Abstract
Aims
To assess the haemodynamic, electrocardiographic and glycaemic effects of piperaquine-dihydroartemisinin (Artekin®) fixed combination therapy in uncomplicated malaria.
Methods
Sixty-two Cambodians (32 children and 30 adults) with falciparum or vivax malaria were given Artekin® given as four age-based oral doses over 32 h. Supine and erect blood pressure, the electrocardiographic QT interval and plasma glucose were measured before treatment and then at regular intervals during a 4-day admission period as part of efficacy and safety monitoring. QT intervals were rate-corrected (QTc) using Bazett's formula.
Results
Artekin® therapy was well tolerated and all patients responded to treatment. Average parasite and fever clearance times were 19 and 12 h, respectively. The pretreatment mean fall in systolic blood pressure on standing was 8 ± 6 mmHg and 6-hourly measurements over 72 h showed no significant change (P = 0.48). There was a significant lengthening of the mean QTc to a maximum of 11 ms0.5 (95% confidence interval 4–18 ms0.5) relative to baseline at 24 h (P = 0.003). The maximal QTc prolongation observed in any patient was 53 ms0.5. There was a mean 0.4 mmol l−1 reduction in the post-absorptive plasma glucose during the first 48 h but no episodes of hypoglycaemia (plasma glucose <3.0 mmol l−1) were observed at any time.
Conclusions
Artekin® is safe and effective combination therapy for uncomplicated malaria in children and adults. Although piperaquine is a long half-life drug related to other quinoline compounds including chloroquine and quinine, no clinically significant cardiovascular or metabolic effects were observed.
Keywords: combination therapy, dihydroartemisinin, hypoglycaemia, piperaquine, postural hypotension, QT interval prolongation
Introduction
Although the combination of an artemisinin derivative with a long-acting schizonticidal drug is currently advocated as the optimal strategy for the treatment of malaria [1, 2], the choice of partner drug can be problematic. Existing candidates are relatively expensive (mefloquine and atovaqone-proguanil), have unpleasant side-effects (mefloquine), are unreliably absorbed (lumefantrine) or face high levels of pre-existing parasite resistance (chloroquine, amodiaquine and sulfadoxine-pyrimethamine). Other ‘forgotten’ antimalarial drugs are being considered. These were developed decades ago but did not achieve sustained use. They include 4-aminoquinoline compounds such as piperaquine (1,3-bis[1-(7-chloro-4′-quinolyl)-4′-piperazinyl]) phosphate.
Piperaquine is available as a relatively inexpensive fixed combination with dihydroartemisinin (Artekin®) that can be given in a short-course regimen. Initial studies from Indochina have shown that it is effective and well tolerated [3]. However, whilst already licensed in some south-east Asian countries, the systematic collection of detailed pharmacokinetic, efficacy and safety data from conventional Phase I–IV trials, as required by regulatory bodies in the developed world [4], has not been carried out. This would include preclinical in vitro and animal studies, volunteer and clinical studies that define absorption, excretion, clearance, metabolic pathways and drug concentrations during treatment, whilst simultaneously monitoring for toxicity and therapeutic response. Some piperaquine animal toxicity data have been published [5, 6] and the therapeutic and prophylactic efficacy of piperaquine monotherapy was documented in clinical trials in the 1970s [7, 8]. However, the therapeutic index of piperaquine remains poorly defined and dosage recommendations, including those for Artekin®, have been devised empirically with little understanding of the relationship between serum concentrations and toxicity.
The first Phase II evaluation of Artekin® was carried out recently in Cambodian patients with uncomplicated falciparum malaria and showed excellent efficacy, with 100% and 92.3% 28-day cure rates in children and adults, respectively [3]. However, one child died unexpectedly during the trial. Whilst the cause of death is likely to have been an unrelated septic illness, a drug reaction could not be excluded. The most serious side-effect of quinoline antimalarial drugs is that of cardiotoxicity, specifically prolongation of the electrocardiographic QT interval [9, 10] and postural blood pressure changes [11, 12]. Since artemisinin drugs may also prolong the QT interval [13, 14], there is the potential for synergistic cardiotoxicity in Artekin®-treated patients. Hypoglycaemia is also a well-recognized complication of falciparum malaria that may be potentiated by quinoline drugs [15]. Although artemisinin derivatives are unlikely to cause hypoglycaemia [16, 17], the effect of piperaquine on glucose homoeostasis is unknown.
