Abstract
Aims
It is well established that there is a wide intra- and interindividual variability in dose requirements for lorazepam and midazolam in intensive care patients. The objective of this study was to compare the population pharmacokinetics of lorazepam and midazolam after long-term continuous infusion in mechanically ventilated critically ill patients.
Methods
Forty-nine critically ill patients randomly received either lorazepam (n = 28) or midazolam (n = 21) by continuous infusion for at least 24 h. Multiple blood samples were obtained for determination of the drug and metabolite concentrations by HPLC. Population pharmacokinetic models were developed using the Non-Linear Mixed Effect Modelling (NONMEM) program. The influence of selected covariates was investigated. The prospective performance of the models was evaluated on the basis of results in separate groups of patients for lorazepam (n = 31) and midazolam (n = 33).
Results
The pharmacokinetics of lorazepam were best described by a two-compartment model. Alcohol abuse, positive end expiratory pressure (PEEP) and age were identified as significant covariates. Total body clearance for patients without alcohol abuse was 4.13 − (PEEP − 5) × 0.42 l h−1, and 0.74 l h−1 for patients with alcohol abuse. The volume of distribution was 0.74 l, the steady state volume of distribution was 56 − (age − 58) × 2.1 l and the intercompartmental clearance was 10 l h−1. The proportional residual error was 15% and the median absolute prediction error was 13.6% with a bias of 1.5%. The pharmacokinetics of midazolam were best described by a two-compartment model with alcohol abuse, APACHE score and age as significant covariates. Total body clearance for patients without alcohol abuse was 11.3 − (age − 57) × 0.14 l h−1, and 7.27 – (age −57) × 0.14 l h−1 for patients with alcohol abuse. The volume of distribution was 7.15 l, the steady state volume of distribution was 431 l, and the intercompartmental clearance was 40.8 − (APACHE score − 26) × 2.75 l h−1. The proportional residual error was 31% with an additive residual error of 32 ng ml−1. The median absolute prediction error was 12.9% with a bias of 1.2%. The prospective performance in the lorazepam evaluation group was better with the covariate adjusted model, but in the midazolam evaluation group it was not better than with the simple model. In all models a tendency to overestimate the lower plasma concentrations was observed.
Conclusions
The pharmacokinetics of both lorazepam and midazolam were well described by a two-compartment model. Inclusion of alcohol abuse and age as covariates improved both models. PEEP was identified as an additional covariate for lorazepam, and the APACHE score for midazolam. For both drugs there is a large interindividual variability in their pharmacokinetics when used for long-term sedation in critically ill patients. However, the intra-individual variability is much lower for lorazepam.
Keywords: intensive care unit, lorazepam, midazolam, pharmacokinetics
Introduction
The aim of sedation for patients in an intensive care unit (ICU) is mainly to reduce fear and stress, and to facilitate mechanical ventilation. The ideal level of sedation keeps the patients lightly asleep but easily arousable. Furthermore, for certain patients amnesia, respiratory depression or an antitussive effect are also desired. Typically this requires careful titration of the dose to the needs of each individual patient [1, 2].
Lorazepam and midazolam are among the widely used sedatives for long-term sedation by continuous intravenous infusion. There is a wide intra- and interindividual variability in dose requirements in ICU patients. In an earlier study, we compared the use of lorazepam and midazolam for the long-term sedation of ICU patients [3]. We found that it was significantly easier to induce and maintain a predefined level of sedation with lorazepam than with midazolam. The wide intra- and interindividual variability in midazolam pharmacokinetics, and the many factors influencing this, might explain the more difficult titration of this drug to the desired level of sedation.
There are important qualitative and quantitative differences in pharmacokinetics between midazolam and lorazepam. Midazolam is metabolized by the hepatic cytochrome P450 (CYP3A4) system to hydroxymetabolites with distinct intrinsic pharmacological activities, which are subsequently eliminated as conjugates. It has been shown that the principal metabolite 1-hydroxymidazolam is almost equipotent to the parent compound [4–6]. In contrast the major route of lorazepam elimination is direct conjugation to its glucuronide metabolite, which has little intrinsic pharmacological activity [7]. It is well established that there can be differences in the intra- and interindividual variability in the clearance of drugs that are eliminated by oxidation vs conjugation pathways, respectively. Cytochromes P450 and other phase I enzymes are generally present in smaller amounts than phase II enzymes and they are more affected by disease. Thus, a much lower variability is observed for drugs that are eliminated by conjugation [8]. However, there may be differences in the variability of the oxidative and conjugating hepatic enzyme systems and their responses to critical illness [8].
Another important difference between the two drugs concerns their hepatic extraction. Midazolam has an intermediate to high hepatic extraction ratio, which makes its pharmacokinetics susceptible to changes in hepatic blood flow. In contrast, the hepatic extraction ratio of lorazepam is much lower, making its pharmacokinetics relatively insensitive to changes in hepatic blood flow.
