Abstract
Aims
The relationship between β2-adrenoceptor polymorphisms and bronchoprotective response with long-acting β2-adrenoceptor agonists is unknown.
Methods
We retrospectively analysed data from six placebo-controlled randomized studies in corticosteroid treated asthmatics where formoterol or salmeterol were administered over a 1–2-week period, with prior 1–2 week washout, assessing the primary end point of methacholine PD20 and adenosine monophosphate PC20, following first and last dose, expressed as doubling dose difference from placebo.
Results
There was no significant heterogeneity between the different studies. Patients who had homozygous or heterozygous genotypes containing the arginine-16 polymorphism (Arg16-Arg16 or Arg16-Gly16) had greater bronchoprotective subsensitivity compared with the homozygous glycine-16 genotype (Gly16-Gly16), amounting to a mean doubling dose difference of 1.49 (95% CI 0.50, 2.48), after the last dose. Subsensitivity of response was greater with formoterol than salmeterol after the last dose in all genotypes, especially with the arginine-16 polymorphism, amounting to a doubling dose difference of 3.00 (95% CI 1.01, 4.99) between formoterol and salmeterol.
Conclusions
Our retrospective analysis showed that the arginine-16 polymorphism was associated with subsensitivity of response for bronchoprotection, which was greater for formoterol than salmeterol. A prospective study will be required in order to further evaluate these findings, particularly to assess whether these differences are mirrored by exacerbations.
Keywords: adenosine monophosphate PC20, bronchial hyper-responsiveness, formoterol, genotype, haplotype, long-acting β2-adrenoceptor agonist, methacholine PD20, salmeterol, subsensitivity, β2-adrenoceptor polymorphism
Introduction
Regular use of β2-adrenoceptor agonists results in β2-adrenoceptor down-regulation and associated subsensitivity to their effects on airway smooth muscle and mast cells, which occurs to a greater degree for bronchoprotection than for bronchodilatation [1]. The so-called functional antagonism exhibited by β2-adrenoceptor agonists may be demonstrated by increasing bronchomotor tone using either direct or indirect bronchoconstrictor stimuli such as methacholine or adenosine monophosphate, respectively. The glycine-16 polymorphism of the β2-adrenoceptor has been shown to be associated with an increased β2-adrenoceptor agonist promoted down-regulation in vitro[2, 3] and a greater degree of bronchodilator subsensitivity to salbutamol and formoterol in vivo[4–6].
However, the relationship between β2-adrenoceptor polymorphism and bronchoprotection with long-acting β2-adrenoceptor agonists has been given relatively little attention. In one study, there was no effect of β2-adrenoceptor polymorphism at position 16 or 27 on the acute protection afforded by a single dose of formoterol against methacholine-induced bronchoconstriction [7]. In another study, the glycine-16 polymorphism was associated with significant blunting of salbutamol-induced protection against methacholine after a single dose of formoterol or salmeterol [8]. It is unclear whether β2-adrenoceptor polymorphisms determine the development of subsensitivity during regular exposure to long-acting β2-adrenoceptor agonists in corticosteroid treated patients. Furthermore, formoterol has much higher β2-adrenoceptor intrinsic activity than salmeterol, which is particularly evident in the presence of increased bronchomotor tone [9], although it is unknown whether this may be of clinical relevance in terms of the propensity for β2-adrenoceptor agonist-induced down-regulation and subsensitivity.
Methods
We retrospectively analyzed data from six randomized double-blinded placebo-controlled studies where long-acting β2-adrenoceptor agonists such as formoterol or salmeterol were administered over 1–2 weeks, with a prior 1–2 week washout, in corticosteroid treated mild to moderate persistent asthmatics, as summarized in Table 1[10–15]. All studies employed a cross-over design apart from one parallel group study. The selection criteria for the six studies previously performed in our unit were based on asthmatic patients with previous genotyping who were receiving add-on therapy with long-acting β2-adrenoceptor agonists to inhaled corticosteroids for at least a one-week period, and where the primary end-point was either methacholine or adenosine monophosphate provocative dose/dilution causing a 20% fall in forced expiratory volume in 1 second (FEV1) from baseline (MCh PD20 or AMP PC20). No studies from our unit pertaining to the study of genotype have been excluded from the analysis. Methacholine and adenosine monophosphate bronchial challenges were performed as previously described according to standard protocols [16, 17]. Bronchoprotection was measured after the first and last doses of formoterol or salmeterol. β2-adrenoceptor polymorphisms at position 16 and 27 were determined as previously described [4]. All patients gave informed consent for genotyping and the study was approved by the Tayside Committee on Medical Research Ethics.