In order to investigate further the potential toxicity of Artekin®, we performed a clinical evaluation of Artekin® in Cambodian children and adults with either Plasmodium falciparum or P. vivax infections with particular emphasis on haemodynamic, electrocardiographic and glycaemic monitoring.
Methods
Ethical approval
The study protocol was approved by the National Ethics Committee for Health Research, Ministry of Health, Cambodia and by the Human Rights Committee of the University of Western Australia. Written informed consent was obtained from all adult patients or from the parents or guardians of the children. All hospital-related costs were covered and, in addition, patients or parents/guardians received compensation to cover loss of earnings and other expenses incurred through participation in the study.
Study site and recruitment procedures
The study was conducted in August and September of 2002 at the Cambodian Government health centre in Snoul district, Kratie Province in eastern Cambodia. The local population is predominantly ethnic Khmer. Recruitment was by active or passive case detection, the former during visits to villages when screening of those with symptoms was performed and the latter through patients being referred to, or seeking treatment at, the health centre.
Giemsa-stained thick film blood smears were prepared and examined by an experienced microscopist and designated as negative after examination of>100 fields at ×1000 magnification. Speciation was determined by thin film microscopy. Symptomatic patients in the age groups 2–10 years and ≥16 years who had a positive blood film (parasite density 1000–150 000 µl−1 whole blood) were eligible. In the children, only those with P. falciparum infections were studied while adults with either P. vivax or P. falciparum were enrolled. Exclusion criteria included pregnancy, significant comorbidity, regular medication use, receipt of recent antimalarial treatment (specifically, quinine or an artemisinin drug within the last 7 days, chloroquine within the last 14 days, or sulfadoxine-pyrimethamine and mefloquine within the last 28 days), or clinical manifestations of severe or complicated malaria [18].
Clinical procedures
Patients were requested to remain in hospital for at least 4 days. After enrolment, each subject was weighed, a full physical examination was performed and vital signs (axillary temperature, heart rate, respiratory rate, lying and standing systolic and diastolic blood pressures, and conscious state) were recorded. In addition to a blood smear, each patient had a venous blood sample taken for white cell count, plasma glucose and haematocrit. A 12-lead electrocardiogram (ECG) was recorded.
Artekin® (Holleykin Pharmaceuticals Co. Ltd, Phnom Penh, Cambodia) in the form of tablets (320 mg piperaquine phosphate plus 40 mg dihydroartemisinin) or granules (120 mg piperaquine phosphate and 15 mg dihydroartemisinin) was administered in accordance with the manufacturer's instructions in age-based doses given by mouth at times 0, 6, 24 and 32 h. Children under the age of 7 years were treated with granules (2–3 years, 4 × 1 sachets; 4–6 years, 4 × 1.5 sachets). Children aged 7–10 years and all adults (> 16 years) received tablets (7–10 years, 1 × 4 tablets; adults, 4 × 2 tablets). Treatments were supervised and patients were observed closely for at least 1 h after the first dose. Those vomiting during this time were re-treated and excluded from the present study.
Pulse and respiratory rates, axillary temperature and conscious state were recorded 6-hourly over the first 72 h. Blood smears were taken 6-hourly until two consecutive slides were negative. Venous haematocrit and white blood cell count were recorded daily for the first 3 days. Blood pressure was measured at baseline and every 6 h for 72 h. After recording the supine blood pressure, the measurement was repeated after the patient had been standing erect for 1 min.
