DDCT, DCT and CT were 4.2, 6.8, 7.8, and 9.3 min, respectively. Standard curves were analysed in the concentration range 10–200 nm in plasma and 0.1–10 µm in urine. The interday coefficients of variation for CT, DCT and DDCT at a concentration of 10.5 nm were 13.4%, 10.1% and 9.4%, respectively. Those at 55 nm were 8.8%, 7.4% and 2.7%, respectively. The limit of quantification was 5 nm for all compounds.
Chiral assay of CT and its metabolites
Quantification of the enantiomers was performed with chiral HPLC in combination with the above-described method. The LC fractions containing CT and the two metabolites were manually collected after UV detection. The volume of each fraction was reduced to about 100 µl in a vacuum centrifuge (Speed Vac SC 110 A; Savant Instruments) before injection onto a chiral HPLC column as described by Rochat et al.[16]. The enantiomers were separated on a cyclobond I 2000 column (250 × 4.6 mm) [17]. The mobile phase consisted of 22% acetonitrile and 88% aqueous triethylamine buffer 1%, adjusted to pH 6.2 with acetic acid (v/v 22 : 78). The flow rate was 0.8 ml min−1 and the column was maintained at 30 °C. The detection wavelength was 239 nm. The concentrations of the separate S- and R-enantiomers were calculated from their chromatographic area ratios. The total, achiral concentration was determined previously. The accuracy was assessed by analysis of all compounds in the S/R concentration ratio range of 0.2–1.0 for CT, 0.6–1.2 for DCT, and 0.4–1.0 for DDCT. The limit of quantification was 1.5 nm for each enantiomer.
Statistical and pharmacokinetic methods
All statistical tests were carried out using the computer program STATISTICA version 5.5 (StatSoft Inc, Tulsa, OK, USA). Differences between the three groups were calculated using the Kruskal–Wallis anova ranks test and normally distributed pharmacokinetic parameters were compared using the paired t-test. Difference of frequencies was calculated with the two-tailed Fisher's exact test. Correlation coefficients were calculated using the Spearman rank order test.
CT, DCT and DDCT pharmacokinetics were analysed using noncompartmental techniques. The area under the plasma concentration vs time (0–12 h) curve, AUC0−12, was calculated using the trapezoidal rule. Recovery of the enantiomers of CT, DCT and DDCT in urine was calculated as the ratio between urinary molar amount of the measured substance (obtained from the concentration of 0–12 h postdose urine collection and the urine volume) and the molar amount of each CT enantiomer administered in the racemate given. Renal clearance was estimated from urinary recovery divided by plasma AUC. In the PMDe/PMMe subject, the terminal half-life was calculated as ln2/Ke, where Ke is (lnC1 lnC−2)/(T1–T2), C1 is the first concentration measured, C2 is the second, and T1 and T2 are the corresponding time-points.
Calculations of estimated frequencies of the PMDe/PMMe phenotype in different populations are based upon the frequency of PM CYP2C19 of 3% and 20% and CYP2D6 of 7% and 1%, in Europeans and Asians [18], respectively, and analogous calculation in Tanzanians are based upon the frequencies PM CYP2C19 7.5% [19] and PM CYP2D6 7% [20].
Results
The single subject with a PMDe/PMMe phenotype was given half the dose of CT that the other 18 subjects received. However, on day 2 this subject developed diarrhoea with abdominal cramps, accompanied on day 3 by restlessness and pronounced yawning every 2–5 min, and by malaise and a feeling of unreality on day 5. The subject withdrew from the study and recovered uneventfully. This subject had an estimated CT half-life of 95 h based on two plasma concentration measurements. In this subject the S/R CT ratio was 1.0 at both sampling times. The corresponding mean ± s.d. S/R-CT ratios immediately predose were 0.56 ± 0.07, 0.71 ± 0.30 and 0.99 ± 0.03, for the EMDe/EMMe, PMDe/EMMe and EMDe/PMMe panels, respectively.