Table 1.
Study details.
| Reference | Severity FEV1 % predicted | Challenge time postβ2-adrenoceptor agonist | Duration | ICS | Primary outcome following first dose | Primary outcome following last dose |
|---|---|---|---|---|---|---|
| Lipworth [10] | Mild/Moderate | 1 h | 1 week | Yes | MCh PD20 | MCh PD20 |
| 77% | FM = SM > PL | FM = SM > PL | ||||
| Lipworth [11] | Mild/Moderate | 12 h | 1 week | Yes | MCh PD20 | MCh PD20 |
| 77% | FM = SM > PL | FM = SM > PL | ||||
| Lipworth [12] | Mild/Moderate | 1 h | 2 weeks | Yes | MCh PD20 | MCh PD20 |
| 87% | FM = TB > PL | FM = TB = PL | ||||
| Sims [13] | Mild/Moderate | 12 h | 2 weeks | Yes | AMP PC20 | AMP PC20 |
| 79% | FM = ML > PL | ML > PL = FM | ||||
| Aziz [14] | Mild/Moderate | 12 h | 1 week | Yes | AMP PC20 | AMP PC20 |
| 82% | FM > PL | FM = PL | ||||
| Wilson [15] | Mild/Moderate | 12 h | 2 weeks | Yes | AMP PC20 | AMP PC20 |
| 79% | ML = SM > PL | ML > PL = SM |
FM Formoterol, SM Salmeterol, TB Terbutaline, ML Montelukast, PL Placebo, ICS Inhaled corticosteroid, MCh Methacholine, AMP Adenosine monophosphate.
Statistical analysis
The power was set at 80% and α-error set at 0.05 (two-tailed) in order to detect a one doubling dose/dilution difference in MCh PD20 or AMP PC20 between genotypes with 20 patients in each group [18]. The primary outcome variable was either MCh PD20 or AMP PC20 in all studies (Table 1). To normalize distribution, data for MCh PD20 or AMP PC20 were logarithmically transformed, and analyses were performed using Statgraphics® statistical software package (STSC Software Publishing Group, Rockville, USA). Log-transformed data were calculated as doubling dose/dilution change from placebo. Comparisons were performed between different genotypes (at position 16 or 27) and haplotypes (at position 16 and 27 together), as well as comparing formoterol vs salmeterol at each genotype. In order to obviate multiple haplotype comparisons with small numbers, we elected only to compare the same three haplotypes as in the previous study from Dishy et al.[19].
Data were analysed for the composite end point of MCh PD20 and AMP PC20 together and also for the end point of either MCh PD20 alone or AMP PC20 alone for genotype comparisons. However, only data for the composite end point of MCh PD20 and AMP PC20 together were analysed for haplotype comparisons, due to insufficient numbers with data for either MCh PD20 alone or AMP PC20 alone. An overall multifactorial analysis of variance was performed using patients, treatments, genotypes, and haplotypes, as factors, followed by Bonferroni multiple range testing set at 95% CI. In order not to confound the overall α-error, differences are all quoted as either being significant (P < 0.05, Bonferroni corrected) or not. Thus, 95% CI for the doubling dose/dilution difference from placebo which excluded zero would indicate a significant difference between genotypes. In addition, a chi-square heterogeneity testing of variance across different studies was performed with further analysis according to bronchial challenge, peak or trough measurement, long-acting β2-adrenoceptor agonist, and genotype (Table 2).
Table 2.
Homogeneity across the different studies for first and last dose bronchoprotection, as change from placebo, according to bronchial challenge, timing of challenge, full or partial agonist activity, and genotype.
| First dose | Last dose | |||
|---|---|---|---|---|
| χ2 | P | χ2 | P | |
| MCh | 1.83 | 0.40 | 2.20 | 0.33 |
| AMP | 0.70 | 0.70 | 0.91 | 0.63 |
| Peak | 1.58 | 0.21 | 2.16 | 0.14 |
| Trough | 0.99 | 0.80 | 1.25 | 0.74 |
| Formoterol | 2.05 | 0.84 | 3.09 | 0.69 |
| Salmeterol | 0.51 | 0.78 | 0.06 | 0.97 |
| Gly16-Gly16 | 2.67 | 0.75 | 4.35 | 0.50 |
| Arg16-Gly16 or Arg16-Arg16 | 2.17 | 0.71 | 1.63 | 0.80 |
| Glu27-Glu27 | 5.24 | 0.39 | 5.88 | 0.32 |
| Gln27-Glu27 or Gln27-Gln27 | 2.12 | 0.83 | 1.67 | 0.89 |
Results
Heterogeneity comparison
Chi-square heterogeneity analysis showed no significant differences between the different studies in first (χ2 = 2.90, P = 0.72) and last (χ2 = 2.22, P = 0.82) dose bronchoprotection as change from placebo. In addition, further heterogeneity analysis according to bronchial challenge, timing of challenge, full or partial agonist activity, and genotype, also showed no significant differences (Table 2).