ECGs were performed with the patient resting in bed at baseline (0 h), and 24 and 48 h after administration of the first dose. These times were chosen so that (i) serum piperaquine concentrations would be close to maximal during the administration regimen employed [19], and (ii) the effects of wakefulness and circadian variation on ECG indices were minimized [20, 21]. Because of its relative accuracy and robustness [22], manual measurement of the QT interval using a paper speed of 25 mm s−1 was performed. The QT interval was measured from the beginning of the Q wave to the point at which the terminal limb of the T wave returned to baseline. When U waves were present, the QT interval was measured to the nadir of the T and U waves. The lead showing the longest interval in the baseline ECG was used for this reading (usually I, II, aVL, V5 or V6) and the same lead was used for subsequent measurements in each patient. The corrected QT interval (QTc) was derived by adjusting for heart rate according to Bazett's formula (QTc = QT/√RR, where the RR interval is the time interval between the peaks of two consecutive QRS complexes [23]). The QTc was considered prolonged if it was>450 ms0.5 or if, during treatment, it was>60 ms0.5 more than the baseline value [24, 25].
Capillary glucose measurements were performed using a portable meter (Lifescan One Touch®; Johnson and Johnson, Fremont, CA, USA). Measurements were performed approximately 2 h after a breakfast meal on a daily basis for the first 3 days after treatment had started. Patients were also questioned about symptoms of hypoglycaemia at other times and additional plasma glucose determinations were performed if necessary.
Patients were discharged from hospital after 3 days of observation provided they were asymptomatic and aparasitaemic. All patients were followed up on days 7, 14, 21 and 28 and were advised to return if they became symptomatic at any other time. Each follow-up visit included clinical assessment and documentation of any new or persistent symptoms. Axillary temperature and thick and thin blood films were also taken at these times.
Statistical analysis
Data were analysed using the computer package SPSS for Windows (SPSS Inc., Chicago, IL, USA). Data are, unless otherwise stated, presented as mean and [95% confidence intervals (CI)]. Two-sample comparisons were by means of Student's t-test. Repeated measures analysis of variance was used to assess changes in mean QTc, blood glucose, blood pressure, heart rate, venous haematocrit and leucocyte count during the study. A two-tailed level of significance of P < 0.05 was used in all tests.
Results
Clinical course
A total of 62 patients (30 children and 32 adults) were recruited. Of the adults, 22 had falciparum and 10 had vivax malaria. The present subjects formed part of a group of patients who participated in a pharmacokinetic study of piperaquine [37]. Details of the present patients are summarized in Table 1. All responded promptly to Artekin® treatment [parasite clearance time 19 (16–21) h; fever clearance time 12 (11–14) h]. No recrudescences were observed during the 28 days of follow-up.
Table 1.
Baseline characteristics of the patients
| Children | Adults | Total | |
|---|---|---|---|
| Number of patients | 30 | 32 | 62 |
| Age (years) | 7.5 ± 2.5 | 30.0 ± 11.2 | 19.1 ± 14.0 |
| Sex (% males) | 53 | 47 | 50 |
| Body weight (kg) | 17.3 ± 4.5 | 46.4 ± 6.0 | 32.3 ± 15.6 |
| Axillary temperature (°C) | 38.4 ± 0.6 | 38.2 ± 0.6 | 38.3 ± 0.6 |
| Parasitaemia (µl−1) | 12 800 (3100–33 342)* | 7700 (1000–110 000) | 10 400 (2400–75 600) |
| Pulse rate (min−1) | 118 ± 19*** | 89 ± 8 | 103 ± 20 |
| Supine systolic/diastolic blood pressure (mmHg) | 104 ± 6**/65 ± 5* | 109 ± 7/68 ± 5 | 106 ± 7/66 ± 5 |
| Standing systolic/diastolic blood pressure (mmHg) | 97 ± 9*/57 ± 7* | 101 ± 8/62 ± 9 | 99 ± 9/58 ± 8 |
| QTc (ms0.5) | 438 ± 25* | 424 ± 25 | 431 ± 26 |
| QTc>440 ms0.5 (%) | 11 (37%) | 8 (25%) | 19 (31%) |
| Plasma glucose (mmol l−1) | 5.8 ± 1.0 | 5.5 ± 1.0 | 5.7 ± 1.0 |
| Venous haematocrit (%) | 31.8 ± 5.2** | 36.4 ± 4.8 | 34.2 ± 5.5 |
| Total leucocyte count (× 106 l−1) | 6 730 ± 1520* | 5820 ± 1510 | 6 260 ± 1570 |
Data are presented as percentages, mean ± SD, or median and (interquartile range).
