Abstract
Aims
To compare the antihypertensive effect, and tolerability and safety of once daily doses of KT3-671 with that of placebo in patients with mild to moderate uncomplicated essential hypertension.
Methods
A randomised, multicentre, double blind, parallel-group comparison of KT3-671 with placebo. Hypertensive patients [Ambulatory Blood Pressure Monitoring (ABPM), mean daytime DBP > 90 mmHg, Office sitting mean DBP 95-114 after a 7–28 day washout period] entered a 2-week, single blind, run-in phase. Patients eligible for the double-blind phase were randomised to receive KT3-671 40 mg, 80 mg, 160 mg or placebo once daily over 4 weeks. The primary end-point was trough mean sitting office DBP. The study had 90% power to detect a 5 mmHg change between treatments and placebo at the 5% level of significance. The secondary end-points were 24 hour, daytime and night time mean ABPM.
Results
Office DBP was significantly lower with KT3-671 40 mg but not the other 2 dosage groups (−3.2; 95% CL −6.1 : −0.3 P < 0.03). Office SBP was significantly reduced with all dosage groups (40 mg −5.9, 95% CL −11 : −0.9; 80 mg −4.9, 95% CL −9.9 : 0.1 and 160 mg −5.7, 95% CL −10.8 : −0.7 P < 0.05). All doses of KT3-671 reduced systolic and diastolic ABPM. The number of patients with treatment related adverse events were comparable to placebo (38.8% KT3-671 vs 32.8% placebo). There was some evidence of a dose-response relationship with fall in nocturnal ABPM.
Conclusions
Oral KT3-671 was well tolerated. KT3-671 reduced office systolic BP at all doses and diastolic BP at some of the doses. Due to greater precision and power, the falls in mean ambulatory systolic and diastolic pressure were all significantly lower than placebo.
Keywords: angiotensin II, blood pressure, hypertension, receptors
Introduction
Blockade of the renin-angiotensin system by ACE inhibitors is an established treatment of many cardiovascular disorders. Angiotensin II receptor blockers differ from ACE inhibitors in three important respects. These drugs block angiotensin II whether generated by the classical ACE dependent pathway or by means of other enzymes such as chymase. Angiotensin II receptor blockers act highly selectively at angiotensin1 (AT1) receptor subtypes thought to be responsible for the detrimental effects of angiotensin while leaving potentially beneficial effects mediated at the AT2 receptor unimpaired. Unlike ACE inhibition, AT1 receptor blockers have no effects on bradykinin or substance P and are free of the major undesirable side-effects (cough and angioedema).
KT3-671 is a novel chemical entity, which has been shown to be a potent, orally active, specific AT1 receptor antagonist. KT3-671 has undergone in vitro and in vivo pharmacological studies in which it demonstrated a selective affinity for AT1 receptors in a variety of animal models where a dose dependent reduction in blood pressure (BP) was observed [1–7].
A previous phase II randomized double-blind study of patients with mild to moderate essential hypertension using KT3-671 20–80 mg suggested a shallow dose–response curve for change in sitting trough BP, although only falls in sitting office diastolic BP were significantly greater than those following placebo. Changes in ambulatory blood pressure (ABP) were more marked than those for office trough BP [8]. A study with higher doses might reproduce the dose–response curve as seen in animal studies.
This study was designed to compare the antihypertensive effect of once daily doses of KT3–671 up to 160 mg with that of placebo in patients with mild to moderate uncomplicated essential hypertension. The tolerability of KT3-671 at these doses was also examined.
Methods
The study protocol was approved by the Multicentre Research and Ethics Committee for Scotland and subsequently by local research ethics committees of the centres involved. The study was conducted according to the ethical principles for medical research involving human subjects of the World Medical Association Declaration of Helsinki and in accordance with the Guidelines for Good Clinical Practice. All patients were given information about the study before participation and gave written voluntary informed consent.