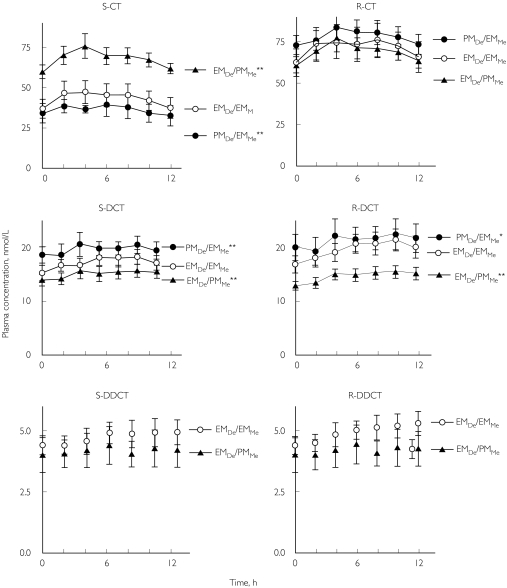
All subjects in panels EMDe/EMMe, PMDe/EMMe, and EMDe/PMMe completed the study. CT concentrations showed only minor variation during a dosage interval for both enantiomers and its metabolites within each panel, except for R-DCT and R-DDCT that showed a slight increasing tendency (Figure 1). Cmax of CT was most often attained 2 or 4 h after the last tablet intake in all three panels. There were significant differences between the panels in AUC of S-CT (Kruskal–Wallis anovaP = 0.02). There was no significant difference between groups in AUC S-DCT, but a strong trend for R-DCT with P = 0.05 (Table 2).
Figure 1.
Plasma concentrations of enantiomers of citalopram (CT) and its metabolites desmethylcitalopram (DCT) and didesmethylcitalopram (DDCT) in the panels EMDe/EMMe, PMDe/EMMe, and EMDe/PMMe during a dosage interval (0–12 h) after racemic CT had been given for seven days. AUC statistical difference is marked, indicating paired t-test significance levels of *P < 0.05 and **P < 0.01 compared with the panel EMDe/EMMe. Total concentrations of DDCT (S- plus R-DDCT) were below the limit of quantification in panel PMDe/EMMe.
Table 2.
AUC (mean ± s.d.) of the enantiomers of citalopram (CT) and its metabolites desmethylcitalopram (DCT) and didesmethylcitalopram (DDCT) during steady-state of citalopram (racemic citalopram 20 mg daily for 7 days) in healthy Swedish volunteers divided into the three different panels (n = 6 in each panel).
| AUC, nmol h−1l−1 | S-CT | R-CT | S-DCT | R-DCT | S-DDCT | R-DDCT |
|---|---|---|---|---|---|---|
| EMDe/EMMe | 530 ± 183 | 868 ± 249 | 208 ± 37 | 233 ± 46 | 37 ± 8 | 59 ± 10 |
| PMDe/EMMe | 451 ± 155 | 941 ± 158 | 237 ± 43 | 251 ± 66 | NQ | NQ |
| EMDe/PMMe | 830 ± 128 | 839 ± 145 | 182 ± 33 | 172 ± 26 | 34 ± 9 | 50 ± 18 |
| Kruskal–Wallis anova, P | 0.02 | 0.61 | 0.11 | 0.05 | – | – |
NQ, Not quantifiable.
As shown in Table 2, mean AUC of (S) CT was significantly higher for panel EMDe/PMMe compared to panels EMDe/EMMe (P < 0.01) and PMDe/EMMe (P < 0.01). The mean AUC difference between EMDe/EMMe and the two other panels was 340 nmol*h/L (95% CI 186-495). (R) CT was not significantly different between the panels with mean difference between EMDe/PMMe and PMDe/EMMe of 70 nmol*h/L (95% CI-91-230), and 29 nmol*h/L (95% CI-121-180) between EMDe/EMMeand PMDe/EMMe was and 102 (95% CI-150-359) between PMDe/EMMeand EMDe/PMMe. In contrast to (S) CT, the AUC of (S) DCT was lower in panel EMDe/PMMe with mean difference 29 nmol*h/L (95% CI 6-52) (P < 0.01) and higher in panel PMDe/EMMe with mean difference 27 nmol*h/L (95% CI-43- 97) (P < 0.01) compared to panel EMDe/EMMe. The panels differed for (R) DCT in a similar way as for (S) DCT. The enantiomers of DDCT could be quantified in the two panels with EMDe (Figure 1). All subjects of panel PMDe/EMMe had detectable levels of DDCT, however below the limit of quantification of 5 nm of the sum of the two enantiomers.
