Abstract
Aims
Inhalers combining long acting β2-adrenoceptor agonists (LABA) and corticosteroids (ICS) are indicated at Step 3 of current asthma guidelines. We evaluated the relative effects of LABA + ICS combination vs ICS alone on pulmonary function, bronchoprotection, acute salbutamol recovery following methacholine bronchial challenge, and surrogate inflammatory markers in patients with moderate persistent asthma.
Methods
Twenty-nine patients with mean FEV1 (± SEM) of 78 ± 3% predicted completed a randomized, double-blind, double-dummy, cross-over study. Patients received either 4 weeks of budesonide 400 µg + formoterol 12 µg (BUD + FM) combination twice daily followed by 1 week of BUD 400 µg alone twice daily, or 4 weeks of fluticasone propionate 250 µg + salmeterol 50 µg (FP + SM) combination twice daily followed by 1 week of FP 250 µg alone twice daily. Measurements were made at baseline and following each randomized treatment.
Results
FEV1 increase from pretreatment baseline as mean (± SEM) % predicted was significantly higher (P < 0.05) for BUD + FM (8 ± 1%) vs BUD (2 ± 1%), and for FP + SM (8 ± 1%) vs FP (2 ± 1%). The fall in FEV1 following methacholine challenge as percentage change from prechallenge baseline FEV1 was not significantly different in all four groups; BUD + FM (22 ± 1%), BUD (24 ± 1%), FP + SM (23 ± 1%) and FP (23 ± 1%). Salbutamol recovery over 30 min following methacholine challenge as area under curve (AUC %.min) was significantly blunted (P < 0.05) with BUD + FM (486.7 ± 35.5) vs BUD (281.1 ± 52.8), and with FP + SM (553.1 ± 34.1) vs FP (368.3 ± 46.7). There were no significant differences between respective combination inhalers or between respective ICS alone. Decreases in exhaled nitric oxide (NO) and serum eosinophilic cationic protein (ECP) from baseline were not significantly different between treatments.
Conclusions
Combination inhalers improve pulmonary function without potentiating anti-inflammatory effects on exhaled NO and serum ECP as compared with ICS alone, but delay acute salbutamol recovery after bronchoconstriction.
Keywords: asthma, budesonide, combination inhalers, fluticasone, formoterol, inflammation, methacholine challenge, pulmonary function, recovery, salbutamol, salmeterol
Introduction
Current guidelines [1–3] recommend the use of long-acting β2-adrenoceptor agonists (LABA) such as formoterol (FM) and salmeterol (SM) in conjunction with inhaled corticosteroids (ICS) to improve asthma control at Step 3 in moderate to severe persistent asthma [4–6]. The functional antagonism attributed by LABA against bronchoconstrictor stimuli (so called ‘bronchoprotection’) may have a role in stabilization of airway smooth muscle and may be a possible explanation for their effects in reducing exacerbations, as they do not exhibit any meaningful anti-inflammatory activity [7, 8]. We have previously shown that both FM 12 µg twice daily and SM 50 µg twice daily as add on therapy to ICS in mild to moderate asthmatics resulted in significant residual trough protection amounting to less than one doubling dose [9]. The effects of FM and SM as add on therapy in more severe patients are currently unknown, where differences in their β2-adrenoceptor intrinsic activity may result in differences in their relative bronchoprotection. The pros and cons for using LABA at Step 3 have been extensively reviewed elsewhere [10].
Subsensitivity to the bronchoprotective effects of LABA have been demonstrated irrespective of treatment with ICS [11–14]. Indeed even administering a single 1600 µg bolus of salbutamol (eight times the usual dose) does not overcome this bronchoprotective tolerance induced by LABA [15]. Moreover other studies have shown that the acute response to salbutamol may be blunted in patients treated with LABA and ICS [11, 16, 17, 18, 19]. Inhalers combining LABA and ICS are currently advocated in asthma therapy in view of data showing additive effects of LABA and ICS on asthma control which were as effective as increasing the dose of ICS [4, 5, 20].
We therefore aimed to evaluate the relative effects of FM and SM in combination inhalers as compared with ICS alone on pulmonary function, bronchoprotection, salbutamol recovery following methacholine challenge, and surrogate inflammatory markers, in patients with moderate persistent asthma.