Placebo comparison
Absolute values for MCh PD20 (± SEM) following placebo were not significant comparing genotypes at position 16 and position 27; Arg16-Arg16/Arg16-Gly16: 89.6 ± 25.9 µg, Gly16-Gly16: 75.4 ± 13.6 µg, Gln27-Gln27/Gln27-Glu27: 67.6 ± 14.5 µg and Glu27-Glu27: 93.0 ± 20.1 µg.
Genotype comparison
When expressing the data as change from placebo, a negative value for the doubling dose/dilution difference indicates that the genotype is worse than placebo whereas a positive value indicates that the genotype is better than placebo. For the composite end point of MCh PD20 and AMP PC20 together, there was significantly greater subsensitivity of response for homozygous or heterozygous genotypes containing the arginine-16 polymorphism (Arg16-Arg16 or Arg16-Gly16) compared with the homozygous glycine-16 genotype (Gly16-Gly16), after the last dose, amounting to a mean doubling dose/dilution difference in MCh PD20/AMP PC20 of 1.49 (95% CI 0.50, 2.48) (Table 3). For the same genotype comparison, with the single end point of MCh PD20 alone, after the last dose, there was a significant mean doubling dose difference of 2.55 (95% CI 1.11, 3.99) (Table 3). There were no significant differences between genotypes at position 27 for the composite end point of MCh PD20 and AMP PC20 together and for the single end point of MCh PD20 alone (Table 3). It can be seen from Figure 1 that there was greater subsensitivity of response after both the first and last doses for the Arg16-Arg16 or Arg16-Gly16 genotype, compared with the Gly16-Gly16 genotype. There was a greater degree of protection loss between first and last doses, as change from placebo, for the Arg16-Arg16 or Arg16-Gly16 genotype, compared with the Gly16-Gly16 genotype, for both the composite end point of MCh PD20 and AMP PC20 together, and for the single end point of either MCh PD20 alone or AMP PC20 alone.
Table 3.
Doubling dose/dilution shift as change from placebo comparing different genotypes.
| Doubling dose/dilution difference from placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| MCh PD20 and AMP PC20(±SEM) | MCh PD20 alone (±SEM) | AMP PC20 alone (±SEM) | |||||||
| Genotype | (n) | First dose | Last dose | (n) | First dose | Last dose | (n) | First dose | Last dose |
| (a)Gly16-Gly16 | 100 | 0.60 ± 0.26 | −0.05 ± 0.29 | 71 | 0.47 ± 0.33 | −0.04 ± 0.38 | 29 | 0.87 ± 0.36 | −0.08 ± 0.36 |
| (b) Arg16-Gly16 or Arg16-Arg16 | 52 | −0.10 ± 0.36 | −1.50 ± 0.41* | 28 | −1.10 ± 0.52* | −2.60 ± 0.61* | 24 | .05 ± 0.40 | −0.31 ± 0.40 |
| (c) Glu27-Glu27 | 63 | 0.32 ± 0.33 | −0.38 ± 0.38 | 49 | 0.31 ± 0.41 | −0.16 ± 0.48 | 14 | 0.33 ± 0.51 | −1.16 ± 0.50 |
| (d) Gln27-Glu27 or Gln27-Gln27 | 89 | 0.37 ± 0.28 | −0.70 ± 0.32 | 50 | −0.26 ± 0.40 | −1.35 ± 0.48 | 39 | 1.18 ± 0.31 | 0.17 ± 0.30* |
denotes P < 0.05 for first dose (a vs b) and last dose (a vs b, c vs d).
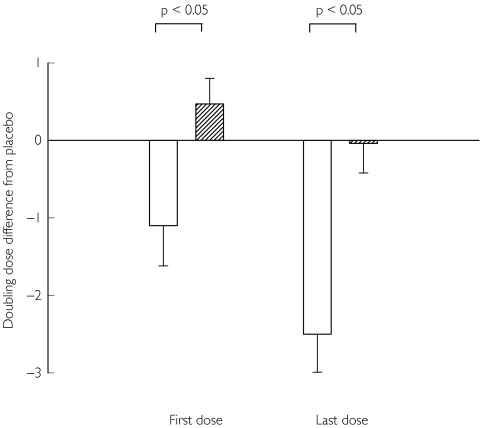
Figure 1.