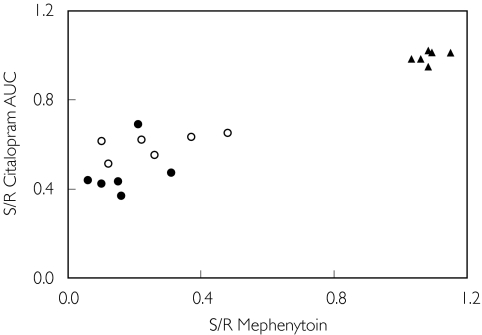
The plasma S/R-CT AUC ratio was significantly correlated with the urinary S/R-mephenytoin ratio in the 18 subjects of all three panels (rs = 0.85; P < 0.01) (Figure 2). However, when CYP2C19 PMs were excluded, the rs value was ony 0.49 (Figure 2).
Figure 2.
Correlation between plasma S/R AUC 0–12 h citalopram (CT) ratio and urinary S/R mephenytoin 0–8 h ratio in 18 subjects who were previously phenotyped with mephenytoin in a screening programme and who now received racemic CT 20 mg daily for 7 days until steady-state (Spearman r = 0.85; Spearman r2 = 0.72; P < 0.01). EMDe/EMMe (○); PMDe/EMMe (•); and EMDe/PMMe (▴).
There were no differences between the three panels regarding the amount of CT or DCT excreted, with the single exception of R-DCT (P = 0.05) (Table 3). Differences between groups for DDCT could not be estimated since the concentrations could not be quantified in the urine samples from the PMDe/EMMe panel. Total recovery of CT, DCT plus DDCT in urine was about 35% of the administered dose in all subjects. The mean renal clearances of S-CT and R-CT were about 4 l h−1, and did not vary significantly between the panels. The apparent renal clearances of the metabolites DCT and DDCT were higher than those of CT, and did not differ between the panels.
Table 3.
Urinary recovery (Ae) and renal clerance (ClRen) (mean ± s.d.) of enantiomers of citalopram (CT) and its metabolites desmethylcitalopram (DCT) and didesmethylcitalopram (DDCT) in 18 healthy Swedish volunteers divided into three panels (n = 6 in each panel). Racemic CT was given until steady-state concentration, 10 mg twice daily for 7 days. Urine was collected during a dose interval (0–12 h) after the last dose. NQ, Not quantifiable.
| Total | ClRen, l h−1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ae,% dose | S-CT | R-CT | S-DCT | R-DCT | S-DDCT | R-DDCT | Ae% | S-CT | R-CT | S-DCT | R-DCT | S-DDCT | R-DDCT |
| EMDe/EMMe | 7.2 ± 3.3 | 12.9 ± 5.1 | 6.7 ± 1.6 | 6.7 ± 1.6 | 1.0 ± 0.6 | 2.3 ± 1.3 | 36.8 ± 1.7 | 4.3 ± 1.6 | 4.7 ± 1.6 | 11.1 ± 4.3 | 9.8 ± 3.7 | 10.8 ± 4.0 | 15.5 ± 5.8 |
| PMDe/EMMe | 5.8 ± 1.7 | 14.0 ± 4.6 | 7.1 ± 1.9 | 7.2 ± 2.4 | NQ | NQ | 34.1 ± 1.9 | 4.1 ± 1.3 | 4.7 ± 1.7 | 9.6 ± 2.3 | 9.3 ± 2.3 | NQ | NQ |
| EMDe/PMMe | 10.4 ± 3.5 | 11.7 ± 3.8 | 4.5 ± 1.1 | 4.1 ± 1.1 | 0.9 ± 0.4 | 1.8 ± 0.7 | 33.4 ± 1.8 | 3.9 ± 1.3 | 4.3 ± 1.4 | 8.1 ± 2.4 | 8.1 ± 2.4 | 9.4 ± 3.9 | 12.1 ± 4.4 |
| Kruskal–Wallis | 0.07 | 0.51 | 0.06 | 0.05 | – | – | – | 0.51 | 1.0 | 0.13 | 0.14 | – | – |
| anova, P | |||||||||||||
The following adverse events were reported: nausea (6 subjects), diarrhoea (4), dry mouth (4), fatigue (3), headache (3), yawning (3), and constipation (1). Four subjects did not report any adverse events. The only serious event was that reported by the single PMDe/PMMe subject. Interestingly, three individuals reported a feeling similar to an alcohol hangover. Two of these (one from the PMDe/EMMe panel and one from the EMDe/PMMe panel) also reported frequent yawning.
