Abstract
Aims
Current guidelines recommend the use of first-generation antihistamines for the treatment of cough due to rhinitis/postnasal drip syndrome. The antitussive activity of the second-generation antihistamine, fexofenadine, has not been investigated. Therefore, we evaluated the effect of fexofenadine on capsaicin-induced cough in healthy volunteers and in subjects with acute viral upper respiratory tract infection (URI).
Methods
Twelve healthy volunteers and 12 subjects with URI underwent pulmonary function testing and capsaicin cough challenge on two separate days, 2 h after ingesting 180 mg fexofenadine or matched placebo. Subjects inhaled single, vital-capacity breaths of capsaicin aerosol, administered in incremental doubling concentrations, until the concentration inducing five or more coughs (C5) was determined.
Results
In both subject groups, C5 was not significantly different after fexofenadine compared to placebo. In subjects with URI, pulmonary function studies were also similar. In healthy volunteers, however, FEV1 and FEF25−75, pulmonary function parameters reflecting the degree of airway dilatation, were significantly increased after fexofenadine. Mean (95% CI) values for FEV1(L) after fexofenadine and placebo were 3.16 (2.77, 3.55) and 3.08 (2.69, 3.47), respectively (P = 0.017). Mean values for FEF25−75(L/s) were 3.49 (3.10, 3.88) and 3.26 (2.79, 3.72), respectively (P = 0.029).
Conclusions
Fexofenadine demonstrated no antitussive activity against capsaicin-induced cough in healthy volunteers and subjects with URI. The ineffectiveness of fexofenadine in suppressing cough probably reflects the lack of anticholinergic activity and central nervous system penetrance that is characteristic of first-generation antihistamines. The mild bronchodilation induced by fexofenadine in healthy volunteers is of unclear clinical significance and requires further investigation.
Keywords: antihistamines, capsaicin, cough, fexofenadine, pulmonary function tests
Introduction
Cough is the most common complaint for which patients in the United States seek medical attention [1]. Multiple prospective studies have demonstrated that rhinitis/postnasal drip syndrome (PNDS) is among the most common causes of chronic cough [2–4]. Recent guidelines published by the American College of Chest Physicians (ACCP) recommend the use of a first-generation antihistamine/decongestant combination for the treatment of chronic cough due to PNDS, given the observed ineffectiveness of newer-generation, relatively non-sedating antihistamines in acute cough associated with the common cold [5].
Fexofenadine, the active metabolite of terfenadine, is a second-generation, highly selective, peripheral histamine H1-receptor antagonist without cardiac effects. Fexofenadine has been demonstrated to inhibit allergen-induced symptoms in subjects with seasonal allergic rhinitis [6, 7], as well as nasal congestion in subjects with perennial allergic rhinitis [8].
To our knowledge, the effect of fexofenadine on cough reflex sensitivity has not been investigated. Therefore, we performed a prospective, randomized, placebo-controlled, crossover study to evaluate the effect of a single dose of fexofenadine on cough reflex sensitivity to inhaled capsaicin and pulmonary function in healthy volunteers as well as in subjects with acute viral upper respiratory tract infection (URI). Capsaicin, the pungent extract of red peppers, has been shown, in humans, to induce cough in a reproducible and dose-dependent manner [9], thereby rendering it an excellent tool for the evaluation of the effect of a pharmacological intervention on the sensitivity of the cough reflex.