Doubling dose shift for the single endpoint of MCh PD20 alone, for the effect of genotype on bronchoprotection with long-acting β2-adrenoceptor agonists, as change from placebo, with SEM. A positive value indicates an increase whereas a negative value indicates a decrease in protection for a given genotype, relative to placebo. There was a greater degree of protection loss between first and last doses, for the Arg16-Arg16 or Arg16-Gly16 genotype ( ), compared with the Gly16-Gly16 genotype (
), compared with the Gly16-Gly16 genotype ( ).
).
Haplotype comparison
Haplotype comparisons at position 16 and 27 together showed significantly greater subsensitivity of response for homozygous arginine-16/glutamine-27 compared with homozygous glycine-16/glutamine-27, after the last dose, with a mean doubling dose/dilution difference of 3.00 (95% CI 0.39, 5.61), for the composite end point of MCh PD20 and AMP PC20 together (Table 4).
Table 4.
Doubling dose/dilution shift as change from placebo comparing different haplotypes for 16/27 together.
| Doubling dose/dilution difference from placebo MCh PD20 and AMP PC20(±SEM) | |||
|---|---|---|---|
| Haplotype 16/27 | (n) | First dose | Last dose |
| (a)Gly16-Gly16/Glu27-Glu27 | 60 | 0.32 ± 0.09 | −0.34 ± 0.10 |
| (b) Gly16-Gly16/Gln27-Gln27 | 11 | 1.58 ± 0.10 | 0.88 ± 0.13 |
| (c) Arg16-Arg16/Gln27-Gln27 | 5 | −0.28 ± 0.44 | −2.13 ± 0.48* |
Denotes P < 0.05 for last dose (b vs c).
Drug comparison
When expressing the data as change from placebo, a negative value for the doubling dose/dilution difference indicates the drug is worse than placebo whereas a positive value indicates that the drug is better than placebo. Comparing the two drugs after the last dose, formoterol exhibited significantly greater subsensitivity of response than salmeterol in all genotypes, with the most difference observed in patients who possessed the Arg-16 polymorphism where there was a mean doubling dose/dilution difference of 3.00 (95% CI 1.01, 4.99) for the composite end point of MCh PD20 and AMP PC20 together (Table 5). It can be seen from Figure 2 in patients with Arg16-Arg16 or Arg16-Gly16 genotype, that for formoterol, the mean response was below the zero line, which would indicate a worse response compared with placebo, for both the first and last doses, whereas for salmeterol, the respective responses were both above the line, which would indicate a better response compared with placebo. However, in terms of the degree of protection loss between first and last doses, there was no difference between the drugs. The scatter plot in Figure 3 shows that for formoterol, there were many more patients with responses below the zero line compared with salmeterol, which in turn would indicate a worse response compared with placebo, in patients with the Arg16-Arg16 or Arg16-Gly16 genotype, for both first and last dose effects.
Table 5.
Doubling dose/dilution shift as change from placebo comparing different drugs.
| Doubling dose/dilution difference from placebo | |||||||
|---|---|---|---|---|---|---|---|
| Formoterol | Salmeterol | ||||||
| Genotypes | (n) | First dose | Last dose | (n) | First dose | Last dose | Mean doubling dose/dilution difference for formoterol vs salmeterol after last dose (95% CI) |
