Abstract
Aim
To investigate the effect of diet upon liver function tests and serum lipids within the restricted environment of a Phase I unit.
Methods
An open randomized three-way crossover study was designed with subjects consuming three types of diet. The diets comprised, a balanced normal calorie diet, a high-carbohydrate high-calorie diet and a high-fat high-calorie diet. Each diet was consumed in a randomized sequence over 8 days with a recovery period of 14 days between periods. The blood concentrations of various laboratory parameters were measured at intervals throughout each dietary period and during the recovery periods.
Results
Blood transaminase activity and triglyceride concentrations increased significantly whilst subjects consumed a high-carbohydrate high-calorie diet but not when fed either a high-fat high-calorie diet or a balanced normal calorie diet.
Conclusions
The rises in transaminases and triglycerides were caused by the carbohydrate content of the diet rather than its calorific value. Sucrose rather than starch was the carbohydrate which caused the rise in transaminases and triglycerides. The importance of controlling diet in Phase I studies is stressed.
Keywords: diet, liver function tests, lipids, Phase I
Introduction
The liver is the site at which many drugs are removed from the circulation and subsequently metabolized and excreted. By virtue of its position astride the portal circulation the liver is exposed to greater concentrations of orally administered drugs than any other organ apart from the intestine itself. It is not surprising therefore that many drugs can produce hepatic injury including hepatocellular damage, cholestasis and even tumour production [1]. Consequently a major safety concern in any clinical study of a new chemical entity (NCE) will be the effect of the drug upon the liver. Such effects are monitored by changes in the so-called liver function tests (LFTs). These tests include the transaminases [alanine transaminase (ALT) and aspartate transaminase (AST)], alkaline phosphatase (ALP), bilirubin, total protein and albumin with, in addition, gamma glutamyl transferase (γGT), a commonly measured sensitive marker of cholestasis.
In early clinical studies of NCEs in healthy volunteers (Phase I trials) it is important to provide an environment in which any potential effects of nondrug factors upon LFTs are minimized. The reason for this is that nondrug-related changes in LFTs might (incorrectly) be attributed to an effect of the drug itself resulting in the delay, or even termination, of the development of a potentially useful compound. Changes in LFTs of obscure origin have been noted during prolonged periods of residence at the Pfizer Phase I units (unpublished data) and have also been reported in Phase I studies at other centres. Such findings have been attributed to several factors including excess calorie intake due to lack of exercise [2] or to prestudy factors, namely ALT>AST, a high obesity index and/or a high γGT activity [3]. The prestudy activities of ALT and AST/ALT ratios have been proposed to be useful discriminators between ‘ALT-susceptible’ and ‘ALT-nonsusceptible’ volunteers in a retrospective meta-analysis of 13 studies of volunteers taking placebo [4]. Alternatively, Rosenzweig, Brohier and Zipfel [5] tentatively attributed transaminase changes in healthy volunteers taking placebo to ‘dietary factors and rest’, and a more detailed paper from the same group, once again studying healthy volunteers taking placebo, came to a similar conclusion [6]. Indeed, there is evidence in the literature that diet can have an effect upon hepatic enzymes both in animals [7–9] and in healthy humans [2, 10, 11]. However, in patients receiving enteral nutrition, the observed changes in LFTs were considered more likely to be associated with clinical complications rather than with the enteral nutritional support itself [12].
Many of the healthy volunteers in Phase I studies are manual workers who retain large appetites despite much reduced levels of physical activity, compounded by boredom, during long periods of residence within the Phase I environment. Unless carefully monitored these volunteers will eat far in excess of their calorific requirements and they have been noted to consume large amounts of sweet high-carbohydrate foods. We have also observed that restriction of these sweet foods appeared to decrease the incidence of spurious LFTs. With these empirical observations in mind and on the basis of the evidence outlined above, it was decided to investigate formally the effect of diet upon laboratory parameters.
A study was designed to test the effects of three types of diet [high carbohydrate, high calorie (HCHC); high fat, high calorie (HFHC); and balanced normal calorie diets] upon LFTs and also upon antipyrine clearance (as a measure of hepatic microsomal metabolizing capacity). In addition, since elevation of transaminase activities, particularly AST, in the blood can result from muscle damage, the enzyme creatinine kinase (CK), a relatively specific and sensitive marker of muscle damage, was also measured to discriminate between possible effects of diet upon muscle and liver. The calorific values of the high-calorie diets used were excessive but this was done deliberately to maximize the effects of excess calorie intake on the one hand and dietary composition on the other.
It was postulated over 40 years ago that dietary composition, particularly carbohydrate content, could affect blood lipid profiles [13], but there have been conflicting reports in the literature on whether or not this is so [14]. Therefore, as a secondary objective, the effects of the three types of diet upon serum cholesterol and triglyceride levels were also investigated.
