Abstract
Aims
To determine the population pharmacokinetics of artemether and dihydroartemisinin in African children with severe malaria and acidosis associated with respiratory distress following an intramuscular injection of artemether.
Methods
Following a single intramuscular (i.m.) injection of 3.2 mg kg−1 artemether, blood samples were withdrawn at various times over 24 h after the dose. Plasma was assayed for artemether and dihydroartemisinin by gas chromatography-mass spectrometry. The software program NONMEM was used to fit the concentration–time data and investigate the influence of a range of clinical characteristics (respiratory distress and metabolic acidosis, demographic features and disease) on the pharmacokinetics of artemether and dihydroartemisinin.
Results
A total of 100 children with a median age of 36.4 (range 5–108) months were recruited into the study and data from 90 of these children (30 with respiratory distress and 60 with no respiratory distress) were used in the population pharmacokinetic analysis. The best model to describe the disposition of artemether was a one-compartment model with first-order absorption and elimination. The population estimate of clearance (clearance/bioavailability, CL/F) was 14.3 l h−1 with 53% intersubject variability and that of the terminal half-life was 18.5 h. If it was assumed that artemisin displays ‘flip-flop’ kinetics, the elimination half-life was estimated to be 21 min and the corresponding volume of distribution was 8.44 l, with an intersubject variability of 104%. None of the covariates could be identified as having any influence on the disposition of artemether. The disposition of dihydroartemisinin was fitted separately using a one-compartment linear model in which the volume of distribution was fixed to the same value as that of artemether. Assuming that artemether is completely converted to dihydroartemisinin, the estimated value of CL/F for dihydroartemisinin was 93.5 l h−1, with an intersubject variability of 90.2%. The clearance of dihydroartemisinin was formation rate limited.
Conclusions
Administration of a single 3.2 mg kg−1 i.m. dose of artemether to African children with severe malaria and acidosis is characterized by variable absorption kinetics, probably related to drug formulation characteristics rather than to pathophysiological factors. Use of i.m. artemether in such children needs to be reconsidered.
Keywords: artemether, children, intramuscular injection, malaria, population pharmacokinetics
Introduction
Mortality from severe malaria among African children ranges from 5 to 15% [1, 2]. Among the factors associated with poor prognosis in severe malaria is respiratory distress; a sign of metabolic acidosis [1]. The latter often resolves with administration of intravenous fluids [3], indicating that dehydration, which is an established cause of metabolic acidosis [4], is associated with malaria in children. Artemisinin derivatives have been introduced in Africa for treatment of malaria, and several formulations, including an oil-based injection for i.m. use are available. A study of the pharmacokinetics of i.m. artemether in African children with severe malaria reported decreased bioavailability in children with respiratory distress compared with those with no respiratory distress, which could be due to decreased peripheral perfusion in those with respiratory distress [5].
The aim of the present study was to apply a population pharmacokinetic approach to investigate the influence of respiratory distress and other covariates on the disposition of artemether and its metabolite, dihydroartemisinin following i.m. administration of artemether to African children.
Methods
Protocol and patients
The study was conducted in the paediatric ward of Kilifi District Hospital in Kenya. The study was approved by the Kenya Medical Research Institute (KEMRI) Ethics Committee. Eligible children were those admitted with peripheral Plasmodium falciparum parasitaemia detected by light microscopy and signs of severe disease [1]. The parents/guardians gave informed consent for their children to be included in the study. Children with salicylate toxicity were excluded from the analysis.
Patients were managed in the high dependency unit (HDU) of the KEMRI unit in Kilifi. A full initial history and clinical examination, including temperature, blood pressure, pulse rate, respiratory rate, assessment of Blantyre coma score (BCS) was undertaken, [6, 7] and the diagnosis of respiratory distress [1] was made. All children received intravenous (i.v.) quinine for treatment of malaria [8]. Teflon cannulae were inserted into a vein of each arm, one for i.v. fluids and drugs, and the other for blood sampling. A loading dose of artemether of 3.2 mg kg−1 was given by deep intramuscular (i.m.) injection.