Study design
This study was a randomized, double-blind, parallel group comparison of KT3-671 with placebo. Patients who were already taking antihypertensive drugs had a 7–28 day washout period prior to entry to a 2-week, single-blind, run-in phase. Patients were eligible for the double-blind phase if they met both the inclusion and exclusion criteria. These patients were randomized to receive KT3-617 40 mg, 80 mg, 160 mg or placebo once daily for 4 weeks at which point trough office and ambulatory blood pressure (ABP) was assessed. At the end of the 4-week treatment period, patients entered a 2-day washout phase. Subjects were reviewed weekly during the first 2 weeks of the 4 week treatment phase with office blood pressure, drug compliance and adverse event monitoring. If blood pressure rose in the clinician's opinion to unsafe levels the patient was withdrawn from the study. Figure 1 shows a flow diagram of the study.
Figure 1.
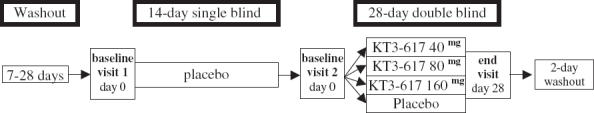
Flow diagram of study
Inclusion criteria
Patients were eligible if they had untreated hypertension or previous antihypertensive therapy (which could be safely discontinued for 7 days) and were males or females without child bearing potential aged 18–80 years. Non-child bearing potential was defined as postmenopausal (12 months without menstruation), surgically sterile, or using medically accepted birth control, and with a negative pregnancy test.
Entry to the single- and double-blind phases of the study was dependant upon patients meeting the following criteria before and after the single-blind run-in phase, respectively.
sitting mean diastolic BP of 95–114 mmHg inclusive
sitting mean systolic BP ≤ 190 mmHg.
In addition progression to the double-blind phase required that there was
less than 8 mmHg difference in diastolic BP between baselines
ambulatory blood pressure monitoring (ABPM) criteria satisfied
overall success rate > 80%
at least one valid recording per hour in the daytime interval
at least one valid recording every 2 h in the night time interval
weekly compliance with therapy of 80% −120%.
Patients were excluded for the following reasons:
history of malignant or secondary hypertension
the use of any drugs that affect blood pressure
psychiatric disorders or drug, medication or alcohol abuse
presence of any clinically significant cardiovascular target organ damage apart from
left ventricular hypertrophy
single uncomplicated MI more than 1 year earlier
first degree heart block or
non specific T wave abnormalities
any other major diseases including
diabetes mellitus
active malignancy
collagen vascular disorder
pregnancy.
Blood pressure measurement
Office blood pressure
The primary efficacy measure was change in office blood pressure. This was defined as the change from baseline to the end of treatment in trough mean sitting office diastolic blood pressure. Measurements at each visit were taken at approximately the same time of day, using the nondominant arm and using the same BP measuring device (an Omron HEM 705CP). Three sitting BP measurements were taken after at least 5 min rest, each measurement separated by intervals of 2 min. The first measurement was discarded and the mean of the last two measurements was recorded. If the second and third diastolic measurements differed by more than 5 mmHg, a further two measurements were taken, and a mean calculated from either the second & third or fourth and fifth measurements.
Secondary efficacy measures were change from baseline to end of treatment in trough mean sitting office systolic BP.
Ambulatory blood pressure monitoring (ABPM)
Ambulatory BP readings were also secondary efficacy parameters. These were change from baseline to end of treatment in 24 h, daytime, and night-time ambulatory systolic and diastolic BP. The trough to peak ratio was calculated according to the method of Staesson et al[9]. ABPM was performed noninvasively for at least 24 h using Spacelabs 90207 (Redford USA) monitors. Monitors were programmed to record BP every 20 min during the daytime interval (08.00–22.00 h) and every 30 min during the night-time interval (22.00–08.00 h).
Tolerability
Laboratory data (full blood count, biochemistry (urea and electrolytes, liver function tests, lipid profile, creatinine kinase and lactate dehydrogenase) and urinalysis), physical examination, monitoring of heart rate (HR), 12-lead electrocardiogram (ECG) and recording of adverse events were performed before entering the single-blind placebo run in & after the double-blind treatment phase.
Statistical methods
The primary and secondary efficacy variables were analysed using analysis of covariance (ancova) on an intension to treat basis including terms for baseline, treatment and study centre. The final model from this analysis was used to assess the dose–response relationship.