Methods
The study
This was an open, randomized, three-way crossover study which involved 12 healthy male subjects aged 20–41 years eating each of three diets in a randomized order to avoid sequence effects. Subjects spent 8 days (9 nights) at the Pfizer Research Clinic eating the randomly selected diet with a recovery period of 14 nonresident days between each dietary period. The 8-day dietary period was selected mainly on personal experience from previous Phase I studies, although there is also evidence in the literature indicating that 8 days is a sufficient period to produce enzyme changes [11]. Similarly the 14-day recovery period was based empirically upon in-house experience, although Porikos and Van Itallie discovered that raised transaminase activities returned to baseline 12 days after switching from a sucrose to an aspartame-containing diet [11]. Whilst nonresident, the subjects consumed their usual diets without restriction. All aspects of the study were conducted in accordance with Good Clinical Practice (GCP) following a carefully designed protocol together with a volunteer information sheet and consent form, all of which had been approved by the Kent and Canterbury Local Hospital Research Ethics Committee. Volunteers were given a full explanation of the study and time for consideration prior to signing the consent form.
Before entry into the study, each subject had a medical screen including a full medical history and physical examination with routine biochemical (including LFTs) and haematological profiles, a hepatitis B screen and a urinary drugs of abuse screen. Fully informed consent for the study was obtained at that time. Subjects were allowed to enter the study only if their medical screen was acceptable, including laboratory profiles which were within the appropriate reference ranges. They were also required to fulfil a number of other criteria which included a body weight which was within the range of 18–28 by Quetelet's index [(weight (kg)/height (m)2], smoking less than 5 cigarettes/day and drinking less than 21 units of alcohol/week. In addition, the subjects had to remain free of any medication (excluding ibuprofen for minor ailments) for at least 3 weeks prior to the start of and throughout the study. The subjects were not allowed to consume alcohol or caffeine, or undertake any unaccustomed exercise for a minimum of 2 days prior to each admission to the unit. Unaccustomed exercise is defined as any exercise which the subject does not routinely take as part of his normal week. Apart from this, during periods of nonresidency, diet and exercise were unrestricted. A dietary history was taken from each subject to ensure that their usual diet was satisfactory and that they were not malnourished.
On the evening of each admission the subjects had a physical examination, were screened for drugs of abuse (urine) and breathalysed for alcohol. Routine urinalysis was also performed at that time. The following morning, prior to commencing the diet, the subjects were weighed, fasting blood samples (baseline) were taken and 600 mg of antipyrine were administered orally for the measurement of antipyrine clearance (see below). Antipyrine was also administered on day 8 of each dietary period.
The diets used in this study with the relative amounts of fat, carbohydrate and protein and the split of carbohydrate between simple sugars and starch are shown in Table 1. The HFHC diet was approximately isocaloric to the HCHC diet, both diets providing more than twice the calories provided by the standard diet. The diets were devised using readily available everyday food and no special dietary supplements were used. The diets were given as three main meals a day together with a light supper. Whilst subjects were on the high-calorie diets they were also given supplementary snacks (e.g. crisps for fat, biscuits for carbohydrate) at appropriate times during the day. The eating of meals/snacks was carefully supervised to ensure that all food and drink was consumed. Subjects received each diet for 8 days. On days 3, 5, 7 and 9 (day of discharge) of each dietary period, fasting morning blood samples were collected, centrifuged and the serum saved for subsequent biochemical analysis. Similarly treated additional fasting blood samples were taken on the mornings of days 6 and 8. These samples were analysed retrospectively only if the results from the other days were of interest. In addition to this, subjects were asked to return to the Clinic following discharge 12 and 16 days after commencing each diet when further fasting blood samples were taken for biochemical analysis. A follow-up examination was conducted on day 16 after the start of the final dietary regimen. This included a physical examination and collection of blood and urine samples for analysis.
Table 1.
Composition of diets
| Diet | Daily kCal | Fat | Protein | Total | Carbohydrate Sucrose | Starch |
|---|---|---|---|---|---|---|
| Standard | 1900 | 798 (42%) | 228 (12%) | 874 (46%) | 266 (14%) | 608 (32%) |
| HFHC | 4500 | 2610 (58%) | 585 (13%) | 1305 (29%) | 180 (4%) | 1125 (25%) |
| HCHC | 4400 | 1320 (30%) | 484 (11%) | 2596 (59%) | 1408 (32%) | 1188 (27%) |
Values are kCal (% of total) provided by macronutrients. HFHC, High fat, high calorie; HCHC, high carbohydrate, high calorie.
Laboratory analyses
Blood samples for routine biochemical and haematological assessment were collected at the prestudy medical screen, prior to the start of each dietary regimen and at follow-up. These tests included routine urea and electrolytes, LFTs (including γGT), creatinine, calcium, CK, total cholesterol and triglycerides together with a full blood count (FBC) with white cell differential. Additional tests, performed at the prestudy screening examination only, were a hepatitis B screen, a fasting glucose and a reticulocyte count. All test results were reviewed by a physician prior to inclusion of the volunteer in the study.