| MCh PD20and AMP PC20(± SEM) | |||||||
| (a) Gly16-Gly16 | 62 | 0.27 ± 0.37 | −0.56 ± 0.42 | 38 | 1.10 ± 0.20 | 0.79 ± 0.21* | 1.35 (0.36, 2.34)† |
| (b) Arg16-Gly16 or Arg16-Arg16 | 35 | −1.11 ± 0.49 | −2.52 ± 0.56 | 17 | 1.96 ± 0.30* | 0.48 ± 0.32* | 3.00 (1.01, 4.99)† |
| (c) Glu27-Glu27 | 39 | −0.09 ± 0.48 | −0.97 ± 0.55 | 24 | 0.98 ± 0.25 | 0.57 ± 0.27* | 1.54 (0.27, 2.81)† |
| (d) Gln27-Glu27 or Gln27-Gln27 | 58 | −0.32 ± 0.39 | −1.47 ± 0.45 | 31 | 1.67 ± 0.22* | 0.79 ± 0.23* | 2.26 (0.88, 3.64)† |
| MCh PD20alone (± SEM) | |||||||
| (a)Gly16-Gly16 | 41 | 0.06 ± 0.50 | −0.64 ± 0.59 | 30 | 1.02 ± 0.21 | 0.79 ± 0.20* | 1.43 (0.19, 2.67)† |
| (b) Arg16-Gly16 or Arg16-Arg16 | 23 | −1.75 ± 0.67 | −3.30 ± 0.79 | 5 | 1.88 ± 0.51* | 0.67 ± 0.50 | 3.97 (−0.28, 8.22) |
| (c) Glu27-Glu27 | 29 | −0.12 ± 0.61 | −0.77 ± 0.73 | 20 | 0.95 ± 0.26 | 0.73 ± 0.25 | 1.50 (−0.05, 3.05) |
| (d) Gln27-Glu27 or Gln27-Gln27 | 35 | −0.97 ± 0.56 | −2.28 ± 0.66 | 15 | 1.41 ± 0.30* | 0.83 ± 0.29* | 3.11 (0.81, 5.41)† |
| AMP PC20alone (± SEM) | |||||||
| (a) Gly16-Gly16 | 21 | 0.67 ± 0.41 | −0.41 ± 0.44 | 8 | 1.40 ± 0.66 | 0.81 ± 0.71 | 1.22 (−0.50, 2.93) |
| (b)Arg16-Gly16 or Arg16-Arg16 | 12 | 0.11 ± 0.53 | −1.02 ± 0.50 | 12 | 2.00 ± 0.53* | 0.40 ± 0.50 | 1.43 (−0.03, 2.89) |
| (c)Glu27-Glu27 | 10 | −0.01 ± 0.61 | −1.54 ± 0.50 | 4 | 1.15 ± 0.97 | −0.22 ± 0.79 | 1.32 (−0.71, 3.35) |
| (d) Gln27-Glu27 or Gln27-Gln27 | 23 | 0.67 ± 0.38 | −0.24 ± 0.40 | 16 | 1.91 ± 0.45* | 0.76 ± 0.48 | 1.00 (−0.25, 2.26) |
denotes P < 0.05 for formoterol vs salmeterol (first or last dose) within a given genotype (a, b, c or d);
95% CI which exclude zero indicate a significant (P < 0.05) difference for formoterol vs salmeterol after last dose.
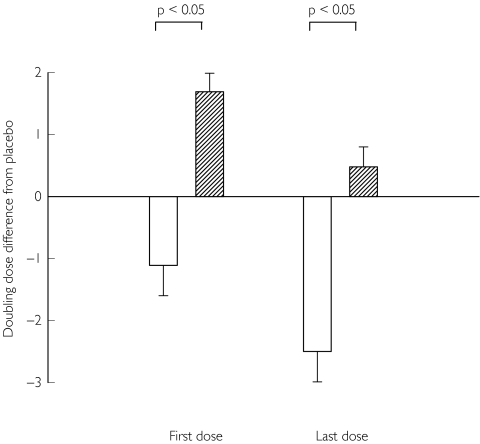
Figure 2.
Doubling dose/dilution shift for the composite end point of MCh PD20 and AMP PC20 together, in patients who possess either homozygous (Arg16-Arg16) or heterozygous (Arg16-Gly16) genotypes containing the Arg-16 allele, comparing formoterol ( ) and salmeterol (
) and salmeterol ( ), with SEM. A positive value indicates an increase whereas a negative value indicates a decrease in protection for either formoterol or salmeterol, relative to placebo. There was no significant difference between the formoterol and salmeterol for the degree of protection loss between first and last doses.
), with SEM. A positive value indicates an increase whereas a negative value indicates a decrease in protection for either formoterol or salmeterol, relative to placebo. There was no significant difference between the formoterol and salmeterol for the degree of protection loss between first and last doses.
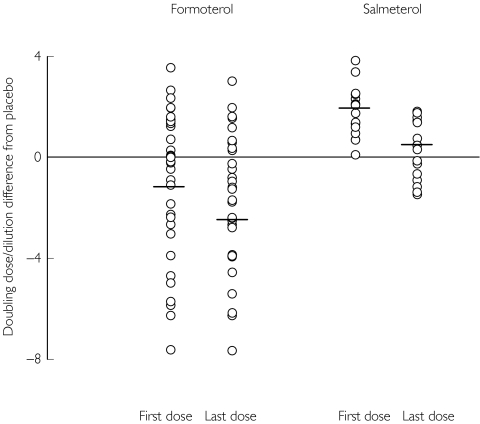
Figure 3.
Scatter plot of individual data for doubling dose/dilution shift, as change from placebo, with mean values as horizontal lines, for composite end point of MCh PD20 and AMP PC20 together, in patients who possess either homozygous (Arg16-Arg16) or heterozygous (Arg16-Gly16) genotypes containing the Arg-16 allele. The corresponding mean (± SEM) values for each drug are given in Table 5.