Methods
Subjects
Twelve healthy volunteers [(5M, 7F; age 35.8 ± 1.4 years (mean ± SEM)] and 12 otherwise healthy subjects with symptoms consistent with acute viral URI (3M, 9F; age 30.8 ± 1.5 years) were recruited and provided written, informed consent for the study, which was approved by the Institutional Review Board of Montefiore Medical Center. All subjects were nonsmokers without history of pulmonary disease and without history or symptoms suggestive of gastroesophageal reflux. Healthy volunteers denied recent (within 4 weeks) symptoms of URI, seasonal allergies, or rhinitis/PNDS. No use of any medication known to affect the sensitivity of the cough reflex was reported by healthy volunteers. Subjects with URI abstained from all medications for at least 24 h prior to study enrolment; seven subjects had used no medication at all for their illness. Predominant symptoms reported by subjects with URI included: cough (10 cases), rhinorrhea (10), nasal congestion (10), sneezing (10), sore throat (7), hoarseness (7), headache (3), sinus pain/pressure (3), subjective fever (2), chills (2), and myalgias (1). Symptoms had been present for a mean of 3.5 ± 0.5 days (range 2–7 days) prior to study enrolment.
Study protocol
Upon enrolment, subjects received capsules containing 180 mg fexofenadine (Allegra™; Aventis, Bridgewater, NJ, USA) or matched placebo in a randomized, double-blind, crossover fashion on two separate days. In subjects with URI, mean interval between studies was 1.7 ± 0.3 days (range 1–4 days). In healthy volunteers, mean interval between studies was 2.7 ± 0.6 days (range 1–7 days). At approximately the same time each day, spirometry and capsaicin cough challenge testing were performed 2 h after ingestion of study drug, to correlate with high plasma levels of fexofenadine [10].
Capsaicin cough challenge
Solutions of capsaicin were prepared and administered as previously described [11]. Briefly, subjects inhaled single, vital-capacity breaths of capsaicin aerosol from a compressed air-driven nebulizer (Model 646; DeVilbiss Health Care Inc., Somerset, PA, USA) controlled by a dosimeter (KoKo DigiDoser; PDS Instrumentation, Inc., Louisville, CO, USA). The nebulizer used in these studies was modified by the addition of an inspiratory flow regulator valve (RIFR, PDS Instrumentation, Inc.) that limited inspiratory flow rate to 0.5 l s−1 regardless of inspiratory force, thereby guaranteeing a consistent and reproducible amount of solution delivered with each breath. Single breaths of capsaicin aerosol were administered in incremental, doubling concentrations, with inhalations of saline randomly interspersed to increase challenge blindness, until the concentration inducing five or more coughs (C5) was reached. Breaths were delivered at 1-min intervals, and the number of coughs occurring in the 15 s after each inhalation of capsaicin was recorded. Subjects were unaware that the endpoint of the study was the number of coughs induced.
Spirometry
Prior to capsaicin cough challenge, subjects performed three acceptable spirometric efforts (KoKo Spirometer, PDS Instrumentation) according to American Thoracic Society criteria [12].
Data analysis
Mean and 95% confidence intervals (95% CI) for log C5 and spirometric data (FVC, FEV1, and FEF25−75) after fexofenadine and placebo were calculated and compared by a paired Student's t-test for dependent samples. P < 0.05 was considered significant.
Results
The induction of five or more coughs was achieved in all participants. In subjects with URI, there were no significant differences in cough reflex sensitivity (C5) and pulmonary function values (FVC, FEV1, and FEF25−75) after fexofenadine and placebo. Values for log C5 are shown in Figure 1. Mean (95% CI) log C5 after fexofenadine and placebo were 0.75 (0.41, 1.09) and 0.73 (0.39, 1.06), respectively (P = 0.83).
Figure 1.