Discussion
Based on blood sampling at two time-points, the PMDe/PMMe subject had a CT terminal half-life of 95 h. This is longer than the median half-lives of 37 h in healthy subjects (n = 12), 50 h in patients with significant renal impairment (n = 7), and 83 h in patients with liver cirrhosis (n = 9) [21]. Since the S/R-CT ratio was foud to be 1.0 in the two blood samples from this subject, the two enantiomers were eliminated at the same rate. In this regard this PMDe/PMMe subject was similar to individuals in the EMDe/PMMe panel who lacked CYP2C19. EMMe showed stereospecific elimination of CT by CYP2C19. Although the PMDe/PMMe geno- and phenotype is rare (approximately two per thousand in a European or Asian population and five per thousand in Tanzanians), clinicians should be aware that these individuals may need a lower dose of CT.
After 7 days, predose concentrations of CT were similar at time points 0 and 12 h for all subjects, reflecting steady state conditions. For DCT and DDCT, there was a tendency for a slight increase in trough values after 12 h in the majority of subjects, and thus steady-state conditions had probably not quite been achieved.The plasma concentrations of DCT were higher than those of DDCT, as reported previously [22].
The higher plasma concentrations of S-CT and lower concentrations of S-DCT PMMe indicate that the N-demethylation of S-CT is catalysed by CYP2C19. However, the difference in the AUC of S-DCT between the two phenotypes was smaller than the difference in the AUC of S-CT. This discrepancy may be due to inhibition of further CYP2D6 metabolism of DCT by high concentrations of CT in the PMMe panel. CT is a known CYP2D6 inhibitor with a Ki of 5.1 µm in vitro. However, racemic CT was used in the latter study, and it is not known whether the enantiomers differ in their inhibitory potency. As the plasma concentrations of R-DCT were low in EMDe/PMMe, R-CT also might be partly N-demethylated by CYP2C19, despite the fact that there was no difference in R-CT between the panels (Figure 1). The PMDe panel had higher concentrations of both S-DCT and R-DCT compared to the other panels. In addition, S- and R-DDCT were not detected in the PMDe/EMMe panel. This indicates that both enantiomers of DCT are partially N-demethylated by CYP2D6. This finding confirms previous studies, that the first N-demethylation of CT is catalysed mainly by CYP2C19 [9], and the second mainly by CYP2D6 [10, 11]. Furthermore, CYP3A4 also contributes to the N-demethylation of CT [15, 23].
The AUC S/R ratio of CT correlated significantly with the S/R ratio of mephenytoin, suggesting that the S-enantiomers, but not the R-enantiomers of CT are metabolized by CYP2C19.
Urinary recovery or renal clearance of CT and its metabolites did not differ significantly between the phenotyped panels. Total recovery was about 35%, which is similar to the results of other studies [15, 21, 24]. There were no differences between the panels regarding renal clearance.
Three individuals from three different panels reported hangover feeling. Two of these also reported frequent yawning, which is mediated via oxytocinergic neurones in the hypothalamus and other neuronal systems with acetylcholine or serotoninergic receptors [25]. One study has shown increased yawning in young rats treated with CT [23]. The three subjects with a hangover feeling had the highest concentrations of the two CT enantiomers (Fisher's test, P < 0.001). This might indicate a possible clinical correlation. Speculatively, this may be related to the occurrence of the mania recently associated with high-dose SSRI treatment [26, 27].