P < 0.05;
P < 0.01;
P < 0.001 vs. adults.
Haemodynamic measures
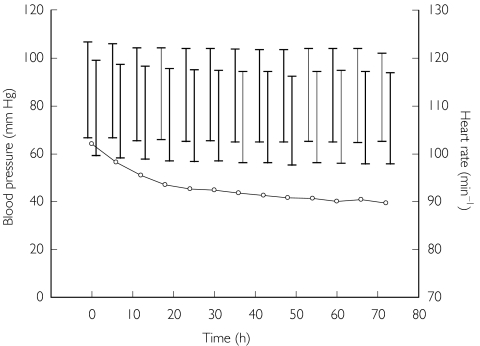
The mean heart rate fell progressively over time in children and adults (P < 0.001 by anova) in parallel with changes in axillary temperature, especially during the first 24 h of treatment (see Figure 1). The mean pretreatment fall in systolic blood pressure on standing in the sample as a whole was 8 mmHg and there was no difference in this measure between children and adults (P = 0.88). Subsequent measurements of the postural fall in systolic blood pressure over 72 h showed no significant change (P = 0.48 by anova; see Figure 1). No clinically significant orthostatic hypotension (> 20 mmHg difference between supine and erect systolic blood pressure) was observed in any patient during the study.
Figure 1.
Mean blood pressure and heart rate during and after treatment. The mean supine blood pressure is on the left of each pair of vertical bars and the erect blood pressure on the right; the upper crossbar indicates systolic and the lower diastolic pressure. Mean heart rate is shown as open circles.
Electrocardiographic measures
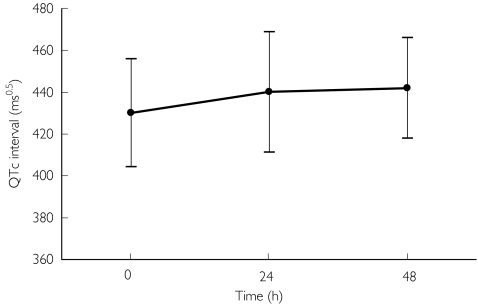
The children had longer QTc intervals than the adults at baseline (P = 0.043; see Table 1). Compared with baseline there was a statistically significant lengthening of the mean QTc at 24 h of 11 (4–18) ms0.5 (P = 0.003) in the sample as a whole and a nonsignificant lengthening of 7 (−1.5–15) ms0.5 (95% CI −1.5, 15, P = 0.105) at 48 h (see Figure 2). Similar time-related changes were seen in adults and children (data not shown). In no patient at any time after treatment was started was the QTc increased by>60 ms0.5. The maximal QTc prolongation observed (53 ms0.5) occurred in a female child at 24 h. One child had ECG evidence of frequent atrial ectopics at 24 h post-treatment and one adult had ventricular ectopics at the same time point, but no other arrhythmias or additional ECG abnormalities were observed.
Figure 2.
Mean ± SD QTc interval before treatment (0 h) and at 24 and 48 h.
Plasma glucose
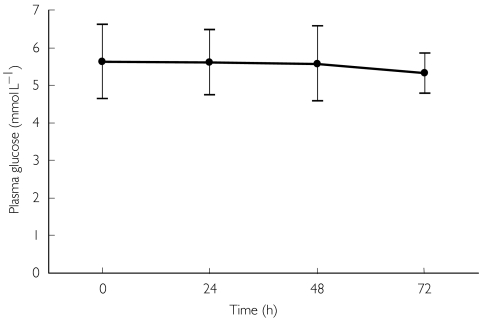
The mean capillary plasma glucose concentration decreased progressively from 5.7 mmol l−1 at baseline to 5.3 mmol l−1 at 72 h amongst the 62 subjects (P = 0.008; see Figure 3). There were no age-related differences in plasma glucose at baseline (see Table 1) or subsequently. No significant episodes of hypoglycaemia (suggestive symptoms and/or plasma glucose concentration <3.0 mmol l−1) were observed during regular clinical assessments or at any other time during the study.