A blood sample (5 ml) obtained before treatment was sent for a full blood count, thick and thin blood films, blood culture, blood gas, electrolytes, and for the determination of creatinine, glucose, lactate and salicylate concentrations. When lumbar puncture was indicated for diagnostic purposes, this was performed when the child was neurologically stable, as some of the children had raised intracranial pressure [9]. Vital signs, BCS and blood glucose were measured 4 hourly after the initial admission assessment. Oxygen was available if required, but there were no facilities for artificial ventilation. Fluids were given for maintenance at 4 ml kg −1 day−1 or as a bolus of physiological saline (0.9%) if respiratory distress was severe. Resuscitation with blood transfusion was initiated if the patient was severely anaemic [10, 11].
Sampling protocol and drug assay
Blood samples (0.5 ml) were collected pretreatment, and at 0.5, 1, 2, 4, 6, 12, 18 and 24 h after the start of treatment. There were one to five samples from each patient. Blood was centrifuged (1500 g; 5 min) and the plasma separated, dispensed into labelled cryo-tubes and snap-frozen in liquid nitrogen until assayed for artemether and dihydroartemisinin by a gas chromatography-mass spectrometry method [12]. The recoveries for artemether and dihydroartemisinin were 94.9 ± 1.6 and 92.2 ± 4.1%, respectively. The limit of quantification in plasma was 5 ng ml−1 both for artemether and dihydroartemisinin. The within-day coefficients of variations (CVs) were 3–10.4% and 7.7–14.5% for artemether and dihydroartemisinin, respectively. Between-day CVs were 6.5–15.4 and 7.6–14.1% for artemether and dihydroartemisinin, respectively.
Data analysis
Admission parameters
Mean parameters on admission to hospital for children with respiratory distress were compared with those for children without respiratory distress using the 95% confidence interval [13].
Pharmacokinetic analysis
Inspection of the data suggested that a one-compartment open model with first-order absorption would best characterize the plasma profiles. Artemether plasma concentrations were initially modelled alone in the absence of metabolite concentrations. Nonlinear mixed effects modelling was performed by extended least squares regression using the NONMEM program (double precision, version V, level 1.1 [14]), with first-order conditional estimation (FOCE). The pharmacokinetic parameters used to characterize the model were absorption rate constant (ka), apparent clearance (CL/F) and apparent volume of distribution (Vz/F). Dihydroartemisinin plasma concentrations were modelled in a sequential fashion, using the individual artemether parameters as input. Interpatient variability in the pharmacokinetic parameters was modelled with use of an exponential error model as follows:
where pj is the pharmacokinetic parameter (p) for the jth individual as predicted by the regression model,
is the typical population value of p, and ηpj represents the persistent difference between the jth individual's p value and that predicted by the regression model, and was modelled as an independent, identically distributed random variable. A diagonal covariance matrix was implemented, as the data would not support a full variance–covariance matrix. The magnitude of interpatient variability was expressed as a coefficient of variation (%CV), approximated by the square root of the variance estimate. The magnitude of residual variability in artemether and dihydroartemisinin plasma concentrations was modelled with an additive error model. Model assessment was informally based on the population predicted artemether and dihydroartemisinin plasma profiles. Also, goodness of fit was assessed from the scatter plots of individual and population predicted vs. observed concentrations. Plots of weighted residuals (WRES) and residuals (RES) vs. predicted concentrations and vs. time were also used for informal model assessment.
Results
A total of 100 children were recruited into the study. Ten children were excluded from the analysis: Three of these had died immediately after recruitment, whereas the remaining seven had no detectable artemether and dihydroartemisinin in plasma. The clinical and laboratory features of the patients on admission to hospital are given in Table 1. Several of these measurements were significantly different between children with respiratory distress and those without (Table 1). All patients recruited recovered fully after treatment.
Table 1.