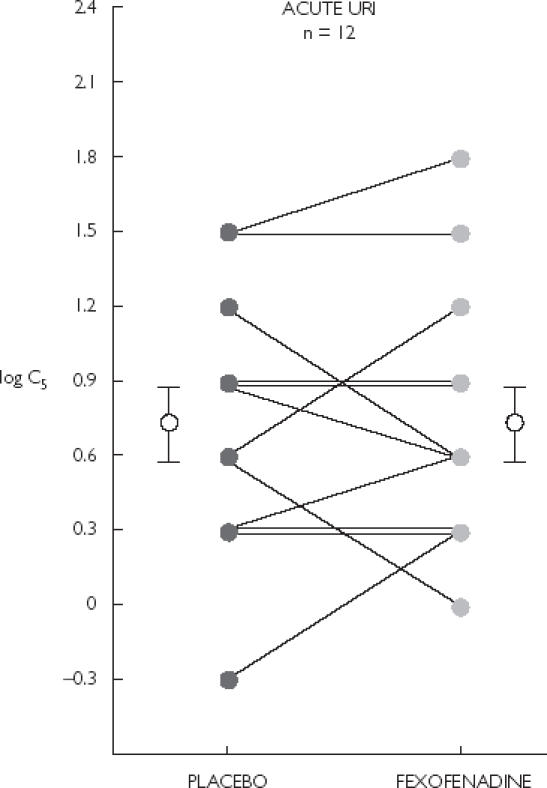
Cough reflex sensitivity (C5) in 12 subjects with URI. Mean (95% CI) log C5 after placebo, 0.73 (0.39, 1.06) and fexofenadine, 0.75 (0.41, 1.09) were not significantly different (P = 0.83). Error bars indicate mean ± SEM.
In healthy volunteers, no differences were noted in cough reflex sensitivity (Figure 2). Mean values for log C5 after fexofenadine and placebo were 0.95 (0.62, 1.28) and 0.93 (0.65, 1.20), respectively (P = 0.67). In terms of pulmonary function, however, FEV1 and FEF25−75 were significantly increased after fexofenadine compared to placebo. Mean values for FEV1 (L) after fexofenadine and placebo were 3.16 (2.77, 3.55) and 3.08 (2.69, 3.47), respectively (P = 0.017). Mean values for FEF25−75 (l s−1) after fexofenadine and placebo were 3.49 (3.10, 3.88) and 3.26 (2.79, 3.72), respectively (P = 0.029). Mean values for FVC were not different between the two study periods.
Figure 2.
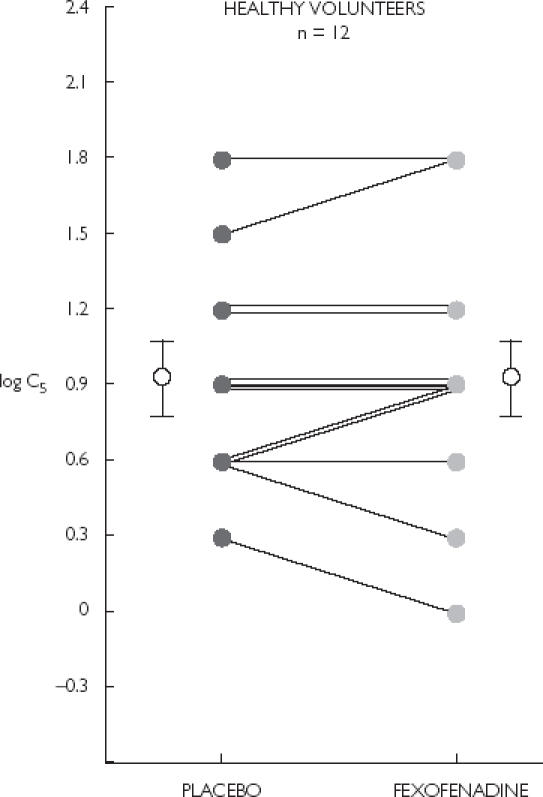
Cough reflex sensitivity (C5) in 12 healthy volunteers. Mean (95% CI) log C5 after placebo, 0.93 (0.65, 1.20) and fexofenadine, 0.95 (0.62, 1.28) were not significantly different (P = 0.67). Error bars indicate mean ± SEM.