Methods
Patients
Eligible patients had stable moderate persistent asthma [1] for at least 3 months prior to the study and none had received a course of oral corticosteroids or antibiotics during this period. All were nonsmokers and were receiving inhaled short-acting β2-adrenoceptor agonists for symptomatic relief at least once daily and ICS therapy such as beclomethasone dipropionate (BDP) (n = 21), budesonide (BUD) (n = 4) and fluticasone propionate (FP) (n = 4), either alone or in combination with second line controller therapy such as LABA (n = 9), theophylline (n = 3) or a leukotriene receptor antagonist (n = 3). All patients were required to exhibit hyper-responsiveness to methacholine on bronchial challenge testing with a provocative dose causing a 20% reduction from baseline FEV1 (PD20) of less than 500 µg. All gave informed consent and the Tayside Committee on Medical Research Ethics approved the study.
Study design (Figure 1)
Figure 1.
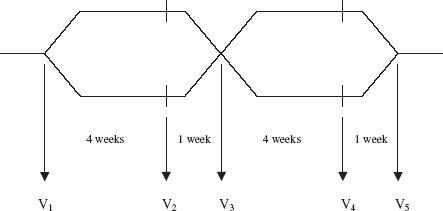
Study flow diagram. Patients had baseline measurements on the first study visit (V1) on their usual ICS therapy and were then randomized to receive either BUD + FM for 4 weeks followed by the same dose of BUD for 1 week, or FP + SM for 4 weeks followed by the same dose of FP for 1 week. Patients then crossed over to the other randomized treatment arm. Measurements were taken at V1– V5
The study was conducted in a randomized, double-blind, double-dummy, cross-over fashion. There was an initial step down period for patients who were receiving more than 800 µg of ICS daily (n = 5), which was halved once every 2 weeks until a maintenance dose of no more than 800 µg day−1 was achieved. Patients receiving second line controller therapy (n = 11) had them stopped during a 1-week period prior to the study.
Upon recruitment, patients had their usual asthma therapy stopped for the remainder of the study period and were given study medications instead. Patients were issued with a standard salbutamol 100 µg per actuation dry powder inhaler which had a dose counter (Asmasal Clickhaler®, Celltech Pharmaceuticals Ltd, Slough, UK) as their rescue therapy during the study and were randomized to receive either 4 weeks of inhaled combination of BUD 200 µg + FM 6 µg (Symbicort 200/6 Turbohaler®, AstraZeneca UK Ltd, Luton, UK) two puffs twice daily followed by 1 week of BUD 200 µg (Pulmicort 200 Turbohaler®, AstraZeneca UK Ltd, Luton, UK) two puffs twice daily or 4 weeks of inhaled combination of FP 250 µg + SM 50 µg (Seretide 250 Accuhaler®, Allen and Hanburys Ltd, Uxbridge, UK) one puff twice daily followed by 1 week of FP 250 µg (Flixotide 250 Accuhaler®, Allen and Hanburys Ltd, Uxbridge, UK) one puff twice daily. The dose of ICS as FP in Flixotide® or Seretide® was chosen to be equipotent to that of BUD in Pulmicort® or Symbicort®. With each active Turbohaler®, patients also received a placebo Accuhaler® and vice versa. All active and placebo Turbohaler® and Accuhaler® devices were identical in external physical appearance.
Measurements
Measurements were made at baseline and following each randomized treatment with patients having taken the last dose of study medication 12 h prior to attending the laboratory, i.e. all measurements were made at trough at the end of the dosing interval.
Nitric oxide
Measurement of exhaled breath nitric oxide (NO) was performed as described by Kharitonov et al.[21] using an integrated LR2000® clinical real-time NO gas analyser (Logan Research, Rochester, UK) with a flow rate of 250 ml min−1 and an accuracy of two parts per billion (p.p.b.) NO with a response time of 2 s. The normal exhaled NO cut-off value in our laboratory is less than 6 p.p.b. for nonatopic nonasthmatic subjects (i.e. < 2 SD from mean).