FEV1 comparison
There were no significant differences in all genotypes when comparing mean prechallenge FEV1 values, as change from placebo, after first or last dose, respectively; Arg16-Arg16/Arg16-Gly16: 0.20 ± 0.06 l, 0.14 ± 0.08 l, Gly16-Gly16: 0.14 ± 0.03 l, 0.16 ± 0.05 l, Gln27-Gln27/Gln27-Glu27: 0.20 ± 0.04 l, 0.17 ± 0.06 L and Glu27-Glu27: 0.11 ± 0.03 l, 0.13 ± 0.05 l. There were also no significant differences in mean FEV1 values as change from placebo comparing formoterol and salmeterol in any of the genotypes.
Discussion
Our results demonstrated that patients who possessed the Arg-16 β2-adrenoceptor polymorphism as either homozygous or heterozygous genotypes were predisposed to enhanced subsensitivity of bronchoprotective response following regular β2-adrenoceptor agonist use as compared with the homozygous Gly-16 genotype. This difference was significant after the last dose for both the composite end point of MCh PD20 and AMP PC20 together as well as for the single end point of MCh PD20 alone. This trend was also observed for the single end point of AMP PC20 alone, although statistical significance was not achieved, which was likely to be due to a type 2 error as a consequence of the relatively smaller sample size in this group of patients. Moreover, for both the composite end point of MCh PD20 and AMP PC20 together and the single endpoint of either MCh PD20 alone or AMP PC20 alone, we found that the degree of protection loss between first and last doses to be greater for the arginine-16 polymorphism compared with the homozygous glycine-16 polymorphism (Figure 1).
In this retrospective analysis, where the stimuli and time of challenges were different, it was important to show that there was no heterogeneity across studies in the protection afforded by long-acting β2-adrenoceptor agonists. We believe the lack of heterogeneity justified using the composite end point of MCh PD20 and AMP PC20 together, as well as evaluating peak and trough responses. Indeed when comparing genotypes for effects on MCh PD20 alone, the observed differences were more marked than the composite end point of MCh PD20 and AMP PC20 together.
The homozygous Arg16/Gln27 haplotype exhibited significantly greater subsensitivity of response for the composite end point of MCh PD20 and AMP PC20 together after the last dose compared with the homozygous Gly16/Gln27 haplotype. As the homozygous Gln-27 genotype is common to both of these haplotypes, this in turn points to the Arg-16 polymorphism being responsible for the greater degree of β2-adrenoceptor down-regulation and associated subsensitivity. However, we do acknowledge that the haplotype analysis was performed on a relatively small sample size in a particular group of haplotypes. Other data have suggested that the acute bronchodilator response to salbutamol is related to certain complex haplotypes, although these are relatively uncommon [20].
The development of subsensitivity following the first dose of long-acting β2-adrenoceptor agonist as demonstrated in our study would be in keeping with a previous study which showed that subsensitivity to the bronchoprotective effect of salmeterol on methacholine bronchial challenge developed within just 12 h of starting salmeterol [21]. Although responses, relative to placebo, for first and last dose effects for formoterol were both in a negative direction, and that for salmeterol were both in a positive direction, the actual difference between first and last dose, which would indicate the degree of protection loss, was not different comparing the two drugs (Figure 2). Nonetheless, the absolute degree of bronchoprotective subsensitivity after the last dose was significantly greater with formoterol than salmeterol in all genotypes for the composite end point of MCh PD20 and AMP PC20 together, with the difference being most evident in patients who possessed the Arg-16 polymorphism. Indeed, from inspection of the scatter plot (Figure 3), it was evident that there were considerably more patients worse off than placebo with formoterol than salmeterol, and that this was the case for both first and last dose effects. These differences occurred despite concomitant inhaled corticosteroid therapy in all patients. It is conceivable that the presence of increased bronchomotor tone as with methacholine or adenosine monophosphate bronchial challenge, may be more likely to uncover differences in intrinsic β2-adrenoceptor agonist activity between formoterol and salmeterol [9], which in turn would be accentuated in genetically susceptible individuals. We felt it would be invalid to compare formoterol and salmeterol for the single end point of MCh PD20 alone in patients with the Arg-16 polymorphism as this would have greatly reduced the available sample size, where there were 23 patients in the formoterol group compared with only five patients in the salmeterol group.
Our results also showed a lack of development of subsensitivity of the bronchodilator response in all genotypes following regular β2-adrenoceptor agonist, as evident by the similar prechallenge FEV1 values. However, we do acknowledge that as none of the studies was powered on FEV1, it would be invalid to make any proper assessment of the bronchodilator response. Nevertheless, as differences in bronchoprotection were disconnected from FEV1, our findings cannot simply be due to differences in airway calibre per se.