Recently, Barr et al.[9] have compared the pharmacokinetics of midazolam and lorazepam in ICU patients for 12–72 h postoperatively. However, no studies have been reported in critically ill patients after longer-term infusion. Therefore, the objective of the present investigation was to characterize and compare the population pharmacokinetics of midazolam and lorazepam in critically ill patients during long-term sedation. A specific objective was to analyse the influences of several covariates on their pharmacokinetics. Identification of specific covariates might ultimately provide a rational basis for the individualization of doses between and within individual patients.
An important feature of the present study was that there were two datasets available for both drugs, of which one was assigned as a learning set and the other an evaluation set. This allowed assessment of the predictive abilities of the population pharmacokinetic models.
Methods
Study design
Data on the plasma concentrations of midazolam and lorazepam were available from two separate but comparable studies in ICU patients. The first one was a two-treatment, open label, randomized, parallel group study in 66 critically ill patients. This group is referred to as the ‘learning’ group. The protocol for this study was approved by the Ethics Committee of the Vrije Universiteit Medical Center, Amsterdam, the Netherlands. Written informed consent was obtained from the next of kin for eligible patients in the age range 18–85 years, who were expected to require mechanical ventilation in the medical ICU for a minimum of 3 days. Patients were randomly assigned to treatment with either lorazepam or midazolam by continuous infusion. Patients were excluded if they had neurotrauma or tetanus, were in coma, needed muscle relaxants or any sedative other than lorazepam or midazolam, had a history of allergic response to either drug, were pregnant or were mechanically ventilated for less than 24 h due to early extubation or death. Patients were excluded from the pharmacokinetic analysis if the sedative was given for less than 24 h or if less than three blood samples had been taken after 24 h.
Age, sex, weight and duration of sedation were recorded for every patient. The severity of illness was assessed in each patient using the APACHE II score [10]. Concurrent treatments were given as required.
Lorazepam and midazolam were delivered via a volumetric infusion pump at a concentration of 0.16 mg ml−1 and 5 mg ml−1, respectively. For lorazepam two ampoules (2 ml = 8 mg) were diluted with 46 ml of saline, but the midazolam ampoules were used undiluted. The dose rates were individually titrated. To this end the infusion rate was kept as low as possible and adjusted to the desired level of sedation on the basis of the Addenbrooke sedation scale [3]. The desired level of sedation was determined at least once daily by the attending physician and adjusted to keep the patient comfortable and tolerating treatments. If the desired sedation level could not be obtained with the maximum dose of 5.3 mg h−1 or 80 mg h−1 for lorazepam and midazolam, respectively, the infusion was stopped and the case was classified as a therapeutic failure.
There was no standard protocol for the administration of a bolus dose of lorazepam or midazolam. Typically the infusion was started at the rate of 2 ml h−1 of the study medication. If a patient was not adequately sedated a bolus dose of 2 ml was administered and the dose rate was increased by 2 ml h−1. The efficacy of sedation was then reassessed 1 and 2 h later. A new change in dose rate was performed if the level of sedation was still inadequate. If a patient was over-sedated, the dose rate was decreased by 2 ml h−1. At the lowest dose rate of 2 ml h−1 a decrease to 1 or 0.5 ml h−1 was performed if necessary during the weaning phase.
The administration of fentanyl was necessary to provide adequate analgesia. Typically an infusion was started at 0.1 mg h−1 and adjusted to the patients' requirements for analgesia. The amount administered was recorded for every patient. All other drugs administered to the patient were also recorded.
Blood samples for the determination of lorazepam and midazolam were taken at 0, 15, 30, 45 min and 1, 2, 4 and 8 h after starting the continuous infusion. Thereafter, a blood sample was taken every 24 h until termination of therapy. After the infusion had been stopped blood samples were taken at 0, 15, 30 min and 1, 2, 8 and 24 h. The blood was centrifuged and the plasma was separated and stored at −20 °C until analysis.
A second group (the ‘evaluation’ group) consisted of patients from an earlier study [3]. However, fewer data points were available for analysis. The study design, inclusion and exclusion criteria were essentially the same in both the learning and the evaluation groups. In the latter group the concentration of the lorazepam solution was 0.33 mg ml−1, but the midazolam concentration was the same as in the learning group. The treatment schedule was the same in both groups. Blood samples were taken before starting the infusion and at the moment the desired sedation score was reached. Blood samples were also taken before every change in infusion rate and at the time the desired sedation score was reached.
Analytical methods
The plasma concentrations of lorazepam, midazolam and 1-hydroxymidazolam were determined by high performance liquid chromatography with ultraviolet detection [11]. In our previous study [3] the maximum 4-hydroxymidazolam concentration was 233 ng ml−1 which is only approximately 4% of the corresponding concentrations of midazolam. Because 4-hydroxymidazolam is not believed to contribute to the pharmacodynamic effects nor to interfere with the pharmacokinetics of midazolam, its concentrations were not determined [4]. The detection limit of all compounds was 10 ng ml−1. Calibration curves were linear over the range 10 to 1000 ng ml−1 for lorazepam and 10–10 000 ng ml−1 for midazolam. The interassay and intra-assay coefficients of variation were less than 10% for the entire concentration range.