Spirometry
Spirometry was performed according to the American Thoracic Society criteria [22] using a Vitalograph® compact spirometer (Vitalograph Ltd, Buckingham, UK) with a computer assisted pneumotachograph head and pressure transducer. The spirometer was calibrated daily with a Vitalograph® 1 l precision syringe.
Methacholine bronchial challenge
Methacholine bronchial challenge was performed using a standardized computer assisted dosimetric method as previously described by Beach et al.[23]. In brief, methacholine was administered at 5 min intervals in doubling cumulative doses from 3.125 µg to 6400 µg until a 20% reduction in FEV1 was recorded.
Salbutamol recovery
Immediately following methacholine bronchial challenge, patients received inhaled salbutamol 200 µg (Ventolin 200 Accuhaler®, Allen & Hanburys Ltd, Uxbridge, UK) and then measurements of FEV1 were recorded at 5 min intervals for 30 min.
Serum eosinophilic cationic protein
Patients had blood samples taken for serum eosinophilic cationic protein (ECP) prior to any study procedures which were analysed using a radioimmunoassay kit (Pharmacia & Upjohn Diagnostics, Milton Keynes, UK) with an interassay coefficient of variability of 3.1%. The normal serum ECP cut-off value in our laboratory is less than 12 µg l−1 for nonatopic nonasthmatic subjects (i.e. < 2 SD from mean).
Peak flow and rescue diary
Patients kept a daily record of morning domiciliary peak expiratory flow (PEF) rates using a Mini-Wright® peak flow meter (Clement Clarke International Ltd, Harlow, UK) along with documentation of daily rescue inhaler use for the duration of the study.
Statistical analysis
The study was powered at 80% with alpha error set at 0.05 (2 tailed) and beta error of 0.2, in order to detect a 25% difference in salbutamol recovery (the primary outcome variable) between treatments, with a sample size of 24 patients in a crossover design. An overall analysis of variance and multiple range testing with Bonferroni correction were performed and a probability value of less than 0.05 (two tailed) considered significant. Comparisons were made between treatments as change from baseline. To normalize distribution, data for methacholine PD20 were logarithmically transformed, and analyses were performed using Statgraphics® statistical software package (STSC Software Publishing Group, Rockville, USA).
Results
Patients
Forty-six patients were initially screened for the study of whom 32 patients fulfilled the eligibility criteria for entry into the study. Following randomization, three patients dropped out due to personal reasons. Twenty-nine patients (16 females and 13 males) with mean age (± SEM) of 46 ± 3 years completed the study per protocol. The mean ICS dose at randomization was 538 ± 46 µg (BDP = 552 ± 41 µg, BUD = 525 ± 63 µg and FP = 475 ± 65 µg).
Pulmonary function (Figures 2 and 3)
Figure 2.
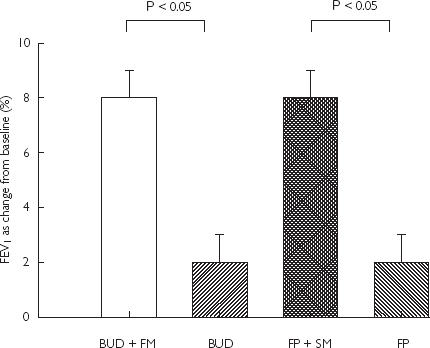
Mean FEV1 (% predicted) as change from baseline with SEM
Figure 3.