Inter-group comparison revealed no significant differences in baseline pulmonary function. In subjects with URI, mean values for FVC, FEV1 and FEF25−75 in terms of predicted percentage, were 99.9 (93.6, 106.2), 96.3 (91.4, 101.1), and 88.5 (73.4, 103.6), respectively. In healthy volunteers, mean values were 94.1 (87.9, 100.2), 90.8 (83.9, 97.6), and 87.9 (74.8, 101.0), respectively.
Discussion
Our results indicate that the second-generation antihistamine, fexofenadine, does not affect cough reflex sensitivity in health, nor during URI. These data support the recently published ACCP clinical guidelines, which recommend the use of a first-generation antihistamine, in combination with a decongestant, for the treatment of chronic cough due to PNDS.
Presumably, since cough is an entirely vagally mediated phenomenon [13], the contrast in the antitussive effects of the first- and second-generation antihistamines is explained by their relative anticholinergic activity. Both types of antihistamines are competitive antagonists to histamine at the H1-receptor site. However, the first-generation antihistamines also demonstrate competitive antagonism of acetylcholine at neuronal and neuromuscular muscarinic receptors. In addition, first-generation antihistamines, due to their lipophilicity, pass the blood–brain barrier, whereas the relatively lipophobic second-generation antihistamines do not [14]. Further supporting this concept are recent animal data demonstrating that the antitussive actions of antihistamines are not directly related to histamine H1-receptor blockade [15]. Interestingly, that study also showed an independence of the antitussive actions of antihistamines and their sedative effects.
Relatively few clinical trials have examined the antitussive effects of antihistamines. A literature review uncovered only three studies that included experimental cough challenge. In healthy volunteers, the first-generation antihistamine, diphenhydramine, inhibited cough induced by citric acid [16], whereas the non-sedating, second-generation antihistamine, terfenadine, was ineffective in suppressing capsaicin-induced cough [17]. Another second-generation agent, loratadine, was also ineffective in healthy volunteers, but did suppress cough induced by ultrasonically nebulized distilled water in patients with nasal disease and unexplained chronic cough [18].
Furthermore, in terms of pathological cough, diphenhydramine has been shown to reduce cough frequency, compared to placebo, in subjects with cough due to chronic bronchitis [19]. Loratadine was demonstrated to be ineffective against cough in a rhinovirus challenge model [14], but did reduce subjectively measured cough scores in subjects with cough associated with allergic rhinoconjunctivitis [20]. It should be noted, however, that the inability of fexofenadine to suppress capsaicin-induced cough in this study does not exclude a possible role for this agent in the treatment of cough due to PNDS.
An unexpected finding of our study was the mild bronchodilation (statistically significant increase in FEV1 and FEF25−75 compared to placebo) by fexofenadine in healthy volunteers, but not in subjects with URI. Although the changes demonstrated were small and of questionable clinical significance, the possibility of an intrinsic bronchodilator action of fexofenadine is raised.
In summary, the second-generation antihistamine, fexofenadine, does not affect cough reflex sensitivity to inhaled capsaicin in healthy volunteers, nor in subjects with URI. The possible bronchodilator activity of fexofenadine suggested in this study requires further investigation.