On fixed days after the start of each dietary regimen (see earlier), the LFTs, CK, total cholesterol and triglycerides were measured on serum from fasting morning blood samples. The biochemical analyses were performed using a Kodak Ektachem automated analyser (Kodak Ltd UK Supplers, Orthochnical Diagnostics, Amersham, UK) and haematological tests with a Coulter counter (Beckman Coulter UK Ltd., High Wycombe, UK). Standard urinalysis (pH, protein, glucose, ketones and blood) was performed with a stix test (BM-Test-5 l; Boehringer, Mannheim, Germany). Urine was analysed for drugs of abuse (cocaine, barbiturate, methadone, benzodiazepines, opiates, cannabinoids and amphetamines) on each admission with a Triage 8® kit (Biosite® Diagnostics, Biosite Inc., San Diego, CA, USA).
Antipyrine clearance was measured by the simplified method described by Dossing et al.[15]. This involved collecting one sample of saliva for antipyrine assay 24 h after administering an oral antipyrine dose (i.e. on day 2 and day 9 of each study period). Antipyrine was assayed in the saliva using high-performance liquid chromatography with UV detection at a wavelength of 254 nm. The overall inaccuracy (bias%) for QC samples within each analytical batch ranged from 8.80 to 9.67% and overall imprecision (CV%) varied from 1.47 to 2.52%; the coefficients of variation (CV%) for calibration standards within each analytical batch ranged from 2.13 to 4.40%.
Statistical analysis
All analyses were performed using a statistical software programme (SAS, version 6.09; SAS Institute Inc., Cary, NC, USA).
For all parameters (except antipyrine clearance), the natural logarithm (ln) of the value which deviated the most from baseline in each study period (day 1 to day 16) was analysed using analysis of variance (anova). This was the maximum observed value for each subject on each diet for all parameters except bilirubin, which used the minimum observed value. The anova model included terms for subject, diet and period. Differences between diets were estimated using pairwise contrasts. Antipyrine clearance values from day 2 and day 9 of each study period were logged (ln) and analysed using anova as above, but included an additional term for day and a day by diet interaction.
The mean, difference between means and standard error (of the difference) values were back transformed (exponentiated) to give geometric means and approximate 95% confidence intervals (CIs). To confirm model specification, scatter plots of residuals and absolute residuals against fitted values were checked.
Results
Subjects remained well and there were no changes of note in any of the physical examinations performed throughout the study. All drugs of abuse screens performed on each admission were negative and there was nothing of note in any of the routine urinalyses.
Body mass, recorded on day 1 and day 9 of each period, is summarized in Table 2. Comparing day 1 with day 9 values, both the HFHC and HCHC diets caused significant rises in body mass (P < 0.001), with mean increases of 2.5 kg (95% CI 1.9, 3.2) and 2.1 kg (95% CI 1.5, 2.8) for the HFHC and HCHC diets, respectively.
Table 2.
Effect of the diets on body mass (kg) (values mean ± SD).
| Day of diet | Standard | HFHC | HCHC |
|---|---|---|---|
| 1 | 76.6 ± 10.5 | 76.4 ± 10.2 | 76.6 ± 10.0 |
| 9 | 75.8 ± 9.9 | 78.9 ± 9.7 | 78.7 ± 10.5 |
| Difference | −0.8* | 2.5** | 2.1** |
P < 0.05;
P < 0.001. HFHC, High fat, high calorie; HCHC, high carbohydrate, high calorie.
In contrast, the standard (normal calorie) regimen resulted in a small but significant (P < 0.05) mean fall in body mass of 0.8 kg (95% CI −1.5, −0.2).
Antipyrine clearance
Antipyrine clearance values are shown in Table 3. Mean values fell consistently for each diet from day 2 to day 9 by about 12% (95% CI −17, −6; P < 0.001), although there was no evidence of a day by diet interaction (P = 0.801) indicating that antipyrine clearance was not affected by diet.
Table 3.
Antipyrine clearance (values mean ± SD)
| Day of diet | Standard | HFHC | HCHC |
|---|---|---|---|
| 2 (ml min−1) | 56.4 ± 12.1 | 58.3 ± 8.4 | 55.9 ± 10.3 |
| 9 (ml min−1) | 48.0 ± 7.3 | 52.4 ± 5.5 | 49.7 ± 9.3 |
HFHC, High fat, high calorie; HCHC, high carbohydrate, high calorie.
Liver function tests (LFTs)
Total protein and albumin in serum were not affected by diet (P = 0.264 and P = 0.705, respectively).
The HCHC diet had a small but statistically significant effect on ALP activity (P = 0.007) with values 6% (95% CI 1, 11; P = 0.017) higher compared with the standard diet, and 8% (95% CI 3, 13; P = 0.003) higher compared with the HFHC diet. However, all activities remained within the reference range for ALP.
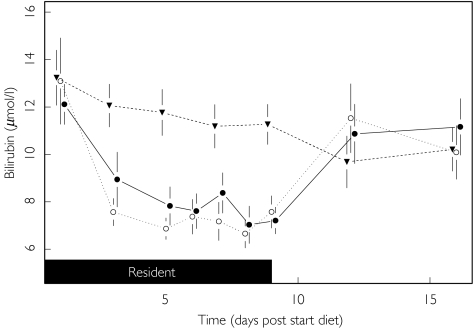
Bilirubin concentrations fell during all dietary periods (see Figure 1). The magnitude of fall was significantly greater for the HCHC (95% CI −35%, −12%) and HFHC (95% CI −38%, −18%) diets compared with the standard diet (P < 0.001) but there was no difference between the HCHC and HFHC diets (95% CI −8%, 23%; P = 0.395). When the subjects reverted to their usual diet (day 9 onwards) bilirubin concentrations rose again to baseline levels.