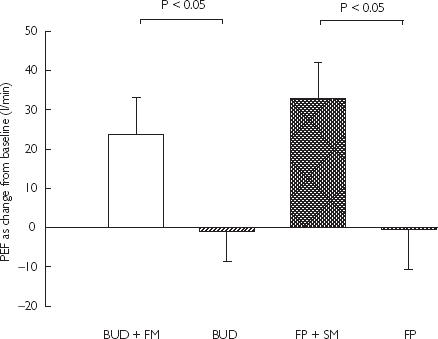
Mean PEF (l min−1) as change from baseline with SEM
Baseline spirometry at randomization showed mean FEV1 of 78 ± 3% predicted. FEV1 increase from baseline was significantly higher (P < 0.05) for BUD + FM combination (8 ± 1%) vs BUD alone (2 ± 1%): mean difference 6% (95% CI 3, 9%), and for FP + SM combination (8 ± 1%) vs FP alone (2 ± 1%): mean difference 6% (95% CI 2, 9%). Baseline mean PEF at randomization was 425 ± 20 l min−1. PEF comparisons as change from baseline were significant (P < 0.05) for FP + SM (33 ± 9 l min−1) vs FP (0 ± 10 l min−1): mean difference 33 l min−1 (95% CI 22, 44 l min−1), and for BUD + FM (24 ± 10 l min−1) vs BUD (−1 ± 8 l min−1): mean difference 24 l min−1 (95% CI 14, 35 l min−1). There were no significant differences in FEV1 and PEF for BUD + FM vs FP + SM, or BUD vs FP. Daily salbutamol rescue use (as puffs per day) was not significantly different for BUD + FM (0.4 ± 0.2) vs BUD (1.0 ± 0.3), or FP + SM (0.4 ± 0.2) vs FP (0.9 ± 0.3).
Bronchial hyper-responsiveness (Figure 4)
Figure 4.
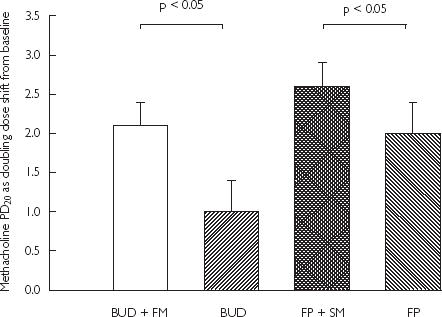
Mean change in methacholine PD20 as doubling dose shift from baseline with SEM
At randomization, patients had a geometric mean baseline methacholine PD20 of 39 ± 10 µg. Increase in methacholine PD20 from baseline (as doubling dose) was significantly different (P < 0.05) comparing BUD + FM (2.1 ± 0.3) vs BUD alone (1.0 ± 0.4): a 1.2 doubling dose difference, or between FP + SM (2.6 ± 0.3) vs FP alone (2.0 ± 0.4): a 0.5 doubling dose difference. There were no significant differences in methacholine PD20 comparing BUD + FM vs FP + SM, or between BUD vs FP.
Salbutamol recovery (Figure 5)
Figure 5.
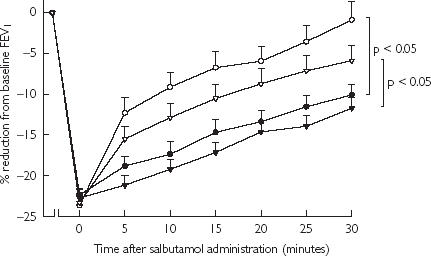
Time profile of salbutamol recovery following methacholine challenge as % mean reduction from baseline prechallenge FEV1 with SEM. BUD + FM (•); BUD (○); FP + SM (▾); and FP (▽).
The fall in FEV1 following methacholine challenge as percentage change from prechallenge baseline FEV1 were not significantly different in all four groups; BUD + FM (22 ± 1%), BUD (24 ± 1%), FP + SM (23 ± 1%) and FP (23 ± 1%). Subsequent salbutamol recovery over 30 min as area under curve (AUC %.min) was delayed significantly (P < 0.05) comparing BUD + FM (486.7 ± 35.5) vs BUD (281.1 ± 52.8), or between FP + SM (553.1 ± 34.1) vs FP (368.3 ± 46.7). There were no significant differences in recovery AUC comparing BUD + FM vs FP + SM, or between BUD vs FP.
Inflammatory markers
Baseline mean exhaled NO and serum ECP at randomization were 12.7 ± 2.8 p.p.b and 42.7 ± 5.5 µg l−1, respectively. Decreases in exhaled NO from baseline were not significant comparing BUD + FM (6.7 ± 2.3 p.p.b) vs BUD (7.5 ± 2.6 p.p.b), or between FP + SM (7.3 ± 2.5 p.p.b) vs FP (7.5 ± 2.5 p.p.b). Similarly, decreases in serum ECP from baseline were also not significant for BUD + FM (11.0 ± 2.8 µg l−1) vs BUD (12.0 ± 2.6 µg l−1), or FP + SM (8.0 ± 2.6 µg l−1) vs FP (7.4 ± 3.0 µg l−1). For exhaled NO and serum ECP, there were no significant differences between BUD + FM vs FP + SM, or BUD vs FP.