In conclusion, PMs of mephenytoin developed significantly higher plasma concentrations of S-CT than EMs, and thus may require a smaller dose to achieve the same efficacy, since S-CT is the active enantiomer. CYP2C19 appears to be the main enzyme contributing to the metabolism of S-CT, and to a very small extent, R-CT.
We acknowledge the participation of the volunteers in the study and the cooperative work of RN Katarina Andersson, RN Eva Götharson, RN Anneli Wahlberg, Dr Per Dalén, Dr Ayman Al-Shurbaji, Ms Lilleba Bohman and Ms Ulla Pettersson. This study was supported by grants from Otto Swärd's Foundation and Swedish Medical Research Council (3902).
References
- 1.Hyttel J. Neurochemical characterization of a new potent and selective serotonin uptake inhibitor: Lu 10–171. Psychopharmacology (Berl) 1977;51:225–233. doi: 10.1007/BF00431629. [DOI] [PubMed] [Google Scholar]
- 2.Milne RJ, Goa KL. Citalopram. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depressive illness. Drugs. 1991;41:450–477. doi: 10.2165/00003495-199141030-00008. [DOI] [PubMed] [Google Scholar]
- 3.Humble M, Wistedt B. Serotonin, panic disorder and agoraphobia: short-term and long-term efficacy of citalopram in panic disorders. Int Clin Psychopharmacol. 1992;6(Suppl 5):21–39. [PubMed] [Google Scholar]
- 4.Masand PS, Gupta S. Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry. 1999;7:69–84. [PubMed] [Google Scholar]
- 5.Naranjo CA, Poulos CX, Bremner KE, Lanctot KL. Citalopram decreases desirability, liking, and consumption of alcohol in alcohol-dependent drinkers. Clin Pharmacol Ther. 1992;51:729–739. doi: 10.1038/clpt.1992.85. [DOI] [PubMed] [Google Scholar]
- 6.Pollock BG, Mulsant BH. Behavioral disturbances of dementia. J Geriatr Psychiatry Neurol. 1998;11:206–212. doi: 10.1177/089198879901100407. [DOI] [PubMed] [Google Scholar]
- 7.Hyttel J, Bogeso KP, Perregaard J, Sanchez C. The pharmacological effect of citalopram residues in the S-(+) -enantiomer. J Neural Transmission–General Section. 1992;88:157–160. doi: 10.1007/BF01244820. [DOI] [PubMed] [Google Scholar]
- 8.Foglia JP, Pollock BG, Kirshner MA, Rosen J, Sweet R, Mulsant B. Plasma levels of citalopram enantiomers and metabolites in elderly patients. Psychopharmacol Bull. 1997;33:109–112. [PubMed] [Google Scholar]
- 9.Sindrup SH, Brosen K, Hansen MG, Aaes-Jorgensen T, Overo KF, Gram LF. Pharmacokinetics of citalopram in relation to the sparteine and the mephenytoin oxidation polymorphisms. Ther Drug Monit. 1993;15:11–17. doi: 10.1097/00007691-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Jeppesen U, Gram LF, Vistisen K, Loft S, Poulsen HE, Brosen K. Dose-dependent inhibition of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol. 1996;51:73–78. doi: 10.1007/s002280050163. [DOI] [PubMed] [Google Scholar]
- 11.Gram LF, Hansen MG, Sindrup SH, et al. Citalopram: interaction studies with levomepromazine, imipramine, and lithium. Ther Drug Monit. 1993;15:18–24. [PubMed] [Google Scholar]
- 12.Kobayashi K, Yamamoto T, Chiba K, Tani M, Ishizaki T, Kuroiwa Y. The effects of selective serotonin reuptake inhibitors and their metabolites on S-mephenytoin 4′-hydroxylase activity in human liver microsomes. Br J Clin Pharmacol. 1995;40:481–485. doi: 10.1111/j.1365-2125.1995.tb05793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crewe HK, Lennard MS, Tucker GT, Woods FR, Haddock RE. The effect of selective serotonin re-uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol. 1992;34:262–265. doi: 10.1111/j.1365-2125.1992.tb04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Moltke LL, Greenblatt DJ, Grassi JM, et al. Citalopram and desmethylcitalopram in vitro: human cytochromes mediating transformation, and cytochrome inhibitory effects. Biol Psychiatry. 1999;46:839–849. doi: 10.1016/s0006-3223(98)00353-9. [DOI] [PubMed] [Google Scholar]