Figure 1.
Effect of diet upon bilirubin. [Graphs (Figures 1–6) represent various laboratory parameters for the three diets (Standard (▾), HFHC (○) and HCHC (•)). Data points are determined from blood samples taken on days 1, 3, 5, 6, 7, 8, 9, 12 and 16 (mean ± SEM). Shaded blocks represent time spent resident at the Research Clinic. Horizontal line(s) denote upper and lower reference limits (ULN and LLN, respectively) where they fall within the scale on the ordinates.]
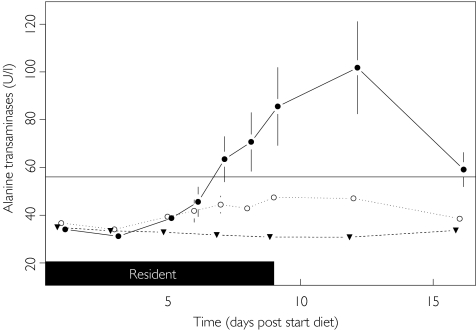
There was powerful evidence for the influence of dietary regimen on transaminase activities. ALT activity remained stable up to day 3 for all diets but subsequently began to rise for both the HFHC and HCHC diets (see Figure 2). Compared with the standard diet, by day 9 and day 12, values had risen significantly to, on average, 38% (95% CI 2, 89; P = 0.040) higher on the HFHC diet and 143% (95% CI 79, 231; P < 0.001) higher on the HCHC diet. The high ALT activities achieved on the HCHC diet were also 76% (95% CI 29, 139; P < 0.001) higher than those on the HFHC diet. This large increase in mean ALT activity on the HCHC diet reflects the fact that eight out of the 12 subjects had ALT values that exceeded the upper limit of the reference range (56 U l−1).
Figure 2.
Effect of diet upon alanine transaminase. ULN (—), Standard (▾), HFHC (○) and HCHC (•).
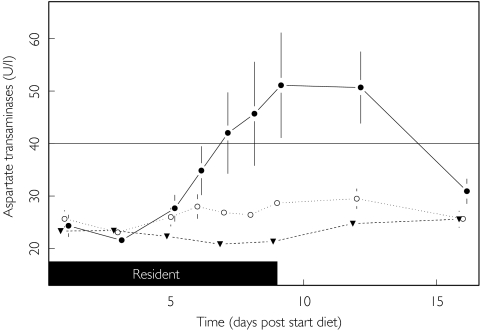
AST activity was also markedly influenced by diet (P < 0.001) but, in contrast to the findings for ALT, no difference was found between the standard and HFHC diets (95% CI −6, 56; P = 0.130). However a similar finding to that for ALT was observed for the HCHC diet compared with the standard diet (Figure 3) with AST activities 90% (95% CI 48, 145) higher. Furthermore, AST activities were 57% (95% CI 22, 103; P < 0.001) higher on the HCHC diet compared with the HFHC diet. Nine out of the 12 subjects on the HCHC diet had AST activities that exceeded the upper limit of the reference range (40 U l−1).
Figure 3.
Effect of diet upon aspartate transaminase. ULN (—), Standard (▾), HFHC (○) and HCHC (•).
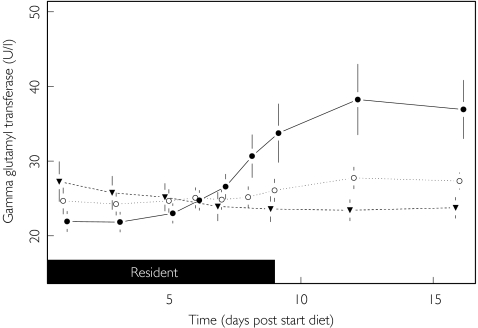
Although the pattern of change was less dramatic, diet also had an effect on γGT activities (P < 0.001). Initially the γGT remained stable, but by day 6 of the HCHC diet had started to rise (see Figure 4). The maximum change from baseline was 45% (95% CI 26, 70) higher than on the standard diet and 32% (95% CI 15, 51) higher than on the HFHC diet (P < 0.001). However, only one of the 12 subjects on the HCHC diet had a γGT activity (80 U l−1) that exceeded the upper limit of the reference range (78 U l−1). No significant difference was found between the HFHC and standard diet, with activities in the former group being slightly higher (95% CI −4%, 27%; P = 0.157).
Figure 4.
Effect of diet upon gamma glutamyl transferase. Standard (▾), HFHC (○) and HCHC (•).
CK activities did not change significantly amongst any of the diets (P = 0.276).