Discussion
Our results showed that each combination inhaler conferred additional bronchodilatation and bronchoprotection against methacholine compared with their respective ICS alone. Acute salbutamol recovery after methacholine-induced bronchoconstriction was significantly delayed with each combination inhaler compared with ICS alone. There was no evidence of any potentiation of anti-inflammatory activity on exhaled NO and serum ECP with combination inhalers vs ICS alone. Our design employed an initial 4 weeks of combination inhaler followed by withdrawal of LABA for the fifth week in order to compare 5 weeks duration of ICS vs 4 weeks of combination therapy.
The bronchodilator properties of LABA as seen with improvement in pulmonary function parameters of FEV1 and PEF in our patients are in keeping with previous studies with combination inhalers vs ICS alone [24–26]. In vitro pharmacological differences in β2-adrenoceptor intrinsic activity between FM and SM [27] were not translated to in vivo differences on their effects on bronchodilatation or bronchoprotection in our study. This is consistent with a previous study where there was no difference in bronchoprotection when adding in FM or SM to ICS [9], while down regulation of peripheral blood lymphocyte β2-adrenoceptors occurs to a comparable degree with both agonists [28].
Our results showed that LABA when taken in combination with ICS did not potentiate anti-inflammatory properties in terms of reduction in exhaled NO and serum ECP. It is important to consider whether we had achieved maximal effects in each of these parameters, in terms of there being further room for improvement with the combination inhalers vs their respective ICS alone. For exhaled NO, mean absolute levels had fallen to 5.2 p.p.b. with ICS alone, which is within the range for normal nonasthmatic subjects (i.e. < 6 p.p.b). We have previously reported a plateau response for exhaled NO with 400 µg daily of BUD, while for serum ECP, there was a dose–response effect up to 1600 µg daily in moderate persistent asthmatics [29]. Mean absolute values for serum ECP in our patients had fallen to 31 µg l−1 with ICS alone, which is considerably higher than normal values for nonatopic nonasthmatic subjects (i.e. < 12 µg l−1).
So it would seem that there was further room for suppression of serum ECP but not exhaled NO in our patients, had there been any potentiation of corticosteroid response with the combination inhalers. In retrospect, we should have perhaps included a comparator group where the dose of BUD and FP alone was doubled to 1600 µg and 1000 µg, respectively, to assess whether there was any further potential for anti-inflammatory suppression. Nonetheless the present data are in agreement with a previous study where we showed no potentiation of serum ECP suppression with FM 12 µg or 24 µg added to BUD 400 or 800 µg daily [30]. In vitro data have previously suggested that LABA such as FM or SM may potentiate ligand independent nuclear translocation of the cytosolic glucocorticoid receptor complex, although this also occurs with salbutamol, probably indicating a class effect of β2-adrenoceptor agonists per se[31, 32]. SM has also been shown to be an effective inhibitor of mediators released from human lung mast cells [33], in keeping with in vitro data showing LABA to influence cell activation, adhesion, chemotaxis and survival [34]. However, in vivo, for mast cell priming using adenosine monophosphate bronchial challenge, with chronic dosing of LABA in ICS treated patients, these effects are lost due to tolerance [13, 14]. We used methacholine bronchial challenge, which reflected the effects on airway smooth muscle. It has been shown with chronic dosing of terbutaline that tolerance was greater for adenosine monophosphate than methacholine bronchial challenge [35]. In vivo data in asthmatic patients have shown SM or FM to exhibit little or no clinical meaningful anti-inflammatory activity [7, 8, 14, 36–38]. Nevertheless, we acknowledge that the anti-inflammatory markers used in our study may not have been sufficient alone to assess properly the anti-inflammatory properties of LABA, and that biopsy studies will be required to evaluate this further.