Clinical and laboratory measurements in children on admission to hospital
| Respiratory distress | ||||
|---|---|---|---|---|
| Parameter | No of patients | Present | Absent | 95% CI for the difference between themeans,† medians or ‡ proportions |
| Sex (M : F) | 90 | 14 : 16 | 32 : 28 | |
| Age (months)† | 90 | 32 (5–67) | 37.5 (8–108) | −15, 1.0 |
| Weight (kg) | 90 | 9.5 (8.5, 10.5) | 11.3 (10.5, 12.1) | −3.1, −0.5* |
| Auxiliary temperature (°C) | 88 | 37.9 (37.6, 38.4) | 38.1 (37.8, 38.4) | −0.3, 0.8 |
| Rectal temperature (°C) | 83 | 38.9 (38.6, 39.3) | 38.7 (38.3, 39.2) | −0.8, 0.4 |
| Pulse rate (beats min−1) | 89 | 155 (146, 164) | 143 (137, 149) | 1.6, 22.3* |
| Respiratory rate (breaths min−1)† | 89 | 50 (32–100) | 38 (18–88) | 8.0, 20.0* |
| Coma (BCS ≤ 2)‡ | 90 | 15/30 (50%) | 34/60 (56.7%) | −0.27, 0.14 |
| Parasitaemia (µl−1); geometric mean | 90 | 49051 (22212, 108317) | 32406 (17048, 61602) | 0.53, 4.34* |
| Haemoglobin (g dl−1) | 90 | 5.97 (5.0, 6.9) | 6.96 (6.4, 7.5) | −2.0, 0.02 |
| Sodium (mmol l−1) | 90 | 135.7 (133.2, 138.2) | 134.9 (133.3, 136.4) | −1.9, 3.6 |
| Potassium (mmol l−1) | 90 | 5.3 (4.8, 5.7) | 4.9 (3.7, 6.3) | −1.6, 2.1 |
| Urea (µmol l−1) | 90 | 9.5 (6.9, 12.1) | 4.2 (3.3, 5.1) | 3.1, 7.4* |
| Creatinine (µmol l−1) | 90 | 102.7 (82.2, 123.2) | 62.2 (54.0, 70.4) | 22.4, 58.7* |
| Blood glucose (≤ 2.2 mmol l−1)‡ | 56 | 9/27 (33.3%) | 7/29 (24.1%) | −0.14, 0.32 |
| Lactate (mmol l−1) | 77 | 5.2 (3.8, 6.7) | 4.23 (3.32, 5.14) | −0.5, 2.5 |
| Salicylate (mg dl−1) | 80 | 2.9 (2.0, 3.8) | 2.7 (1.6, 3.9) | −1.5, 1.8 |
| pH | 89 | 7.24 (7.18, 7.30) | 7.38 (7.34, 7.41) | −0.2, −0.1* |
| Base excess† | 89 | −14.8 (−17.9, −11.7) | −4.8 (−6.5, −3.1) | −12.8, −6.7* |
| Blood transfusion‡ | 90 | 16/30 (50%) | 13/60 (21.7%) | 0.11, 0.52* |
Values are mean (95% confidence interval on the mean) or
median (range) or
number (percentage).
Significantly different between the two groups. BCS, Blantyre coma score.
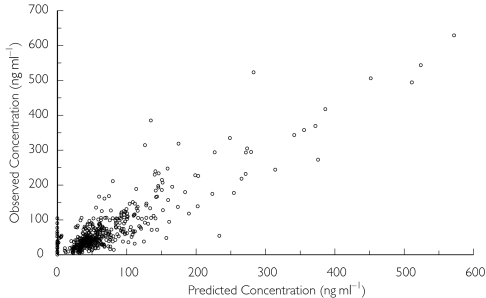
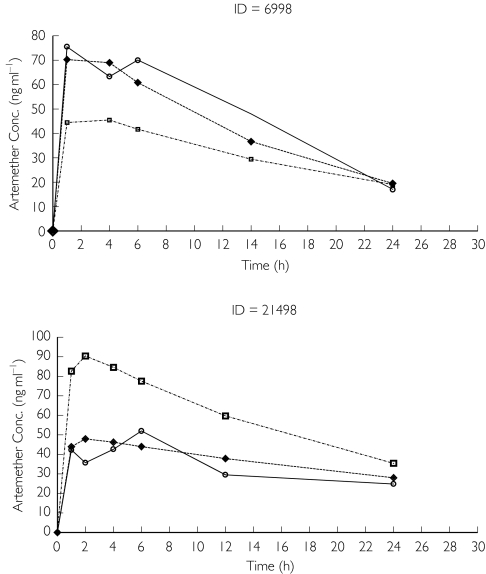
The artemether plasma concentrations were fitted with a one-compartment model with first-order absorption, using first-order estimation. However, there was negligible variability in the estimate of clearance, which did not appear to represent the data. The analysis was repeated using first-order conditional estimation and the population parameter estimates were calculated with improved precision. Parameters were estimated with reasonable precision (≤36.6%) and are shown in Table 2. Based on the estimate of Cl/F and Vz/F the elimination half-life was 18.5 h, whereas the absorption half-life was 0.046 h. Scatter plots indicated that the model fitted the data reasonably well (Figure 1). Plots of weighted residuals vs. predicted concentration and time were uniformly and evenly distributed around zero. Many concentration–time profiles showed ‘erratic’ behaviour (two representative subjects are shown in Figure 2). Alternate absorption models (zero order) and residual error models (proportional and combined additive and proportional) were tried but did not improve the fit or did not converge successfully. It was assumed that ‘flip-flop’ kinetics was occurring and so the model was re-run forcing the elimination and absorption parameters to change round. Cl/F was little affected but the Vz/F estimate became 8.44 l and the elimination half-life was 21 min (Table 2). Graphical analysis did not reveal any clear kinetic–covariate relationships and hence formal covariate analysis using NONMEM was not performed.