- 15.Sidhu J, Priskorn M, Poulsen M, Segonzac A, Grollier G, Larsen F. Steady-state pharmacokinetics of the enantiomers of citalopram and its metabolites in humans. Chirality. 1997;9:686–692. doi: 10.1002/(SICI)1520-636X(1997)9:7<686::AID-CHIR9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Rochat B, Amey M, Baumann P. Analysis of enantiomers of citalopram and its demethylated metabolites in plasma of depressive patients using chiral reverse-phase liquid chromatography. Ther Drug Monit. 1995;17:273–279. doi: 10.1097/00007691-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Kosel M, Eap CB, Amey M, Baumann P. Analysis of the enantiomers of citalopram and its demethylated metabolites using chiral liquid chromatography. J Chromatogr B Biomed Sci Appl. 1998;719:234–238. doi: 10.1016/s0378-4347(98)00384-3. [DOI] [PubMed] [Google Scholar]
- 18.Bertilsson L, Lou YQ, Du YL, et al. Pronounced differences between native Chinese and Swedish populations in the polymorphic hydroxylations of debrisoquin and S-mephenytoin. Clin Pharmacol Ther. 1992;51:388–397. doi: 10.1038/clpt.1992.38. [published erratum appears in Clin Pharmacol Ther 1994 June; 55: 648] [DOI] [PubMed] [Google Scholar]
- 19.Herrlin K, Massele AY, Jande M, et al. Bantu Tanzanians have a decreased capacity to metabolize omeprazole and mephenytoin in relation to their CYP2C19 genotype. Clin Pharmacol Ther. 1998;64:391–401. doi: 10.1016/S0009-9236(98)90070-4. [DOI] [PubMed] [Google Scholar]
- 20.Wennerholm A, Johansson I, Massele AY, et al. Decreased capacity for debrisoquine metabolism among black Tanzanians: analyses of the CYP2D6 genotype and phenotype. Pharmacogenetics. 1999;9:707–714. [PubMed] [Google Scholar]
- 21.Joffe P, Larsen FS, Pedersen V, Ring-Larsen H, Aaes-Jorgensen T, Sidhu J. Single-dose pharmacokinetics of citalopram in patients with moderate renal insufficiency or hepatic cirrhosis compared with healthy subjects. Eur J Clin Pharmacol. 1998;54:237–242. doi: 10.1007/s002280050452. [DOI] [PubMed] [Google Scholar]
- 22.Fredricson Overo K. Kinetics of citalopram in man; plasma levels in patients. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:311–318. doi: 10.1016/s0278-5846(82)80181-4. [DOI] [PubMed] [Google Scholar]
- 23.Urba-Holmgren R, Holmgren B, Leon BA, Ugarte A. Age-dependent changes in serotonergic modulation of yawning in the rat. Pharmacol Biochem Behav. 1992;43:483–486. doi: 10.1016/0091-3057(92)90180-n. [DOI] [PubMed] [Google Scholar]
- 24.Oyehaug E, Ostensen ET, Salvesen B. High-performance liquid chromatographic determination of citalopram and four of its metabolites in plasma and urine samples from psychiatric patients. J Chromatogr. 1984;308:199–208. [PubMed] [Google Scholar]
- 25.Argiolas A, Melis MR. The neuropharmacology of yawning. Eur J Pharmacol. 1998;343:1–16. doi: 10.1016/s0014-2999(97)01538-0. [DOI] [PubMed] [Google Scholar]
- 26.Vesely C, Fischer P, Goessler R, Kasper S. Mania associated with serotonin selective reuptake inhibitors. J Clin Psychiatry. 1997;58:88. doi: 10.4088/jcp.v58n0206e. [letter] [DOI] [PubMed] [Google Scholar]
- 27.Bryois C, Ferrero F. Mania induced by citalopram. Arch General Psychiatry. 1994;51:664–665. doi: 10.1001/archpsyc.1994.03950080076011. [letter] [DOI] [PubMed] [Google Scholar]