Salbutamol recovery following acute bronchoconstriction was significantly delayed in patients receiving the combination inhalers. As can be seen from the minimal rescue consumption in our patients during the study, any subsensitivity response of salbutamol response can be attributed to effects of the LABA combination inhalers as such. The probable explanations for this phenomenon are due to LABA induced β2-adrenoceptor down regulation, induced β2-adrenoceptor uncoupling and associated desensitization, and prolonged β2-receptor occupancy, all of which have been shown in vivo[16, 39, 40, 41, 42]. This finding has clinical implications in that asthmatic patients who are on LABA + ICS combination may potentially have a delayed response to salbutamol during an acute episode of bronchoconstriction. Previous in vivo data in constricted airways have shown similar interaction between LABA and salbutamol [17, 19, 43], which is not overcome by a high dose (1600 µg) of the latter [15].
We observed a significantly higher PD20 after each combination inhaler vs the respective ICS alone, which may be explained by the residual effects of each LABA on airways smooth muscle, resulting in additional antagonism of methacholine-induced bronchoconstriction. Consequently the higher dose of methacholine administered in the presence of combination inhalers may itself have resulted in delayed recovery. However against this hypothesis would be the finding that the percentage fall in FEV1 following methacholine challenge was similar in all four groups, i.e. after each combination inhaler and respective ICS alone. If the delay in recovery was merely due to a higher administered dose of methacholine, one would perhaps expect an associated greater fall in FEV1 with the combination inhalers. Moreover, as this was a cross-over study, one would also anticipate that methacholine sensitivity per se to a given dose of ICS would not have changed appreciably.
In conclusion, our results show that combination inhalers improve pulmonary function compared with their respective ICS given alone, but they do not exhibit any potentiation of anti-inflammatory effects on exhaled NO and serum ECP. Furthermore combination inhalers delay recovery following salbutamol when used for relief of acute bronchoconstriction.
Acknowledgments
This study received no support from the pharmaceutical industry and was funded from a departmental research grant from the University of Dundee.
References
- 1.Expert Panel Report II. Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 1997. National Asthma Education and Prevention Program. [Google Scholar]
- 2.The British Guidelines on Asthma Management. 1995 review and position statement. Thorax. 1997;52(Suppl 1):S1–S21. [Google Scholar]
- 3.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the was adopted by the ATS Board of Directors November 1986. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 4.Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA) Br Med J. 2000;320:1368–1373. doi: 10.1136/bmj.320.7246.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauwels RA, Lofdahl CG, Postma DS, et al. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337:1405–1411. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 6.Matz J, Emmett A, Rickard K, Kalberg C. Addition of salmeterol to low-dose fluticasone versus higher-dose fluticasone: an analysis of asthma exacerbations. J Allergy Clin Immunol. 2001;107:783–789. doi: 10.1067/mai.2001.114709. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun WJ, Hinton KL, Kratzenberg JJ. The effect of salmeterol on markers of airway inflammation following segmental allergen challenge. Am J Respir Crit Care Med. 2001;163:881–886. doi: 10.1164/ajrccm.163.4.2001060. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus SC, Boushey HA, Fahy JV, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285:2583–2593. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 9.Lipworth BJ, Dempsey OJ, Aziz I. Functional antagonism with formoterol and salmeterol in asthmatic patients expressing the homozygous glycine-16 beta (2)-adrenoceptor polymorphism. Chest. 2000;118:321–328. doi: 10.1378/chest.118.2.321. [DOI] [PubMed] [Google Scholar]