Table 2.
Pharmacokinetic parameter estimates for artemether and dihydroartemisinin (DHA) based on a one-compartment open model with first-order absorption using first-order conditional estimation (FOCE) estimation and an additive residual error model
| Parameter | Estimate | Precision (% CV) | |
|---|---|---|---|
| artemether | ka (h−1) | 1.52 | 36.5 |
| [absorption half-life (h)] | (0.46) | ||
| Original run | Cl/F (l h−1) | 14.3 | 12.8 |
| Vz/F (l) | 382.0 | 11.7 | |
| [elimination half-life (h)] | (18.5) | ||
| artemether | ka (h−1) | 0.0435 | 17.6 |
| [absorption half-life (h)] | (15.9) | ||
| ka and k changed round | Cl/F (l h−1) | 16.8 | 11.2 |
| Vz/F (l) | 8.44 | 44.2 | |
| [elimination half-life (h)] | (0.35) | ||
| Interpatient variability in ka (% CV)* | 103.0 | 32.3 | |
| Interpatient variability in Cl/F (% CV) | 52.7 | 27.8 | |
| Interpatient variability in Vz/F (% CV) | 102.0 | 25.0 | |
| Residual variability for drug (ng ml−1) | 39.0 | 21.1 | |
| DHA | Clm/F (l h−1) | 93.5 | 19.3 |
| Interpatient variability in Clm/F (% CV) | 90.3 | 27.7 | |
| Residual variability for metabolite (ng ml−1) | 10.2 | 46.9 |
Values are from the original analysis, but were very similar when ka and k were changed round.
Figure 1.
Plot of observed artemether concentrations vs. posterior predicted concentrations
Figure 2.
Observed (○), population (□) and posterior individual (♦) artemether concentration–time plots for two representative subjects
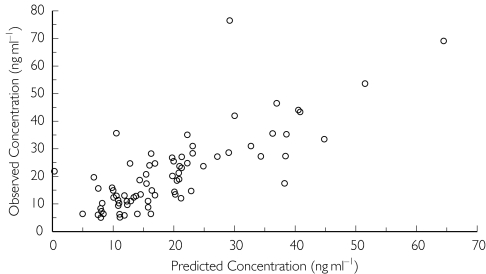
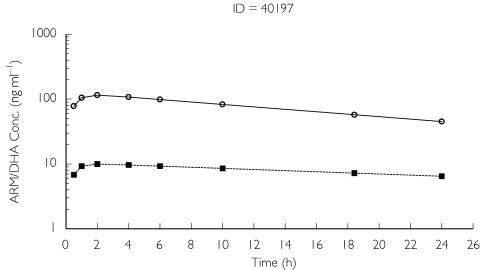
A one-compartment model with all of the drug being eliminated via the metabolite was employed for the analysis of dihydroartemisinin concentrations. However, it was not possible simultaneously to model drug and metabolite concentration profiles. Parameters for the parent drug were fixed to those of the individual posterior estimates obtained from the one-compartment linear model with first-order conditional estimation for artemether. However, despite NONMEM indicating successful minimization with no errors, the estimate of volume for the metabolite (dihydroartemisinin) was negligible. To overcome this, the volume parameter for the metabolite was fixed to be the same as that for the drug (8.44 l) enabling estimation of Clm/F (Table 2). Scatter plots indicated the model characterized dihydroartemisinin concentrations moderately well (Figure 3), but once again many of the profiles were erratic and simply mirrored those predicted for artemether (Figure 4). Graphical analysis did not reveal any clear kinetic–covariate relationships and hence a formal NONMEM covariate analysis for dihydroartemisinin was not performed.