The present results would at first sight appear to be contrary to previous in vitro and in vivo studies which showed greater β2-adrenoceptor agonist-induced down-regulation and subsensitivity in association with the Gly-16 β2-adrenoceptor polymorphism [2–6]. However, the previous in vivo studies evaluated the bronchodilator response to salbutamol or formoterol in the presence of resting bronchomotor tone [4–6], which may not relate to what happens when bronchomotor tone is increased in the setting of methacholine or adenosine monophosphate bronchial challenge. It was interesting that a significant mean difference in MCh PD20 protection of 1.57 doubling doses was seen after the first dose comparing genotypes at position 16, while a difference of 3.63 doubling doses occurred after first dose between treatments in genotypes containing the Arg-16 polymorphism. It is unclear whether this difference after the first dose of long-acting β2-adrenoceptor agonist reflects the development of early β2-adrenoceptor down-regulation or prolonged receptor occupancy as has been shown after a single dose of long-acting β2-adrenoceptor agonist [8, 21]. All of our studies employed a β2-adrenoceptor agonist free washout period prior to each randomized treatment, so that the first dose protection was not confounded by potential down-regulation due to reliever therapy with short-acting β2-adrenoceptor agonists.
Our current findings are however, in keeping with previous longer term studies where asthmatic patients with the homozygous Arg-16 β2-adrenoceptor genotype were associated with worse outcomes of asthma control following regular salbutamol use [22, 23]. Moreover, the homozygous Arg16/Gln27 haplotype has also been shown in vivo to be associated with enhanced β2-adrenoceptor agonist mediated desensitization in vascular tissue following continuous exposure to isoproterenol [19], which is consistent with our own findings. According to the so-called dynamic model of receptor kinetics proposed by Liggett [24], it has been suggested that endogenous catecholamines may down regulate β2-adrenoceptor in their basal state to a greater degree in the Gly-16 than the Arg-16 polymorphism. Thus, subsensitivity to exogenous β2-adrenoceptor agonists would be most apparent in Arg-16 patients as their receptors have not yet been down-regulated to the same degree as the Gly-16 patients.
In conclusion, our findings support an important role for a single nucleotide polymorphism of arginine at position 16 of the β2-adrenoceptor in determining the development of subsensitivity following regular exposure to long-acting β2-adrenoceptor agonist therapy in corticosteroid treated asthmatics, with formoterol exhibiting greater subsensitivity than salmeterol. One may argue that to truly predict the development of subsensitivity in clinical practice, one would need to determine the complex haplotype rather than the genotype of a given individual. Moreover, a prospective study will be required to evaluate better the findings of our retrospective analysis. With increasing use of fixed dose combination inhalers containing a long-acting β2-adrenoceptor agonist and inhaled corticosteroid, we believe our results may assume even greater relevance in terms of whether a full or partial β2-adrenoceptor agonist should be used as long-term therapy.
Acknowledgments
This study was funded by the University of Dundee departmental grant and received no support from the pharmaceutical industry.
References
- 1.Lipworth B, Tan S, Devlin M, et al. Effects of treatment with formoterol on bronchoprotection against methacholine. Am J Med. 1998;104:431–438. doi: 10.1016/s0002-9343(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 2.Green SA, Cole G, Jacinto M, et al. A polymorphism of the human beta 2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J Biol Chem. 1993;268:23116–23121. [PubMed] [Google Scholar]
- 3.Green SA, Turki J, Bejarano P, et al. Influence of beta 2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 1995;13:25–33. doi: 10.1165/ajrcmb.13.1.7598936. [DOI] [PubMed] [Google Scholar]
- 4.Tan S, Hall IP, Dewar J, et al. Association between beta 2-adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet. 1997;350:995–999. doi: 10.1016/S0140-6736(97)03211-X. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FD, Graves PE, Baldini M, et al. Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing. J Clin Invest. 1997;100:3184–3188. doi: 10.1172/JCI119874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima JJ, Thomason DB, Mohamed MH, et al. Impact of genetic polymorphisms of the beta2-adrenergic receptor on albuterol bronchodilator pharmacodynamics. Clin Pharmacol Ther. 1999;65:519–525. doi: 10.1016/S0009-9236(99)70071-8. [DOI] [PubMed] [Google Scholar]