Trough-to-peak ratio and responder rate were summarized by treatment group. Safety data were summarized by treatment group and by visit, as appropriate. Continuous variables were summarized using mean, SD, median and range. Categorical variables were summarized using frequency counts and percentages. All statistical tests were two–sided. Interactions in the ancova model were tested at the 10% significance level. All other statistical tests were carried out at the 5% level.
The study had 90% power to detect a 5 mmHg difference in office trough diastolic blood pressure between treatment and placebo at the 5% level of significance.
Results
Study subjects
A total of 244 patients were randomized at 17 centres in the United Kingdom; at the conclusion 197 patients remained in the per protocol population ( Table 1). Reasons for exclusion were noncompliance, adverse events, development of contraindication or failure to comply with study protocol. The placebo, KT3-671 40 mg, KT3-671 80 mg, KT3-671 160 mg groups had 13, 15, 7 and 12 subjects excluded, respectively, for a variety of reasons, most commonly for receiving less than 26 days of double-blinded treatment. There were no significant differences in exclusion rates between the various treatment groups.
Table 1.
Demographics: study populations.
| Demographic | Placebo n= 61 | KT3-671 40 mg n= 65 | KT3-671 80 mg n= 58 | KT3-671 160 mg n= 60 | ||
|---|---|---|---|---|---|---|
| Age (years) | Mean | 54 | 55 | 53 | 52 | |
| Range | 26–76 | 31–78 | 27–76 | 28–71 | ||
| Sex (%) | Male | 57 | 71 | 72 | 70 | |
| Female | 43 | 29 | 28 | 30 | ||
| Race (%) | Caucasian | 95 | 98 | 96 | 96 | |
| Black | 3 | 0 | 0 | 0 | ||
| Asian | 2 | 2 | 3 | 2 | ||
| Other | 0 | 0 | 0 | 2 | ||
| Weight (kg) | Mean | 83 | 83 | 85 | 83 | |
| Range | 54–110 | 60–123 | 56–144 | 55–150 | ||
| Height (cm) | Mean | 169 | 170 | 171 | 170 | |
| Range | 151–183 | 148–190 | 152–194 | 147–198 | ||
| Smoking (%) | Smoker | 10 | 13 | 26 | 18 | |
| Ex-smoker | 30 | 36 | 36 | 40 | ||
| Non-smoker | 60 | 51 | 38 | 42 | ||
| Alcohol (%) | Yes | 85 | 90 | 78 | 85 | |
| No | 15 | 10 | 22 | 15 | ||
| Antihypertensive treatment | Yes | 38 | 126 | |||
| No | 23 | 57 | ||||
| Analysis populations n (%) | Per protocol | 48 (78.7) | 50 (76.9) | 51 (87.9) | 48 (80) | |
| ITT* | 61 (100) | 65 (100) | 58 (100) | 60 (100) |
ITT: intention to treat.
Blood pressure
Office blood pressure
The baseline DBP and SBP varied from 101.4 ± 4.3 mmHg in the placebo group to 102.4 ± 4.8 mmHg in the KT3-671 40 mg group & 158.2 ± 11.8 mmHg in the placebo group to 162.1 ± 13.6 mmHg in the KT3-671 40 mg group, respectively.
Compared with placebo treatment only KT3-671 40 mg showed a significant reduction from baseline in trough mean sitting DBP. KT3-671 significantly reduced office systolic BP at all doses compared with placebo. A dose–response relationship for the reduction in trough sitting mean diastolic or systolic BP was not demonstrated ( Table 2).
Table 2.