Figure 3.
Mean ± SD plasma glucose before treatment (0 h) and at 24 and 48 h.
Haematological and other variables
The venous haematocrit remained unchanged throughout the period of monitoring (34.2% at baseline vs. 34.6% at 72 h; P > 0.1) and the average difference in haematocrit between children and adults of approximately 5% observed at baseline (see Table 1) persisted during this time. The mean total leucocyte count fell from 6260 × 106 l−1 to 5840 × 106 l−1 over 72 h (P = 0.002). There were no neurological complications present in any patient during the 28-day follow-up period.
Discussion
The present study is the first to have examined the effects of Artekin® on important cardiovascular and metabolic variables during treatment of uncomplicated malaria. Given that one of the components of Artekin®, piperaquine, is chemically similar to quinoline drugs such as quinine and chloroquine, we were particularly interested in changes in postural blood pressure, the QT interval and plasma glucose. There was no evidence that recommended doses of Artekin® given over 32 h were associated with clinically significant postural hypotension, QTc prolongation or propensity to hypoglycaemia. Our data add to available evidence that Artekin® is a safe and well-tolerated antimalarial drug combination.
Treatment with conventional quinoline drugs such as quinine and chloroquine has been shown to exacerbate the postural blood pressure changes resulting from acute falciparum malaria [11, 12]. In addition to worsening already unpleasant symptoms, this can predispose to syncope and vomiting, with significant implications for subsequent management. We found that, prior to Artekin® therapy, mild postural hypotension was present, with a mean fall in systolic blood pressure of <10 mmHg on standing. This did not increase during treatment or subsequently over a period of 3 days. Part of the reason for the stability of blood pressure changes in our patients may have been the prompt fever clearance time (mean 12 h), a characteristic of therapy with artemisinin drugs [13]. Nevertheless, it is reassuring that a regimen that included piperaquine did not produce a single case of clinically significant postural hypotension.
We found minimal prolongation of the QTc interval during and after Artekin® therapy. Furthermore, none of our patients exhibited QTc prolongation of>60 ms0.5, a recognized threshold for drug-induced arrhythmias in other noncardiovascular contexts [24, 25]. Population pharmacokinetic analysis of adult data [37]revealed mean peak serum piperaquine concentrations at 12 h (108 µg l−1), 29 h (95 µg l−1) and 36 h (124 µg l−1) during the present dosing regimen. It is possible therefore that more profound changes in QTc may have been missed because the ECGs were taken at 24 and 48 h (mean serum piperaquine 60 and 78 µg l−1, respectively). However, these concentration data were available to us only after the study had been completed. It should also be noted that the Bazett formula, and in particular the use of √RR in the denominator, may not represent the best way of correcting for heart rate [26]. As a result, lengthening of the QTc may simply reflect a decline in heart rate with recovery from malaria rather than a specific drug effect [26]. Nevertheless, alternative formulae do not necessarily improve on Bazett's correction [27]. In addition, the mean QTc interval increased and then declined over 48 h in our patients while heart rate fell steadily, suggesting that the mild QTc prolongation at 24 h was drug-related.
The QTc changes seen in our patients were equivalent to those reported using similar methodologies in African children with uncomplicated malaria treated with artemether-lumefantrine, pyrimethamine-sulphadoxine or chloroquine [28, 29] and in adult Thai patients treated with artemether-lumefantrine or artesunate-mefloquine [30]. Like other studies of uncomplicated malaria [29], the present study showed that a proportion of patients (30%) had mildly prolonged QTc intervals prior to drug administration. It is therefore possible that subsequent changes reflect the effect of malaria per se rather than drug therapy [29, 30]. Mefloquine is another quinoline antimalarial drug that has minimal effect on ventricular repolarization [31]. However, it has been shown to exhibit a potentially lethal interaction with the more cardiotoxic drug halofantrine [32]. This suggests that Artekin® should be used with care in patients recently treated with halofantrine [32, 33].