Fasting lipids
Diet had a small but statistically significant influence on fasting serum cholesterol (P = 0.001). Cholesterol levels remained relatively stable for all diets until day 7, when levels began to rise slowly for the high-calorie diets. The rises in cholesterol were 15% (95% CI 7, 22; P < 0.001) and 7% (95% CI 1, 15; P = 0.032) higher than the standard diet for the HCHC and HFHC diets, respectively. There was no significant difference between the HCHC and HFHC diets, although it can be seen from the lower limit of the 95% CI (95% CI 0%, 14%; P = 0.057) that higher values achieved for the HCHC diet nearly achieved statistical significance. None of the mean values rose above the desirable range for cholesterol of <5.2 mmol l−1(Table 4).
Table 4.
Maximum deviation of laboratory parameters from baseline.
| Test | Normal range | Standard | HFHC | HCHC |
|---|---|---|---|---|
| Albumin (g l−1) | 39–50 | 46.1 ± 3.2 | 46.3 ± 2.4 | 45.7 ± 2.2 |
| ALP (U l−1) | 38–126 | 79.9 ± 24.3 | 78.0 ± 22.2 | 84.3 ± 25.5 |
| ALT (U l−1) | 7–56 | 36.8 ± 6.4 | 51.8 ± 13.0 | 103.8 ± 63.3 |
| AST (U l−1) | 5–40 | 27.4 ± 4.6 | 33.3 ± 6.7 | 56.8 ± 30.2 |
| Bilirubin (µmol l−1) | 3–22 | 8.47 ± 2.44 | 6.01 ± 1.66 | 6.46 ± 2.00 |
| Cholesterol (mmol l−1) | 0.0–6.5 | 4.50 ± 0.96 | 4.79 ± 0.81 | 5.11 ± 0.84 |
| CK (U l−1) | 57–374 | 170 ± 100 | 252 ± 215 | 400 ± 794 |
| γGT (U l−1) | 8–78 | 26.5 ± 7.0 | 28.7 ± 4.0 | 39.8 ± 15.8 |
| Total protein (g l−1) | 63–82 | 77.1 ± 4.7 | 78.9 ± 4.3 | 78.6 ± 2.6 |
| Triglycerides (mmol l−1) | 0.45–1.82 | 1.77 ± 0.75 | 1.56 ± 0.45 | 3.30 ± 1.50 |
Values presented are the mean (± SD) of the maximum de viation from baseline (any time point) for all subjects and as such not representative of the data illustrated in Figures 1, 1, 2, 3, 4, 5, 6. ALP, Alkaline phosphatase; ALT, alanine transaminase; CK, creatinine kinase; γGT, gamma glutamyl transferase.
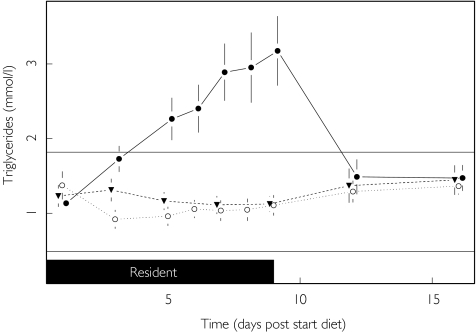
In contrast to cholesterol, diet was found to strongly influence fasting triglycerides (P < 0.001), but only when the subjects were on the HCHC diet. Triglyceride levels on the HCHC diet started to rise markedly from day 1 of the diet and continued rising throughout the dietary period (Figure 5). Fasting triglycerides were 80% (95% CI 42, 131) higher on the HCHC diet than on the standard diet and 99% (95% CI 56, 153) higher than on the HFHC diet (P < 0.001). These high values reflect the fact that 10 out of the 12 subjects on the HCHC diet had values that exceeded the upper limit of the reference range (1.82 mmol l−1).
Figure 5.
Effect of diet upon triglycerides. LLN (—), ULN (—), Standard (▾), HFHC (○) and HCHC (•).
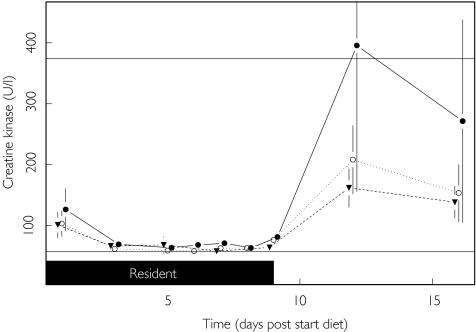
The means of the highest observed values (at any time point) for each parameter (lowest observed value for bilirubin) are shown in Table 4. It can be seen that in general the standard deviation of the mean increases with the value of the mean, and this is the basic assumption that has been made in the model used for the statistical analyses. The only mean values lying outside the reference range are for ALT, AST, CK and triglycerides on the HCHC diet. The highest (and highly variable) activities for CK were achieved following discharge from the clinic (Figure 6), whilst for ALT, AST and triglycerides rises commenced during residency (Figures 2, 3 and 5).
Figure 6.
Effect of diet upon creatinine kinase. LLN (—), ULN (—), Standard (▾), HFHC (○) and HCHC (•).