Trough sitting mean diastolic and systolic BP for the per protocol population.
| Baseline ± SD (mmHg) | Mean change from baseline ± SD (mmHg) | Least-squares difference ± SE (mmHg) | Upper & lower 95% confidence interval | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment group | DBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | SBP |
| Placebo | 101.4 ± −4.3 | −2.6 ± 6.5 | −2.2 ± 13.4 | ||||||
| 40 mg | 102.4 ± 4.8 | −6.0 ± 7.6 | −8.8 ± 13.9 | −3.2 ± 1.5 | −5.9 ± 2.6 | −6.1, −0.3 | −11, −0.9 | < 0.03 | < 0.02 |
| 80 mg | 101.5 ± 4.7 | −5.2 ± 6.9 | −7.6 ± 12.2 | −2.6 ± 1.4 | −4.9 ± 2.5 | −5.4, 0.3 | −9.9, 0.1 | < 0.07 | < 0.05 |
| 160 mg | 102.2 + 4.8 | −4.7 ± 8.4 | −8.4 ± 12.7 | −2.0 ± 1.5 | −5.7 ± 2.6 | −4.9, 0.9 | −10.8, 0.7 | < 0.18 | < 0.02 |
Ambulatory blood pressure
Compared with the placebo group, the three KT3-671 doses produced greater reductions in ambulatory systolic and diastolic BP. The mean differences between the placebo group and all three KT3-671 groups for ambulatory BP were statistically significant. These data are summarized in Table 3. A linear dose–response relationship was evident for nocturnal systolic and diastolic ambulatory BP (Table 4).
Table 3.
Ambulatory BP for the per protocol population.
| Baseline ± SD (mmHg) | Mean change ± SD (mmHg) | Least-squares mean difference ± SE (mmHg) | Upper & lower 95% confidence interval | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Treatment group | DBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP & SBP |
| 24 h | ||||||||
| Placebo | 92.3 ± 5.6 | 0.2 ± 3.6 | 0.2 ± 6.0 | |||||
| 40 mg | 93.5 ± 6.1 | −3.6 ± 5.3 | −5.8 ± 8.2 | −3.7 ± 0.9 | −5.7 ± 1.5 | −5.6, −1.8 | −8.8, −2.7 | < 0.001 |
| 80 mg | 94.2 ± 7.2 | −4.0 ± 5.4 | −7.4 ± 8.7 | −3.9 ± 0.9 | −7.1 ± 1.5 | −5.8, −2.1 | −10.1, −4.0 | < 0.001 |
| 160 mg | 94.9 ± 7.3 | −4.7 ± 5.3 | −8.1 ± 9.1 | −4.4 ± 1.0 | −7.1 ± 1.6 | −6.3, −2.5 | −10.3, −4.0 | < 0.001 |
| Daytime | ||||||||
| Placebo | 98.7 ± 4.9 | −0.6 ± 4.6 | −1.1 ± 7.8 | |||||
| 40 mg | 99.5 ± 6.4 | −4.7 ± 5.6 | −7.2 ± 8.7 | −4.2 ± 1.1 | −5.9 ± 1.7 | 6.2, −2.1 | −9.3, −2.6 | < 0.001 |
| 80 mg | 100.6 ± 7.1 | −5.3 ± 5.8 | −9.6 ± 8.9 | −4.3 ± 1.0 | −7.7 ± 1.7 | −6.4, −2.3 | −11.0, −4.4 | < 0.001 |
| 160 mg | 100.3 ± 7.7 | −4.9 ± 6.3 | −8.8 ± 10.2 | −4.0 ± 1.1 | −6.4 ± 1.7 | −6.1, −1.9 | −9.8, −3.0 | < 0.001 |
| Night-time | ||||||||
| Placebo | 83.5 ± 8.1 | 1.2 ± 4.5 | 1.9 ± 7.2 | |||||
| 40 mg | 84.8 ± 7.4 | −1.9 ± 6 | −3.6 ± 9.2 | −2.8 ± 1.1 | −5.1 ± 1.8 | −5.0, −0.6 | −8.6, −1.5 | < 0.014 |
| 80 mg | 85.1 ± 8.9 | −2.2 ± 6.3 | −4.2 ± 10.1 | −3.2 ± 1.1 | −5.8 ± 1.8 | −5.4, −0.1 | −9.3, −2.3 | < 0.004 |
| 160 mg | 87.3 ± 8.9 | −4.3 ± 6.4 | −7.1 ± 10.6 | −4.7 ± 1.2 | −7.6 ± 1.9 | −7.0, −2.4 | −11.3, −4.0 | < 0.001 |
Table 4.