Hypoglycaemia is not a feature of uncomplicated malaria, but we were interested to see whether, like the chemically related drugs chloroquine and mefloquine [15], the piperaquine component of Artekin® might influence glucose homoeostasis. Although not of a clinically important magnitude, there was a small but statistically significant and progressive fall in plasma glucose during the 72 h following commencement of treatment. Plasma glucose concentrations are elevated in acute falciparum malaria because of insulin resistance due to the stress of the infection [34]. It is likely therefore that the mean 0.4 mmol l−1 fall in plasma glucose in our patients during the first 3 days was primarily a reflection of a reduction in levels of stress hormones such as adrenaline and cortisol rather than an effect of piperaquine. This same phenomenon may also explain the parallel change in peripheral blood white cell count.
We excluded pregnant women from the present study for two reasons. First, no published human data exist regarding the safety of piperaquine in pregnancy. Second, embryonic death and impaired fetal growth and development have been recorded in animals administered artemisinin derivatives early in pregnancy [35]. The World Health Organization currently advises against the use of artemisinin drugs in pregnant women, particularly during the first trimester, where viable alternatives exist [35]. As Artekin® use increases, fetal exposure is likely to occur as a result of unregulated private-sector prescribing and self-medication, as well as inadvertent exposure in women who do not know they are pregnant. It is important that surveillance be instituted in the manner used following the introduction of artesunate-mefloquine combination therapy in Thailand [36].
The 0, 6, 24 and 32-h dosing employed in the present study was that recommended by the manufacturer and one which was presumably developed empirically. Other longer-duration regimens (e.g. 0, 12, 24 and 36 h, or 0, 12, 24 and 48 h) may represent more practical and equally effective alternatives. Optimal dosing regimens should be based on detailed pharmacokinetic and pharmacodynamic data for both piperaquine and dihydroartemisinin. Nevertheless, the safety of the relatively short 32-h schedule used in our patients is reassuring.
Because of its efficacy and relatively low cost, the use of Artekin® is likely to increase in future. The present results provide reassurance of the cardiovascular and metabolic safety of this combination antimalarial therapy in healthy children and young adults with uncomplicated malaria. Although piperaquine can be considered a ‘forgotten’ drug that has been rediscovered, it has not gone through the normal stages of drug development required in Western countries. Because of this, further continuing safety evaluation appears important, especially the assessment of its electrocardiographic interactions with other quinoline antimalarial drugs and consideration of safety in pregnancy.
Acknowledgments
We thank the National Centre for Parasitology, Entomology and Malaria Control, Phnom Penh and the European Commission Cambodia Malaria Control Project, Phnom Penh for invaluable support. We also thank staff at the Snoul health centre in Cambodia for their assistance with the conduct of the field studies. T-Y.H. was the recipient of a Raine Medical Research Foundation Bachelor of Medical Science Scholarship. Valuable financial support also came from the University of Western Australia and the Ramaciotti Research Foundation.