Figure 3.
Plot of observed dihydroartemisinin concentrations vs. posterior predicted concentrations.
Figure 4.
Population predicted drug and metabolite profiles for subject 40197. ARM pop predicted (○) and DHA pop predicted (▪)
Discussion
Artemether absorption following i.m. dosing was very erratic with high interpatient variability. The estimate of Cl/F but not half-life was consistent with literature values [15]. No data following i.v. administration are available, but it was speculated, based upon previously available information after oral administration of artemether [15], that the half-life should be relatively short, in the region of 1–2 h and certainly not as long as 18.5 h found in our original analysis. If we assume that the terminal half-life is the absorption half-life, rather than the elimination half-life, a much lower value for the volume of distribution (8.44 l) is found. This is approximately 0.9 l kg−1, based on the mean weight of the children in this study. A larger apparent volume of distribution of approximately 10 l kg−1 has been reported previously in healthy adults [15]. Artemether is known to be highly bound to plasma proteins, mainly α1-acid glycoprotein and albumin [16]. The concentrations of both proteins might change (increased α1-acid glycoprotein and decreased albumin) in severe malaria, but how these changes may affect the volume of distribution of artemether has not been investigated in children with severe malaria. The absorption of the drug was prolonged and erratic. The appearance of dihydroartemisinin in the circulation was rate-limited by its formation from artemether. Parent drug and metabolite concentrations could not be modelled simultaneously, and thus drug parameters were fixed to those of the posterior estimates obtained following the artemether analysis and Vz/F was made equal to the value for artemether to enable estimation of the Cl/F of dihydroartemisinin. The clearance of dihydroartemisinin was high and variable. None of the possible covariates studied (respiratory distress, age, weight or sex) appeared to affect the pharmacokinetics of artemether.
A number of assumptions were required to allow a satisfactory model fit to be obtained. These assumptions were forced by the sparseness and erratic nature of the data. More intense sampling, albeit logistically difficult, would have aided the analysis but erratic absorption from the i.m. site does not enable implementation of a more complex model.
In a previous study [5] of a smaller group we reported that children with respiratory distress (a sign of metabolic acidosis) had low plasma concentrations of artemether and dihydroartemisinin. Clinically, these patients had both an increased parasite clearance time and an increased coma resolution time. We speculated that respiratory distress and the associated metabolic acidosis might account for the decreased absorption of artemether following i.m. administration. However, the results of the present study suggest that the slow and erratic absorption of artemether following i.m. administration is a property of the drug formulation (an oily injection) and respiratory distress may not play a significant role.
One of the clinical advantages of artemisinin antimalarial compounds is their rapid parasite clearance and resolution of fever. However, this advantage will be realized only if the drug is absorbed rapidly into systemic circulation. A rapid onset of antimalarial action which is sustained for at least three to four life cycles of the parasite is considered essential for successful treatment [15]. Both artemether and dihydroartemisinin have been reported to have relatively short elimination half-lives of 2–5 h following oral administration [15, 17], which accounts partly for the need for extended (≥5 days) oral monotherapy with these compounds to prevent recrudescence. Theoretically, i.m. administration of the oil-soluble artemether should offer an advantage over oral administration due to the expected sustained plasma concentrations of artemether and dihydroartemisinin.