Discussion
This study was designed primarily to investigate the effect of diet on LFTs and to distinguish between the effect of high-calorie intake compared with the effect of dietary composition (high fat or high carbohydrate) upon these laboratory parameters. In addition, the effect of the diets upon serum total cholesterol and triglycerides has been investigated.
The study was performed upon healthy young men in the environment of a Phase I unit under the general conditions that apply to Phase I studies with new chemical entities (i.e. GCP). Since GCP will be observed by all Phase I units, the results obtained are relevant to studies performed at any unit.
Antipyrine clearance
Previous work on animals and on isolated tissue suggested that dietary composition could have marked effects upon cytochrome P450 mixed function oxidases and conjugation enzymes [7, 16]. It is difficult to extrapolate these findings to humans, but such effects might be indicated by any changes found in antipyrine clearance which reflects metabolism by the cytochrome P450 systems.
The lack of effect of any of the diets upon antipyrine clearance (Table 3) suggests that these diets had no direct effect upon the overall oxidative capacity of the liver. Such a finding concurs with earlier work in humans with regard to carbohydrates and lipids, although other dietary factors, such as high protein content, were found to affect both antipyrine and theophylline clearance [17]. The antipyrine clearance values reported in that study compare well with those currently obtained despite the different study conditions.
Creatinine kinase (CK) and transaminases (AST and ALT)
CK is a sensitive marker of muscle damage. Comparison of Figures 2, 3 and 6 indicate that the rises in transaminase activity in resident subjects on the HCHC diet were not of muscular origin. Although clinically there are other potential causes of raised activities of transaminases in the blood (e.g. tissue hypoxia, haemolysis and pancreatitis) these are very unlikely to be pertinent to the healthy subjects in this study. The liver therefore remains by far the most likely source of the large increases in transaminase activities recorded during the HCHC dietary regimen.
The highly variable rises in CK activity following discharge (Figure 6) are likely to be due to a sharp increase in muscular exercise in reaction to the period of inactivity during residency at the Clinic. Many of the subjects normally took regular exercise and missed this whilst resident in the restrictive environment of the Phase I unit.
Liver function tests and diet
The effect of varying the composition of carbohydrate in the diet upon various enzymes (including transaminases) in the blood of healthy humans has been studied previously [10, 11]. However, to our knowledge a direct comparison of the effect of isocaloric high-carbohydrate and high-fat diets (allowing discrimination between calorific value and composition) has not been made.
There is a clear relationship between the marked rises in transaminases and the number of days on the HCHC diet (Figures 2 and 3). Such a powerful relationship was not found with the isocaloric HFHC diet and demonstrates the importance of carbohydrate rather than calories as the prime factor in the changes found. However, some small changes were apparent in ALT when subjects ate the HFHC diet. These effects may be due to the fact that subjects were eating nearly 50% more calories as carbohydrate daily whilst on the HFHC diet compared with the standard diet (Table 1) and are entirely consistent with the primary role of carbohydrate in the recorded changes. This contrasts somewhat with the findings of Porikos and Van Itallie [11], who concluded that both surplus calories and high sucrose intake contributed significantly to the observed rises in transaminase activities. Indeed, surplus calories were attributed to be the sole cause of elevated transaminases found in healthy volunteers after 7 days of residence in a Phase I unit in Japan [2]. However, this study was not directly comparable to the present work since the calorific value of the diet used was not high and remained constant, whereas the level of physical activity of the volunteers was changed. Surplus calories were judged as the difference between calorie intake and their consumption by exercise. We consider that physical exercise during residency in Phase I studies is best avoided since it introduces another variable with the potential of causing changes in laboratory parameters (see earlier).
A much higher proportion of the calories in the carbohydrate fraction of the HCHC diet was due to sucrose than in the other diets. In contrast, the amount of starch in each of the high-calorie diets was nearly the same and about double that in the balanced normal calorie diet (Table 1). This implies that it is the amount of sucrose in the high-carbohydrate diet which mainly underlies the marked rises in transaminases. Such a conclusion concurs with earlier findings [10, 11].
It has been suggested previously that a rise in transaminase activity may be due to the fructose moiety of the sucrose in the diet causing damage to hepatocytes [11] or to lipid deposition in the liver [2]. The antipyrine results suggest that diet does not affect hepatic oxidative function (Table 3) so that although minor hepatic damage cannot be excluded, it seems more plausible that the transaminases are induced by the increased flux of carbohydrate through glycolysis and related pathways. The greater effect upon ALT compared with AST (Figures 2 and 3) might be explained by the fact that the former enzyme is involved directly with pyruvate metabolism whereas AST is more indirectly related to carbohydrate metabolism. Hepatic enzyme induction by increased availability of substrate, such as when certain drugs, e.g. barbiturates, phenytoin, ethanol, etc. are taken regularly, is a well-recognized phenomenon.