References
- 1.White NJ. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia. 1999;41:301–308. [PubMed] [Google Scholar]
- 2.World Health Organisation. Antimalarial Drug Combination Therapy. Geneva: WHO/CDS/RBM; 2001. p. 35. Report of a WHO Technical Consultation. [Google Scholar]
- 3.Denis MB, Davis TME, Hewitt S, et al. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin Infect Dis. 2002;35:1469–1476. doi: 10.1086/344647. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. Guidance for industry. Food and Drug Administration; 1997. http://www.fda.gov/cder/guidance/index.htm. [Google Scholar]
- 5.Zhao HJ, Xia YY, Zheng Z. Pre-clinical toxical study of new antimalarial agents. IV. Liver ultrastructure changes affected by antimalarial agent, compound tablet of piperaquine phosphate and sulfadoxine. Acad J Second Mil Med College. 1981;1:47–48. [Google Scholar]
- 6.Sheng N, Jiang W, Tang HL. Pre-clinical toxical study of new antimalarial agents. II. Piperaquine phosphate and its compound ‘Preventive, 3′. Acad J Second Mil Med College. 1981;1:40–46. [Google Scholar]
- 7.Chen L, Qu FY, Zhou YC. Field observation of prophylactic effect of the new antimalarial piperaquine in Hainan province. Med J PLA. 1979;4:104–108. [Google Scholar]
- 8.Guo XB, Fu LC. Comparative study of artemisinin suppositories and piperaquine phosphate in the treatment of falciparum malaria. Zhong Xi Yi Jie He Za Zhi. 1989;9:473–475. [PubMed] [Google Scholar]
- 9.Karbwang J, Davis TME, Looareesuwan S, Molunto P, Bunnag D, White NJ. A comparison of the pharmacokinetic and pharmacodynamic properties of quinine and quinidine in healthy Thai males. Br J Clin Pharmacol. 1993;35:265–271. [PMC free article] [PubMed] [Google Scholar]
- 10.Touze JE, Heno P, Fourcade L, et al. The effects of antimalarial drugs on ventricular repolarization. Am J Trop Med Hyg. 2002;67:54–60. doi: 10.4269/ajtmh.2002.67.54. [DOI] [PubMed] [Google Scholar]
- 11.Kofi Ekue JM, Phiri DE, Mukunyandela M, Sheth UK, Wernsdorfer WH. Severe orthostatic hypotension during treatment of falciparum malaria. Br Med J. 1988;296:396. doi: 10.1136/bmj.296.6619.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Supanaranond W, Davis TME, Pukrittayakamee S, Nagachinta B, White NJ. Abnormal circulatory control in falciparum malaria: the effects of antimalarial drugs. Eur J Clin Pharmacol. 1993;44:325–329. doi: 10.1007/BF00316467. [DOI] [PubMed] [Google Scholar]
- 13.Hien TT, White NJ. Qinghaosu. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- 14.Brewer TG, Peggins JO, Grate SJ, et al. Neurotoxicity in animals due to arteether and artemether. Trans R Soc Trop Med Hyg. 1994;88(Suppl 1):33–36. doi: 10.1016/0035-9203(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 15.Davis TME. Antimalarial drugs and glucose metabolism. Br J Clin Pharmacol. 1997;44:1–7. doi: 10.1046/j.1365-2125.1997.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hien TT, Day NPJ, Phu NH, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe malaria. N Engl J Med. 1996;335:76–83. doi: 10.1056/NEJM199607113350202. [DOI] [PubMed] [Google Scholar]
- 17.Gribble F, Davis TME, Higham CE, Clark A, Ashcroft F. The antimalarial agent mefloquine inhibits ATP-sensitive K-channels. Br J Pharmacol. 2000;131:756–760. doi: 10.1038/sj.bjp.0703638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warrell DA, Molyneux M, Beales P. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84(Suppl 2):1–69. [PubMed] [Google Scholar]
- 19.Hung TY, Davis TME, Ilett KF. Measurement of piperaquine in plasma by high-performance liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Biomed Sci Appl. 2003. pp. 93–101. [DOI] [PubMed]
- 20.Kostis WJ, Belina JC. Differences in beat-to-beat variability of the QT interval between day and night. Angiology. 2000;51:905–911. doi: 10.1177/000331970005101103. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa M, Saikawa T. Dynamics of the QT interval. Jap J Clin Med. 2002;60:1317–1323. [PubMed] [Google Scholar]