Our study shows that the in vivo plasma concentrations of artemether and dihydroartemisinin, following i.m. artemether, would rapidly exceed the IC50 value (1–6 nm, or 0.3–1.8 ng ml−1) for sensitive parasites [18] in the large majority of patients. Support for this contention comes from the treatment of severe falciparum malaria, where the effect of i.m. artemether is demonstrable on the viability of asexual parasites within 6 h of the first injection [19]. However, the results of this study also suggest that for i.m. artemether a major problem in some patients, especially children with severe malaria, may be its slow and erratic absorption and conversion to dihydroartemisinin. The interpatient variability in artemether and dihydroartemisinin concentrations demonstrated here has also been observed in pharmacokinetic studies in healthy volunteers [20]. Furthermore, it has been inferred from differences in survival in a clinical trial where, in a subgroup of children with respiratory distress, mortality was higher in patients treated with artemether compared with those treated with quinine [21]. With the additional evidence of significant patient-to-patient variability in i.m. artemether absorption provided in the present work, we support the statement by Teja-Isavadharn et al.[20] that a small but significant fraction of patients might have inadequate blood concentrations of artemether and dihydroartemisinin in the critical early phase of treatment. Although artemether is still recommended for the treatment of severe falciparum malaria by WHO, a recent Cochrane review of studies comparing quinine with artemisinin derivatives (including artemether) showed little difference between these treatments [22]. However, the same review [22] reported that use of artemisinin derivatives was associated with more rapid parasite clearance from blood, suggesting that the water-soluble derivative artesunate would be a better alternative, and might be superior to quinine as a treatment of severe malaria in children. In adults with uncomplicated malaria, it has recently been shown that i.m. artesunate is a suitable alternative to i.v. artesunate [23]. In countries with poor resources, i.m. artesunate would be preferred due to ease of administration, but its pharmacokinetics in children with severe malaria would still need to be evaluated.
Acknowledgments
This work is published with the permission of the Director of Kenya Medical Research Institute (KEMRI). The study was supported by a Wellcome Trust project grant (grant no. 048335/A/96/A) to W.W. and a Collaborative Research Initiative Grant from the Wellcome Trust (grant no. 057978/Z/99/Z) to G.O.K. We are grateful to Dr Nobert Peshu (Director, KEMRI-Centre for Geographic Medicine Research-Coast) for his support, and to Dr Jean-Pierre Helenport (Rhone-Poulenc Rorer, France) for the supply of artemether.
References
- 1.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 2.Waller D, Krishna S, Crawley J, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21:577–87. doi: 10.1093/clinids/21.3.577. [DOI] [PubMed] [Google Scholar]
- 3.Taylor TE, Borgstein A, Molyneux ME. Acid-base status in paediatric Plasmodium falciparum malaria. Q J Med. 1993;86:99–109. [PubMed] [Google Scholar]
- 4.Kildeberg P. Metabolic acidosis in infantile gastroenteritis. Acta Paedtr Scand. 1965;54:155–67. doi: 10.1111/j.1651-2227.1965.tb06357.x. [DOI] [PubMed] [Google Scholar]
- 5.Murphy SA, Mberu E, Muhia D, et al. The disposition of intramuscular artemether in children with cerebral malaria: a preliminary study. Trans R Soc Trop Med Hyg. 1997;91:331–4. doi: 10.1016/s0035-9203(97)90097-3. [DOI] [PubMed] [Google Scholar]
- 6.Molyneux ME, Taylor TE, Wirima JJ, et al. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–59. [PubMed] [Google Scholar]
- 7.Newton CR, Chokwe T, Schellenberg JA, et al. Coma scales for children with severe falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:161–5. doi: 10.1016/s0035-9203(97)90207-8. [DOI] [PubMed] [Google Scholar]