The HCHC diet also produced small but significant rises in ALP and γGT activities as suggested previously [10, 18, 19]. In contrast, bilirubin levels fell significantly (Figure 1). These results appear contradictory, since increased ALP and γGT activities usually indicate biliary stasis (which would be expected to result in a rise in bilirubin). However, the high calorie intake may decrease serum bilirubin by stimulating biliary flow, as indicated indirectly by the finding that fasting increases bilirubin levels by decreasing biliary flow [20]. Therefore the current findings are not inconsistent with previous published data and emphasize the complex nature of the effect of diet upon tests which are generally accepted to reflect liver function. However, such tests are not ideal, being affected by many factors [21]. The results should be interpreted with caution, probably reflecting normal adaptive changes in hepatic function in healthy individuals.
Lipids and diet
The finding that the high-carbohydrate diet caused an increase in fasting serum triglycerides is by no means new [13, 22–27], although more recent work has been equivocal [11, 14]. However, the unequivocal and highly statistically significant rise in triglycerides to well above the reference range caused by the HCHC diet compared with the isocaloric HFHC diet cogently supports the hypothesis that carbohydrate rather than calories is the primary cause. It is impossible to ascertain from the results whether the increase in triglycerides is due to an increase in endogenous synthesis by the liver from excess carbohydrate or to a decrease in clearance from the circulation. Nor is the literature clear on this matter [22–25, 27]. Whatever the mechanism, it seems that the sucrose moiety of the diet is responsible since sucrose is the major contributor to total calories in the HCHC diet (Table 1). This finding is in agreement with the results of other groups [23, 26, 28], although this is not undisputed [29]. In contrast, the lack of a substantial effect upon cholesterol levels which are more stable than triglycerides may be because the diets were not maintained for long enough. Reiser et al.[28] found that cholesterol levels increased significantly when sucrose was substituted for starch in the diet, but only after the fifth week.
Conclusion
The marked effect of excess carbohydrate upon the transaminases and also upon triglycerides underlines the importance of diet as a consideration in all Phase I studies. It is strongly recommended that a well-balanced diet with just sufficient calories to maintain body weight be used at all times, especially in studies where volunteers are resident for any length of time. There are many other nondrug factors that can affect laboratory parameters, e.g. exercise, circadian rhythm, stress, posture, time of taking sample, etc. [21, 30] and Phase I units should take these into consideration when planning studies and interpreting results. This will reduce the chance of erroneously attributing changes in laboratory parameters to a drug effect with all of the consequences which might follow from this.
Acknowledgments
The authors thank the staff at the Pfizer Research Clinic, Canterbury, for their hard work during this study.
References
- 1.Stricker BHCH, Spoelstra P. Drug-induced hepatic injury. In: Dukes MNG, editor. Drug-induced disorders. Vol. 1. Amsterdam: Elsevier; 1985. pp. 1–10. [Google Scholar]
- 2.Kanamaru M, Nagashima S, Uematsu T, Nakashima M. Influence of 7-day hospitalisation for Phase I study on the biochemical laboratory tests of healthy volunteers. Jpn J Clin Pharmacol Ther. 1989;20:493–503. [Google Scholar]
- 3.Kobayashi M, Yamada N, Shibata H, Nishikawa T. Elevation of serum transaminase value after administration of non-toxic drugs in some volunteers for Phase I trials: a study on the selection of volunteers. Jpn J Clin Pharmacol Ther. 1991;22:497–500. [Google Scholar]
- 4.Merz M, Seiberling M, Hoxter G, Holting M, Wortha HP. Elevation of liver enzymes in multiple dose trials during placebo treatment: are they predictable? J Clin Pharmacol. 1997;37:791–8. doi: 10.1002/j.1552-4604.1997.tb05626.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig P, Brohier S, Zipfel A. Data on placebo in healthy volunteers: impact of experimental conditions on safety, and on laboratory and physiological variables during phase I trials. Therapie. 1996;51:356–7. [PubMed] [Google Scholar]
- 6.Rosenzweig P, Miget N, Brohier S. Transaminase elevation on placebo during Phase I trials: prevalence and significance. Br J Clin Pharmacol. 1999;48:19–23. doi: 10.1046/j.1365-2125.1999.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bidlack WR, Brown RC, Mohan C. Nutritional parameters that alter hepatic drug metabolism, conjugation and toxicity. Fed Proc. 1986;45:142–8. [PubMed] [Google Scholar]