References
- 1.Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1995. Vital Health Stat. 1997;0:1–38. [PubMed] [Google Scholar]
- 2.Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141:640–647. doi: 10.1164/ajrccm/141.3.640. [DOI] [PubMed] [Google Scholar]
- 3.Pratter MR, Bartter T, Akers S, DuBois J. An algorithmic approach to chronic cough. Ann Intern Med. 1993;119:977–983. doi: 10.7326/0003-4819-119-10-199311150-00003. [DOI] [PubMed] [Google Scholar]
- 4.McGarvey LP, Heaney LG, Lawson JT, et al. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax. 1998;53:738–743. doi: 10.1136/thx.53.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin RS, Boulet L-P, Cloutier MM, et al. Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest. 1998;114(Suppl):133S–181S. doi: 10.1378/chest.114.2_supplement.133s. [DOI] [PubMed] [Google Scholar]
- 6.Allocco FT, Votypka V, de Tineo M, Naclerio RM, Baroody FM. Effects of fexofenadine on the early response to nasal allergen challenge. Ann Allergy Asthma Immunol. 2002;89:578–584. doi: 10.1016/S1081-1206(10)62105-9. [DOI] [PubMed] [Google Scholar]
- 7.Berkowitz RB, Woodworth GG, Lutz C, et al. Onset of action, efficacy, and safety of fexofenadine 60 mg/pseudoephedrine 120 mg versus placebo in the Atlanta allergen exposure unit. Ann Allergy Asthma Immunol. 2002;89:38–45. doi: 10.1016/S1081-1206(10)61909-6. [DOI] [PubMed] [Google Scholar]
- 8.Ciprandi G, Cosentino C, Milanese M, Mondino C, Canonica GW. Fexofenadine reduces nasal congestion in perennial allergic rhinitis. Allergy. 2001;56:1068–1070. doi: 10.1034/j.1398-9995.2001.00191.x. [DOI] [PubMed] [Google Scholar]
- 9.Midgren B, Hansson L, Karlsson J-A, Simonsson BG, Perrson CGA. Capsaicin-induced cough in humans. Am Rev Respir Dis. 1992;146:347–351. doi: 10.1164/ajrccm/146.2.347. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez MA, Estes KS. Pharmacokinetic overview of oral second-generation H1 antihistamines. Int J Clin Pharmacol Ther. 1998;36:292–300. [PubMed] [Google Scholar]
- 11.Dicpinigaitis PV, Grimm DR, Lesser M. Cough reflex sensitivity in subjects with cervical spinal cord injury. Am J Respir Crit Care Med. 1999;159:1660–1662. doi: 10.1164/ajrccm.159.5.9810060. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1216. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 13.Widdicombe JG. Neurophysiology of the cough reflex. Eur Respir J. 1995;8:1193–1202. doi: 10.1183/09031936.95.08071193. [DOI] [PubMed] [Google Scholar]
- 14.Muether PS, Gwaltney JM. Variant effect of first- and second-generation antihistamines as clues to their mechanism of action on the sneeze reflex in the common cold. Clin Infect Dis. 2001;33:1483–1488. doi: 10.1086/322518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLeod RL, Mingo G, O'Reilly S, Ruck L, Bolser DC, Hey JA. Antitussive action of antihistamine is independent of sedative and ventilation activity in the guinea pig. Pharmacology. 1998;57:57–64. doi: 10.1159/000028226. [DOI] [PubMed] [Google Scholar]
- 16.Packman EW, Ciccone PE, Wilson J, Masurat T. Antitussive effects of diphenhydramine on the citric acid aerosol-induced cough response in humans. Int J Clin Pharmacol Ther Tox. 1991;29:218–222. [PubMed] [Google Scholar]
- 17.Studham J, Fuller RW. The effect of oral terfenadine on the sensitivity of the cough reflex in normal volunteers. Pulm Pharmacol. 1992;5:51–52. doi: 10.1016/0952-0600(92)90017-b. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka S, Hirata K, Kurihara N, Yoshikawa J, Takeda T. Effect of loratadine, an H1 antihistamine, on induced cough in non-asthmatic patients with chronic cough. Thorax. 1996;51:810–814. doi: 10.1136/thx.51.8.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lilienfield LS, Rose JC, Princiotto JV. Antitussive activity of diphenhydramine in chronic cough. Clin Pharmacol Ther. 1976;19:421–425. doi: 10.1002/cpt1976194421. [DOI] [PubMed] [Google Scholar]
- 20.Ciprandi G, Buscaglia S, Catrullo A, Marchesi E, Bianchi B, Canonica GW. Loratadine in the treatment of cough associated with allergic rhinoconjunctivitis. Ann Allergy Asthma Immunol. 1995;75:115–120. [PubMed] [Google Scholar]