Trough to peak ratio of the per-protocol population.
| KT3-671 40 mg − placebo | KT3-671 80 mg − placebo | KT3-671 160 mg − placebo | ||||
|---|---|---|---|---|---|---|
| DBP | SBP | DBP | SBP | DBP | SBP | |
| Trough ± SD (mmHg) | −5.7 ± 0.7 | −5.1 ± 0.9 | −4.3 ± 0.3 | −5.1 ± 0.9 | −4.9 ± 1.7 | −8.4 ± 5.0 |
| Peak ± SD (mmHg) | −8.6 ± 1.5 | −11.4 ± 3.1 | −6.7 ± 0.2 | −12.6 ± 1.5 | −8.0 ± 0.4 | −13.7 ± 5.2 |
| Trough : Peak ratio (%) | 66 | 44 | 64 | 41 | 62 | 61 |
Responder rates were not significantly different from placebo for any of the three KT3-671 dosage regimes. There were no significant differences compared with placebo in the mean change from baseline in sitting heart rate after KT3-671.
Tolerability
Of the ITT population (n = 244) that were analyzed for tolerability, 155 experienced one or more adverse events, 65.0% in KT3-671 treated patients compared with 59.0% in placebo-treated patients. There were 287 adverse events in total, most of which were mild or moderate in severity. Only one was considered serious (myocardial infarction) but thought to be unrelated to study medication (KT3-671 40 mg). The most frequently reported adverse events during this study were headache and dizziness. There was a reduction in the number of subjects with headache from 13.1% in the placebo group to 6.7% in the KT3-671 160 mg group with the total number of headache adverse events reduced by half in the KT3-671 160 mg group compared with placebo (12% vs 25%). The number of patients with treatment-related adverse events was 26 (40.0%), 25 (43.1%) & 20 (33.3%) for the KT3-671 40 mg, 80 mg and 160 mg groups, respectively, compared with 20 (32.8%) for the placebo group. Four (6.2%), one (1.7%) and one (1.7%) adverse event led to withdrawal in the respective KT3-671 groups, compared with one (1.6%) in the placebo group. None of the withdrawals was the result of laboratory abnormalities and none of the treatment related adverse events was ascribed to deterioration in blood pressure control.
Compliance
The compliance rates (assessed by return tablet count) for placebo, 40 mg, 80 mg and 160 mg KT3-671 doses were 100%, 96.8%, 100% and 100%, respectively.
Discussion
In this randomized, double-blind study in patients with mild to moderate uncomplicated essential hypertension, KT3-671 40 mg was the only dose that showed a statistically significant difference from the placebo group for the primary efficacy measure. Of the secondary efficacy measures investigated, the change from baseline in office SBP, ambulatory DBP and ambulatory SBP showed statistically significant differences between each of the three KT3-671 groups and placebo.
A dose–response relationship was evident only for night-time ambulatory systolic and diastolic blood pressure. Studies with other angiotensin II antagonists have shown that these drugs generally have a shallow dose–response curve. In hypertensive dogs, a dose–response curve for KT3-671 was found between 3 mg kg−1 and 10 mg kg−1[4]. To achieve a similar dose–response curve the equivalent doses in man would be 225 mg and 750 mg raising the question of whether the dose levels used in the studies were too low.
As with other angiotensin receptor blockers, not all subjects responded to KT3-671. For example losartan and valsartan have responder rates of 55% and 62% [10, 11]. At the doses studied, however, there was little evidence KT3-671 would be a viable antihypertensive drug in the UK population. The responder rates for all three doses up to 160 mg were not significantly different from placebo.
Office and ambulatory readings often show poor consistency [12–14]. However, the greater power and precision resulting from multiple blood pressure readings allows ABPM to provide a more reliable estimate of antihypertensive efficacy. A similar discrepancy between office and ambulatory blood pressure was possibly evident in the HOPE study where changes in office blood pressure of 3/2 mmHg may have been an underestimate due to the nocturnal dosing with ramipril. The substudy by Svensson et al in a small group of subjects with peripheral vascular disease demonstrated a significant difference in nocturnal ambulatory blood pressure of 17/8 mmHg while changes in office blood pressure were not significant (8/2 mmHg) [15].There is evidence that the night/day ratio in ambulatory blood pressure is an important cardiovascular risk predictor [16]. The effects of KT3-671 on nocturnal ambulatory blood pressure are probably clinically significant, but clearly there is a lack of efficacy on daytime diastolic blood pressure with a weak effect on SBP at doses up to 160 mg.