- 8.Dannenberg AJ, Yang EK. Effect of dietary lipids on levels of UDP-glucuronosyltransferase in liver. Biochem Pharmacol. 1992;44:335–40. doi: 10.1016/0006-2952(92)90017-d. [DOI] [PubMed] [Google Scholar]
- 9.Yang EK, Radominska A, Winder BS, Dannenberg AJ. Dietary lipids coinduce xenobiotic metabolising enzymes in rat liver. Biochim Biophys Acta. 1993;1168:52–8. doi: 10.1016/0005-2760(93)90265-b. [DOI] [PubMed] [Google Scholar]
- 10.Irwin MI, Staton AJ. Dietary wheat starch and sucrose. Effect on levels of five enzymes in blood serum of young adults. Am J Clin Nutr. 1969;22:701–9. doi: 10.1093/ajcn/22.6.701. [DOI] [PubMed] [Google Scholar]
- 11.Porikos KP, Van Itallie TB. Diet-induced changes in serum transaminase and triglyceride levels in healthy adult men. Am J Med. 1983;75:624–30. doi: 10.1016/0002-9343(83)90444-8. [DOI] [PubMed] [Google Scholar]
- 12.Richardson RA, Garden OJ, Shenkin A. Enteral nutrition and liver function test abnormalities. J Human Nutrition Dietetics. 1988;1:227–32. [Google Scholar]
- 13.Ahrens EH, Jr, Hirsch J, Oette K, Farquhar JW, Stein Y. Carbohydrate-induced and fat-induced lipaemia. Trans Ass Am Physicians. 1960;74:134–46. [PubMed] [Google Scholar]
- 14.Abbott WGH, Swinburn B, Ruotolo G, et al. Effect of a high-carbohydrate, low-saturated-fat diet on apolipoprotein B and triglyceride metabolism in Pima Indians. J Clin Invest. 1990;86:642–50. doi: 10.1172/JCI114756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dossing M, Poulsen HE, Andreasen PB, Tygstrup N. A simple method for determination of antipyrine clearance. Clin Pharmacol Ther. 1982;32:392–6. doi: 10.1038/clpt.1982.177. [DOI] [PubMed] [Google Scholar]
- 16.Yang CS, Brady JF, Hong J-Y. Dietary effects on cytochromes P 450, xenobiotic metabolism, and toxicity. FASEB J. 1992;6:737–44. doi: 10.1096/fasebj.6.2.1537464. [DOI] [PubMed] [Google Scholar]
- 17.Anderson KE, Pantuck EJ, Conney AH, Kappas A. Nutrient regulation of chemical metabolism in humans. Fed Proc. 1985;44:130–3. [PubMed] [Google Scholar]
- 18.Gordon T. Factors associated with serum alkaline phosphatase level. Arch Pathol Lab Med. 1993;117:187–90. [PubMed] [Google Scholar]
- 19.Nillson O, Helge-Forde O, Brenn T. The Tromso study – distribution and population determinants of γ-glutamyltransferase. Am J Epidemiol. 1990;132:318–26. doi: 10.1093/oxfordjournals.aje.a115661. [DOI] [PubMed] [Google Scholar]
- 20.Dufour DR. Effects of food ingestion on routine laboratory tests. Clin Chem. 1998;44(Suppl 6) A136 (Abstract) [Google Scholar]
- 21.Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. 1. Performance characteristics of laboratory tests. Clin Chem. 2000;46:2027–49. [PubMed] [Google Scholar]
- 22.Reaven GM, Hill DB, Gross RC, Farquhar JW. Kinetics of triglyceride turnover of very low density lipoproteins of human plasma. J Clin Invest. 1965;44:1826–33. doi: 10.1172/JCI105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nestel PJ, Carroll KF, Havenstein N. Plasma triglyceride response to carbohydrates, fats and calorie intake. Metabolism. 1970;19:1–18. doi: 10.1016/0026-0495(70)90112-5. [DOI] [PubMed] [Google Scholar]
- 24.Quarfordt SH, Frank A, Shames DM, Berman M, Steinberg D. Very low density lipoprotein triglyceride transport in type IV hyperlipoproteinaemia and the effects of carbohydrate-rich diets. J Clin Invest. 1970;49:2281–97. doi: 10.1172/JCI106448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nestel PJ. Triglyceride turnover in man. Progr Biochem Pharmacol. 1973;8:125–60. [PubMed] [Google Scholar]
- 26.Hayford JT, Danney MM, Wiebe D, Roberts S, Thompson RG. Tiglyceride integrated concentrations: effect of variation of source and amount of dietary carbohydrate. Am J Clin Nutr. 1979;32:1670–8. doi: 10.1093/ajcn/32.8.1670. [DOI] [PubMed] [Google Scholar]
- 27.Melish J, Le N-A, Ginsberg H, Steinberg D, Brown WV. Dissociation of apoprotein B and triglyceride production in very-low-density lipoproteins. Am J Physiol. 1980;239:E354–E362. doi: 10.1152/ajpendo.1980.239.5.E354. [DOI] [PubMed] [Google Scholar]
- 28.Reiser S, Hallfrisch J, Michaelis OE, Lazar FL, Martin RE, Prather ES. Isocaloric exchange of dietary starch and sucrose in humans 1. Effects on levels of fasting blood lipids. Am J Clin Nutr. 1979;32:1659–69. doi: 10.1093/ajcn/32.8.1659. [DOI] [PubMed] [Google Scholar]
- 29.Mann JI, Truswell AS. Effects of isocaloric exchange of dietary sucrose and starch on fasting serum lipids, postprandial insulin secretion and alimentary lipaemia in human subjects. Br J Nutr. 1972;27:395–405. doi: 10.1079/bjn19720105. [DOI] [PubMed] [Google Scholar]
- 30.Statland BE, Winkel P. Effects of preanalytical factors on the intraindividual variation of analytes in the blood of healthy subjects: consideration of preparation of the subject and time of venipuncture. CRC Crit Rev Clin Lab Sci. 1977;8:105–44. doi: 10.3109/10408367709151694. [DOI] [PubMed] [Google Scholar]