- 10.Lipworth BJ. Antagonism of long-acting β2-adrenoceptor agonism. Br J Clin Pharmacol. 2002;54:231–245. doi: 10.1046/j.1365-2125.2002.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalra S, Swystun VA, Bhagat R, Cockcroft DW. Inhaled corticosteroids do not prevent the development of tolerance to the bronchoprotective effect of salmeterol. Chest. 1996;109:953–956. doi: 10.1378/chest.109.4.953. [DOI] [PubMed] [Google Scholar]
- 12.Lipworth B, Tan S, Devlin M, Aiken T, Baker R, Hendrick D. Effects of treatment with formoterol on bronchoprotection against methacholine. Am J Med. 1998;104:431–438. doi: 10.1016/s0002-9343(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 13.Aziz I, Tan KS, Hall IP, Devlin MM, Lipworth BJ. Subsensitivity to bronchoprotection against adenosine monophosphate challenge following regular once-daily formoterol. Eur Respir J. 1998;12:580–584. doi: 10.1183/09031936.98.12030580. [DOI] [PubMed] [Google Scholar]
- 14.Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. Evaluation of salmeterol or montelukast as second-line therapy for asthma not controlled with inhaled corticosteroids. Chest. 2001;119:1021–1026. doi: 10.1378/chest.119.4.1021. [DOI] [PubMed] [Google Scholar]
- 15.Lipworth BJ, Aziz I. A high dose of albuterol does not overcome bronchoprotective subsensitivity in asthmatic subjects receiving regular salmeterol or formoterol. J Allergy Clin Immunol. 1999;103:88–92. doi: 10.1016/s0091-6749(99)70530-0. [DOI] [PubMed] [Google Scholar]
- 16.Grove A, Lipworth BJ. Bronchodilator subsensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet. 1995;346:201–206. doi: 10.1016/s0140-6736(95)91265-7. [DOI] [PubMed] [Google Scholar]
- 17.van der Woude HJ, Winter TH, Aalbers R. Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting beta2 agonists. Thorax. 2001;56:529–535. doi: 10.1136/thorax.56.7.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuglsang G, Vikre-Jorgensen J, Agertoft L, Pedersen S. Effect of salmeterol treatment on nitric oxide level in exhaled air and dose–response to terbutaline in children with mild asthma. Pediatr Pulmonol. 1998;25:314–321. doi: 10.1002/(sici)1099-0496(199805)25:5<314::aid-ppul5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Storms WW, Bird S, Firriolo KM, Edelman JM. The effect of rescue short-acting beta-agonist bronchodilation in patients on montelukast or salmeterol. J Allergy Clin Immunol. 2001;107:S316. [Google Scholar]
- 20.Condemi JJ, Goldstein S, Kalberg C, Yancey S, Emmett A, Rickard K Salmeterol Study Group. The addition of salmeterol to fluticasone propionate versus increasing the dose of fluticasone propionate in patients with persistent asthma. Ann Allergy Asthma Immunol. 1999;82:383–389. doi: 10.1016/s1081-1206(10)63288-7. [DOI] [PubMed] [Google Scholar]
- 21.Kharitonov S, Alving K, Barnes PJ The European Respiratory Society Task Force. Exhaled and nasal nitric oxide measurements: recommendations. Eur Respir J. 1997;10:1683–1693. doi: 10.1183/09031936.97.10071683. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society. Standardization of spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 23.Beach JR, Young CL, Avery AJ, et al. Measurement of airway responsiveness to methacholine: relative importance of the precision of drug delivery and the method of assessing response. Thorax. 1993;48:239–243. doi: 10.1136/thx.48.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavuru M, Melamed J, Gross G, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2000;105:1108–1116. doi: 10.1067/mai.2000.105711. [DOI] [PubMed] [Google Scholar]
- 25.Aubier M, Pieters WR, Schlosser NJ, Steinmetz KO. Salmeterol/fluticasone propionate (50/500 µg) in combination in a Diskus inhaler (Seretide) is effective and safe in the treatment of steroid-dependent asthma. Respir Med. 1999;93:876–884. doi: 10.1016/s0954-6111(99)90053-7. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro G, Lumry W, Wolfe J, et al. Combined salmeterol 50 µg and fluticasone propionate 250 µg in the diskus device for the treatment of asthma. Am J Respir Crit Care Med. 2000;161:527–534. doi: 10.1164/ajrccm.161.2.9905091. [DOI] [PubMed] [Google Scholar]