The relatively high exclusion rate from the per protocol population for this study compared with other studies with AT1 receptor blockers may have substantially diminished the power of the study to detect changes in the primary efficacy variable office DBP [11]. Another factor that may have decreased the power of the study was the ethical limitation of a 4-week treatment period as most studies demonstrating efficacy of AT1 receptor blockers have been over 8 weeks. Although measurements of the activity of the RAAS were not made during this study, it is known that plasma renin activity predicts the blood pressure response of drugs blocking this system [17, 18]. It is possible therefore that subjects in this study had a relatively low renin activity (predominantly volume dependent blood pressure) making them relatively resistant to the antihypertensive effects of angiotensin II receptor antagonism. The addition of a thiazide diuretic would probably have had an additive effect and the effect may even have been multiplicative considering the relatively poor blood pressure responsiveness in the study subjects [19].
Although shallow, the significant dose–response relationships for noctural ambulatory SBP & DBP support a prolonged duration of action, in keeping with in vitro evidence that KT3-671 is an insurmountable antagonist of the AT1 receptor [1]. This finding together with the trough to peak ratio data also supports a dose-dependent duration of action. The observation of a reduction in headache incidence with KT3-671 when compared with placebo, a feature common to other AT1 receptor blockers [20, 21] is further demonstration of its mode of action. It may be that, at appropriate doses, KT3-671 would be an effective once a day antihypertensive agent. The drug was well tolerated at all three doses in this study, and a feature of AT1 receptor blockers is that adverse effects are not dose-related.
Acknowledgments
This study was supported by a grant from Kotobuki Pharmaceuticals.
References
- 1.Satake N, Imanishi M, Keto Y, et al. The inhibitory effect of KT3-671, a nonpeptide angiotensin-receptor antagonist, on rabbit and rat isolated vascular smooth muscles: a possible involvement of K (ATP) channels. J Cardiovasc Pharmacol. 2000;35:457–467. doi: 10.1097/00005344-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Takata Y, Kurihara J, Yoda T, et al. KT3-671, an angiotensin AT1 receptor antagonist, attenuates vascular but not cardiac responses to sympathetic nerve stimulation in pithed rats. J Cardiovasc Pharmacol. 2001;37:427–436. doi: 10.1097/00005344-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Takata Y, Tajima S, Kato H. Inhibitory effect of KT3-671, a non-peptide angiotensin subtype 1 receptor antagonist, on sympathetic neurotransmission in isolated rabbit aorta. Pharmacol Res. 2000;41:335–340. doi: 10.1006/phrs.1999.0592. [DOI] [PubMed] [Google Scholar]
- 4.Takata Y, Tajima S, Mochizuki S, et al. Antihypertensive activity and pharmacokinetics of KD3-671, a nonpeptide AT1-receptor antagonist, in renal hypertensive dogs. J Cardiovasc Pharmacol. 1998;32:834–844. doi: 10.1097/00005344-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki S, Sato T, Furuta K, et al. Pharmacological properties of KT3-671, a novel nonpeptide angiotensin II receptor antagonist. J Cardiovasc Pharmacol. 1995;25:22–29. doi: 10.1097/00005344-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kawashima K, Amano H, Fujimoto K, et al. Effect of repeated administration of KT3-671, a nonpeptide AT1 receptor antagonist, on diurnal variation in blood pressure, heart rate, and locomotor activity in stroke-prone spontaneously hypertensive rats as determined by radiotelemetry. J Cardiovasc Pharmacol. 1996;27:411–416. doi: 10.1097/00005344-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Amano H, Fujimoto K, Suzuki T, et al. Antihypertensive effect of chronic KT3-671, a structurally new nonpeptide angiotensin AT1-receptor antagonist, in stroke-prone spontaneously hypertensive rats. Jpn J Pharmacol. 1995;69:215–222. doi: 10.1254/jjp.69.215. [DOI] [PubMed] [Google Scholar]
- 8.Kotobuki P. Unpublished data held on file.