- 22.Kautzner J. QT interval measurement. Cardiac Electrophysiol Rev. 2002;6:273–277. doi: 10.1023/a:1016389227576. [DOI] [PubMed] [Google Scholar]
- 23.Ahnve S. Correction of the QT interval for heart rate. Review of the different formulas and the use of Bazett's formula in myocardial infarction. Am Heart J. 1985;109:568–574. doi: 10.1016/0002-8703(85)90564-2. [DOI] [PubMed] [Google Scholar]
- 24.Committee for Proprietary Medicinal Products. 1997. Points to consider: the assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products; http://www.eudra.org/emea.html. [DOI] [PMC free article] [PubMed]
- 25.Elming H, Brendorp B, Kober L, Sahebzadah N, Torp-Petersen C. QTc interval in the assessment of cardiac risk. Cardiac Electrophysiol Rev. 2002;6:289–294. doi: 10.1023/a:1016345412555. [DOI] [PubMed] [Google Scholar]
- 26.Price RN, Nosten F, White NJ. Prolongation of the QTc interval in African children treated for severe malaria. Am J Trop Med Hyg. 1998;59:503. doi: 10.4269/ajtmh.1998.59.503. [DOI] [PubMed] [Google Scholar]
- 27.von Seidlein L, Jaffar S, Greenwood B. Prolongation of the QTc interval in African children treated for severe malaria. Am J Trop Med Hyg. 1998;59:503. doi: 10.4269/ajtmh.1997.56.494. [DOI] [PubMed] [Google Scholar]
- 28.Sowunmi A, Falade CO, Oduola AM, et al. Cardiac effects of halofantrine in children suffering from acute uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 1998;92:446–448. doi: 10.1016/s0035-9203(98)91086-0. [DOI] [PubMed] [Google Scholar]
- 29.von Seidlein L, Bojang K, Jones P, et al. A randomized controlled trial of artemether/benflumetol, a new antimalarial and pyrimethamine/sulfadoxine in the treatment of uncomplicated falciparum malaria in African children. Am J Trop Med Hyg. 1988;58:638–644. doi: 10.4269/ajtmh.1998.58.638. [DOI] [PubMed] [Google Scholar]
- 30.van Vugt M, Ezzet F, Nosten F, et al. No evidence of cardiotoxicity during antimalarial treatment with artemether-lumefantrine. Am J Trop Med Hyg. 1999;61:964–967. doi: 10.4269/ajtmh.1999.61.964. [DOI] [PubMed] [Google Scholar]
- 31.Davis TME, Dembo L, Kaye-Eddie S, Hewitt B, Hislop R, Batty KT. Neurological, cardiovascular and metabolic effects of mefloquine in healthy volunteers: a double-blind, placebo-controlled trial. Br J Clin Pharmacol. 1996;42:415–421. doi: 10.1046/j.1365-2125.1996.04745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nosten F, ter Kuile FO, Luxemburger C, et al. Cardiac effects of antimalarial treatment with halofantrine. Lancet. 1993;341:1054–1056. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- 33.Touze JE, Bernard J, Keundjian A, et al. Electrocardiographic changes and halofantrine plasma level during acute falciparum malaria. Am J Trop Med Hyg. 1996;54:225–228. doi: 10.4269/ajtmh.1996.54.225. [DOI] [PubMed] [Google Scholar]
- 34.Davis TME, Pukrittayakamee S, Supanaranond W, et al. Glucose metabolism in quinine-treated patients with uncomplicated falciparum malaria. Clin Endocrinol. 1990;33:739–749. doi: 10.1111/j.1365-2265.1990.tb03911.x. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organisation. Report of two informal consultations convened by WHO in 2002. Geneva: WHO/CDS/MAL; 2003. Assessment of the safety of artemisinin compounds in pregnancy; p. 1094. [Google Scholar]
- 36.McGready R, Cho T, Keo NK, et al. Artemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug-resistant Plasmodium falciparum. Clin Infect Dis. 2001;33:2009–2016. doi: 10.1086/324349. [DOI] [PubMed] [Google Scholar]
- 37.Hung TY, Davis TNE, Ilett KF, Karunajeewa H, Hewitt S, Denis MB, Lim C, Socheat D. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum of vivax malaria. Br J Clin Pharmacol. 2003 doi: 10.1046/j.1365-2125.2003.02004.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]