- 8.Winstanley P, Newton C, Watkins W, et al. Towards optimal regimens of parenteral quinine for young African children with cerebral malaria: the importance of unbound quinine concentration. Trans R Soc Trop Med Hyg. 1993;87:201–6. doi: 10.1016/0035-9203(93)90494-b. [DOI] [PubMed] [Google Scholar]
- 9.Newton CR, Kirkham FJ, Winstanley PA, et al. Intracranial pressure in African children with cerebral malaria. Lancet. 1991;337:573–6. doi: 10.1016/0140-6736(91)91638-b. [DOI] [PubMed] [Google Scholar]
- 10.English M, Waruiru C, Marsh K. Transfusion for respiratory distress in life-threatening childhood malaria. Am J Trop Med Hyg. 1996;55:525–30. doi: 10.4269/ajtmh.1996.55.525. [DOI] [PubMed] [Google Scholar]
- 11.English M, Waruiru C, Amukoye E, et al. Deep breathing in children with severe malaria: indicator of metabolic acidosis and poor outcome. Am J Trop Med Hyg. 1996;55:521–4. doi: 10.4269/ajtmh.1996.55.521. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed SS, Khalid SA, Ward SA, et al. Simultaneous determination of artemether and its major metabolite dihydroartemisinin in plasma by gas chromatography-mass spectrometry-selected ion monitoring. J Chromatogr B Biomed Sci Appl. 1999;731:251–60. doi: 10.1016/s0378-4347(99)00232-7. [DOI] [PubMed] [Google Scholar]
- 13.Altman D, Machin D, Bryant T, Gardner MJ. Statistics with confidence. 2. Bristol: BMJ Books; 2000. [Google Scholar]
- 14.Beal S, Boeckman A, Sheiner L. NONMEM user's guide. San Francisco: University of California; 1998. Parts I–IV. [Google Scholar]
- 15.Karbwang J, Na-Bangchang K, Congpuong K, et al. Pharmacokinetics and bioavailability of oral and intramuscular artemether. Eur J Clin Pharmacol. 1997;52:307–10. doi: 10.1007/s002280050295. [DOI] [PubMed] [Google Scholar]
- 16.Colussi D, Parisot C, Legay F, et al. Binding of artemether and lumefantrine to plasma proteins and erythrocytes. Eur J Pharm Sci. 1999;9:9–16. doi: 10.1016/s0928-0987(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 17.Lefevre G, Carpenter P, Souppart C, et al. Pharmacokinetics and electrocardiographic pharmacodynamics of artemether-lumefantrine (Riamet) with concomitant administration of ketoconazole in healthy subjects. Br J Clin Pharmacol. 2002;54:485–92. doi: 10.1046/j.1365-2125.2002.01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alin M, Bjorkman A, Ashton M. In vitro activity of artemisinin, its derivatives, and pyronaridine against different strains of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1990;84:635–7. doi: 10.1016/0035-9203(90)90129-3. [DOI] [PubMed] [Google Scholar]
- 19.Murphy S, Watkins WM, Bray PG, et al. Parasite viability during treatment of severe falciparum malaria: differential effects of artemether and quinine. Am J Trop Med Hyg. 1995;53:303–5. doi: 10.4269/ajtmh.1995.53.303. [DOI] [PubMed] [Google Scholar]
- 20.Teja-Isavadharm P, Nosten F, Kyle DE, et al. Comparative bioavailability of oral, rectal, and intramuscular artemether in healthy subjects: use of simultaneous measurement by high performance liquid chromatography and bioassay. Br J Clin Pharmacol. 1996;42:599–604. doi: 10.1111/j.1365-2125.1996.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 21.Murphy S, English M, Waruiru C, et al. An open randomised trial of artemether versus quinine in the treatment of cerebral malaria in African children. Trans R Soc Trop Med Hyg. 1996;90:298–301. doi: 10.1016/s0035-9203(96)90260-6. [DOI] [PubMed] [Google Scholar]
- 22.McIntosh H, Olliaro P. The Cochrane. Oxford: 2003. Artemisinin derivatives for the treatment of malaria (Cochrane Review) Issue I. [Google Scholar]
- 23.Ilett KF, Batty KT, Powell SM, et al. The pharmacokinetic properties of intramuscular artesunate and rectal dihydroartemisinin in uncomplicated falciparum malaria. Br J Clin Pharmacol. 2002;53:23–30. doi: 10.1046/j.0306-5251.2001.01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]