- 9.Staessen JA, Thijs L, Bijttebier G, et al. Determining the trough-to-peak ratio in parallel-group trials. Systolic Hypertension in Europe (SYST-EUR) Trial Investigators. Hypertension. 1997;29:659–667. doi: 10.1161/01.hyp.29.2.659. [DOI] [PubMed] [Google Scholar]
- 10.Pool JL, Glazer R, Chiang YT, Gatlin M. Dose–response efficacy of valsartan, a new angiotensin II receptor blocker. J Hum Hypertens. 1999;13:275–281. doi: 10.1038/sj.jhh.1000788. [DOI] [PubMed] [Google Scholar]
- 11.Hedner T, Oparil S, Rasmussen K, et al. A comparison of the angiotensin II antagonists valsartan and losartan in the treatment of essential hypertension. Am J Hypertens. 1999;12:414–417. doi: 10.1016/s0895-7061(99)00082-5. [DOI] [PubMed] [Google Scholar]
- 12.Waeber B, Rutschmann B, Nussberger J, Brunner HR. Evaluation of antihypertensive therapy: discrepancies between office and ambulatory recorded blood pressure. J Hypertens Suppl. 1991;9:S53–S56. [PubMed] [Google Scholar]
- 13.Lacourciere Y, Leenen F, Rangno R, et al. Discrepancies between clinic & ambulatory blood pressure responses to cilazapril therapy. Can J Cardiol. 1994;10:605–610. [PubMed] [Google Scholar]
- 14.Owens P, Atkins N, O'Brien E. Diagnosis of white coat hypertension by ambulatory blood pressure monitoring. Hypertension. 1999;34:267–272. doi: 10.1161/01.hyp.34.2.267. [DOI] [PubMed] [Google Scholar]
- 15.Svensson P, de Faire U, Sleight P, Yusuf S, Ostergren J. Comparative effects of ramipril on ambulatory and office blood pressures: a HOPE Substudy. Hypertension. 2001;38:E28–E32. doi: 10.1161/hy1101.099502. [DOI] [PubMed] [Google Scholar]
- 16.Verdecchia P. Prognostic value of ambulatory blood pressure: Current evidence and clinical Implications. Hypertension. 2000;35:844–851. doi: 10.1161/01.hyp.35.3.844. [DOI] [PubMed] [Google Scholar]
- 17.Ames RP. Insights from Laragh's review course. the role of the renin-angiotensin system in blood pressure regulation. Am J Hypertens. 2002;15:653–654. doi: 10.1016/s0895-7061(02)02935-7. [DOI] [PubMed] [Google Scholar]
- 18.Hirschl MM, Binder M, Bur A, et al. Impact of the renin-angiotensin-aldosterone system on blood pressure response to intravenous enalaprilat in patients with hypertensive crises. J Hum Hypertens. 1997;11:177–183. doi: 10.1038/sj.jhh.1000404. [DOI] [PubMed] [Google Scholar]
- 19.Weir MR, Smith DH, Neutel JM, Bedigian MP. Valsartan alone or with a diuretic or ACE inhibitor as treatment for African American hypertensives: relation to salt intake. Am J Hypertens. 2001;14:665–671. doi: 10.1016/s0895-7061(01)01296-1. [DOI] [PubMed] [Google Scholar]
- 20.Etminan M, Levine MA, Tomlinson G, Rochon PA. Efficacy of angiotensin II receptor antagonists in preventing headache: a systematic overview and meta-analysis. Am J Med. 2002;112:642–646. doi: 10.1016/s0002-9343(02)01100-2. [DOI] [PubMed] [Google Scholar]
- 21.Tronvik E, Stovner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA. 2003;289:65–69. doi: 10.1001/jama.289.1.65. [DOI] [PubMed] [Google Scholar]