- 27.Linden A, Bergendal A, Ullman A, Skoogh BE, Lofdahl CG. Salmeterol, formoterol, and salbutamol in the isolated guinea pig trachea: differences in maximum relaxant effect and potency but not in functional antagonism. Thorax. 1993;48:547–553. doi: 10.1136/thx.48.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aziz I, McFarlane LC, Lipworth BJ. Comparative trough effects of formoterol and salmeterol on lymphocyte beta2-adrenoceptor regulation and bronchodilatation. Eur J Clin Pharmacol. 1999;55:431–436. doi: 10.1007/s002280050652. [DOI] [PubMed] [Google Scholar]
- 29.Wilson AM, Lipworth BJ. Dose–response evaluation of the therapeutic index for inhaled budesonide in patients with mild-to-moderate asthma. Am J Med. 2000;108:269–275. doi: 10.1016/s0002-9343(99)00435-0. [DOI] [PubMed] [Google Scholar]
- 30.Aziz I, Wilson AM, Lipworth BJ. Effects of once-daily formoterol and budesonide given alone or in combination on surrogate inflammatory markers in asthmatic adults. Chest. 2000;118:1049–1058. doi: 10.1378/chest.118.4.1049. [DOI] [PubMed] [Google Scholar]
- 31.Eickelberg O, Roth M, Lorx R, et al. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem. 1999;274:1005–1010. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- 32.Roth M, Johnson PR, Rudiger JJ, et al. Interaction between glucocorticoids and beta2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet. 2002;360:1293–1299. doi: 10.1016/S0140-6736(02)11319-5. [DOI] [PubMed] [Google Scholar]
- 33.Chong LK, Cooper E, Vardey CJ, Peachell PT. Salmeterol inhibition of mediator release from human lung mast cells by beta-adrenoceptor-dependent and independent mechanisms. Br J Pharmacol. 1998;123:1009–1015. doi: 10.1038/sj.bjp.0701703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson M. Effects of beta2-agonists on resident and infiltrating inflammatory cells. J Allergy Clin Immunol. 2002;110:S282–S290. doi: 10.1067/mai.2002.129430. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor BJ, Aikman SL, Barnes PJ. Tolerance to the nonbronchodilator effects of inhaled beta 2-agonists in asthma. N Engl J Med. 1992;327:1204–1208. doi: 10.1056/NEJM199210223271704. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Ward C, Thien F, et al. An anti-inflammatory effect of salmeterol, a long-acting beta (2) agonist, assessed in airway biopsies and bronchoalveolar lavage in asthma. Am J Respir Crit Care Med. 1999;160:1493–1499. doi: 10.1164/ajrccm.160.5.9811052. [DOI] [PubMed] [Google Scholar]
- 37.Roberts JA, Bradding P, Britten KM, et al. The long-acting beta2-agonist salmeterol xinafoate: effects on airway inflammation in asthma. Eur Respir J. 1999;14:275–282. doi: 10.1034/j.1399-3003.1999.14b07.x. [DOI] [PubMed] [Google Scholar]
- 38.Gardiner PV, Ward C, Booth H, Allison A, Hendrick DJ, Walters EH. Effect of eight weeks of treatment with salmeterol on bronchoalveolar lavage inflammatory indices in asthmatics. Am J Respir Crit Care Med. 1994;150:1006–1011. doi: 10.1164/ajrccm.150.4.7921429. [DOI] [PubMed] [Google Scholar]
- 39.Newnham DM, McDevitt DG, Lipworth BJ. Bronchodilator subsensitivity after chronic dosing with eformoterol in patients with asthma. Am J Med. 1994;97:29–37. doi: 10.1016/0002-9343(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 40.Grove A, Lipworth BJ. Evaluation of the beta 2 adrenoceptor agonist/antagonist activity of formoterol and salmeterol. Thorax. 1996;51:54–58. doi: 10.1136/thx.51.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newnham DM, Grove A, McDevitt DG, Lipworth BJ. Subsensitivity of bronchodilator and systemic beta 2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax. 1995;50:497–504. doi: 10.1136/thx.50.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aziz I, Lipworth BJ. In vivo effect of albuterol on methacholine-contracted bronchi in conjunction with salmeterol and formoterol. J Allergy Clin Immunol. 1999;103:816–822. doi: 10.1016/s0091-6749(99)70425-2. [DOI] [PubMed] [Google Scholar]
- 43.Bhagat R, Kalra S, Swystun VA, Cockcroft DW. Rapid onset of tolerance to the bronchoprotective effect of salmeterol. Chest. 1995;108:1235–1239. doi: 10.1378/chest.108.5.1235. [DOI] [PubMed] [Google Scholar]