- 7.Lipworth BJ, Hall IP, Tan S, et al. Effects of genetic polymorphism on ex vivo and in vivo function of beta2-adrenoceptors in asthmatic patients. Chest. 1999;115:324–328. doi: 10.1378/chest.115.2.324. [DOI] [PubMed] [Google Scholar]
- 8.Aziz I, Lipworth BJ. In vivo effect of albuterol on methacholine-contracted bronchi in conjunction with salmeterol and formoterol. J Allergy Clin Immunol. 1999;103:816–822. doi: 10.1016/s0091-6749(99)70425-2. [DOI] [PubMed] [Google Scholar]
- 9.Linden A, Bergendal A, Ullman A, et al. Salmeterol, formoterol, and salbutamol in the isolated guinea pig trachea: differences in maximum relaxant effect and potency but not in functional antagonism. Thorax. 1993;48:547–553. doi: 10.1136/thx.48.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipworth BJ, Aziz I. A high dose of albuterol does not overcome bronchoprotective subsensitivity in asthmatic subjects receiving regular salmeterol or formoterol. J Allergy Clin Immunol. 1999;103:88–92. doi: 10.1016/s0091-6749(99)70530-0. [DOI] [PubMed] [Google Scholar]
- 11.Lipworth BJ, Dempsey OJ, Aziz I. Functional antagonism with formoterol and salmeterol in asthmatic patients expressing the homozygous glycine-16 beta (2)-adrenoceptor polymorphism. Chest. 2000;118:321–328. doi: 10.1378/chest.118.2.321. [DOI] [PubMed] [Google Scholar]
- 12.Lipworth BJ, Hall IP, Aziz I, et al. Beta2-adrenoceptor polymorphism and bronchoprotective sensitivity with regular short- and long-acting beta2-agonist therapy. Clin Sci (Lond) 1999;96:253–259. [PubMed] [Google Scholar]
- 13.Sims EJ, Jackson CM, Lipworth BJ. Add-on therapy with montelukast or formoterol in patients with the glycine-16 β2-receptor genotype. Br J Clin Pharmacol. 2003;56:104–111. doi: 10.1046/j.1365-2125.2003.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz I, Tan KS, Hall IP, et al. Subsensitivity to bronchoprotection against adenosine monophosphate challenge following regular once-daily formoterol. Eur Respir J. 1998;12:580–584. doi: 10.1183/09031936.98.12030580. [DOI] [PubMed] [Google Scholar]
- 15.Wilson AM, Dempsey OJ, Sims EJ, et al. Evaluation of salmeterol or montelukast as second-line therapy for asthma not controlled with inhaled corticosteroids. Chest. 2001;119:1021–1026. doi: 10.1378/chest.119.4.1021. [DOI] [PubMed] [Google Scholar]
- 16.Beach JR, Young CL, Avery AJ, et al. Measurement of airway responsiveness to methacholine: relative importance of the precision of drug delivery and the method of assessing response. Thorax. 1993;48:239–243. doi: 10.1136/thx.48.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan KS, McFarlane LC, Lipworth BJ. Loss of normal cyclical beta2-adrenoceptor regulation and increased premenstrual responsiveness to adenosine monophosphate in stable female asthmatic patients. Thorax. 1997;52:608–611. doi: 10.1136/thx.52.7.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inman MD, Hamilton AL, Kerstjens HA, et al. The utility of methacholine airway responsiveness measurements in evaluating anti-asthma drugs. J Allergy Clin Immunol. 1998;101:342–348. doi: 10.1016/S0091-6749(98)70246-5. [DOI] [PubMed] [Google Scholar]
- 19.Dishy V, Sofowora GG, Xie HG, et al. The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030–1035. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- 20.Drysdale CM, McGraw DW, Stack CB, et al. Complex promoter and coding region beta2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci USA. 2000;97:10483–10488. doi: 10.1073/pnas.97.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drotar DE, Davis EE, Cockcroft DW. Tolerance to the bronchoprotective effect of salmeterol 12 hours after starting twice daily treatment. Ann Allergy Asthma Immunol. 1998;80:31–34. doi: 10.1016/S1081-1206(10)62935-3. [DOI] [PubMed] [Google Scholar]
- 22.Israel E, Drazen JM, Liggett SB, et al. The effect of polymorphisms of the beta (2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 23.Taylor DR, Drazen JM, Herbison GP, et al. Asthma exacerbations during long term beta agonist use: influence of beta (2)-adrenoceptor polymorphism. Thorax. 2000;55:762–767. doi: 10.1136/thorax.55.9.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liggett SB. The pharmacogenetics of beta2-adrenergic receptors: relevance to asthma. J Allergy Clin Immunol. 2000;105:S487–S492. doi: 10.1016/s0091-6749(00)90048-4. [DOI] [PubMed] [Google Scholar]