Pharmacokinetic model
The Non-Linear Mixed Effect Modelling (NONMEM) program (University of California, San Francisco, CA, version V level 1.1 double precision) was used to analyse the data. This approach estimates the structural pharmacokinetic parameters assuming two levels of random effects, namely interindividual variability of the pharmacokinetic parameters within the population and intraindividual (i.e. residual) error.
Model building using the learning group
Model development was performed in four steps: (1) choice of the structural pharmacokinetic model, (2) covariate analysis, (3) formal testing of the model by eliminating each covariate from the final model and (4) choice of the residual error model.
Initially, we compared one-, two- and three-compartment pharmacokinetic models without any covariates, assuming a proportional, log-normal distribution for both interindividual and intra-individual variability. Empirical Bayes estimates of the individual pharmacokinetic parameters were also computed. Improvements in the goodness of fit were accepted as significant (P < 0.05) when a decrease of > four points in the objective function (−2 × log likelihood) per added model parameter was observed. In addition, the weighted residuals (WR) were also used to evaluate the goodness of fit. The weighted residuals are defined as: WR = (Y−Ŷ)/Ŷ, where Ŷ = the measured concentration and Y = the model predicted value of the concentration.
The median WR (MDWR) was applied for model bias and the median absolute weighted residual (MDAWR) for model precision.
In step (2), the apparent influence of patient covariates on lorazepam or midazolam disposition was analysed by use of the individual Bayesian PK parameter estimates. Continuous (i.e. weight, age, duration of treatment) and categorical (i.e. sex, diagnosis category, alcohol abuse, APACHE score on admission) variables were plotted against the individual pharmacokinetic parameters and examined visually. The influence of the time-dependent variables PEEP (positive end expiratory pressure), albumin concentration, renal function (creatinine and urea concentrations), hepatic function (ASAT, ALAT, AF, gamma-GT, bilirubin, bilirubinglucuronide), CK levels and the amount of fentanyl administered h−1. were plotted against the weighted residual errors. In the midazolam group, the influence of simultaneous medication known to interact with its metabolism by CYP3A4 was also assessed. Covariates selected by this screening were subsequently incorporated one by one into the population model and evaluated by their influence on the objective function. The relative contribution of each factor to the goodness of fit was re-evaluated by deleting it from the full model (step (3)). Only covariates yielding a statistically significant improvement in the goodness of fit (i.e. a reduction in the value of the objective function of more than 4 points) were maintained in the final model.
During step (4), once the final model was adopted, the shape of the intraindividual error distribution was investigated by use of the plot of the individual weighted residual errors vs the individual concentration predictions. Additive, proportional and combined additive-proportional error models were compared.
Validation with the evaluation group
To verify the predictive value of the population model for new individuals, we compared the individual Bayesian predicted drug concentrations obtained with the covariate adjusted models with the observed concentrations measured in the evaluation group. The plot of population predicted vs observed concentrations was examined to assess the absolute size of the prediction error and the existence of systematic bias in the predictions. In addition, the data from the evaluation group were fitted with the final simple and covariate-adjusted models to evaluate whether the error size was in accordance with that described by the original population model.
Results
Patients and data
Initially 66 patients were included in the protocol for the learning group. Seventeen patients were excluded from the pharmacokinetic analysis for the following reasons: six patients died within 24 h, four patients received either midazolam or lorazepam for less than 24 h, one patient was transferred to another ICU and for six patients there were fewer than three blood samples after 24 h. Four of the patients in the latter group died within 72 h, and the sedative was continued until death. For two patients, blood was not sampled when the infusion was stopped. This resulted in a lorazepam learning group containing 28 evaluable patients and a midazolam learning group of 21 patients. The evaluation group contained 31 patients treated with lorazepam and 33 with midazolam. Table 1 summarizes the demographic characteristics of the patients in each group. No statistically significant differences were observed between the study groups.
Table 1.
Demographic characteristics of the study groups (mean ± SD, range).
| Variable | Lorazepam Learning group (n = 28) | Lorazepam Evaluation group (n = 31) | Midazolam Learning group (n = 21) | Midazolam Evaluation group (n = 33) |
|---|---|---|---|---|
| Male/Female | 17/11 | 20/11 | 13/8 | 19/14 |
| Age (years) | 58 ± 17 (21–84) | 61 ± 13 (22–78) | 57 ± 16 (21–84) | 54 ± 18 (20–83) |
| Weight (kg) | 80 ± 25 (40–175) | 76 ± 13 (55–110) | 71 ± 13 (40–90) | 74 ± 15 (44–115) |
| APACHE II-score on admission to ICU | 18 ± 7 (6–36) | 26 ± 10 (9–46) | 26 ± 9 (6–34) | 27 ± 10 (2–43) |
| Number of death | 10 | 10 | 6 | 10 |
| Diagnostic group | ||||
| Trauma | 1 | 4 | 0 | 2 |
| Cardio-respiratory insufficiency | 19 | 16 | 14 | 21 |
| Sepsis | 5 | 6 | 6 | 8 |
| Postoperative | 2 | 3 | 1 | 1 |
| Miscellaneous | 1 | 2 | 0 | 1 |
Table 2 provides the medication data for all groups. In the learning group the 344 measured plasma concentrations of lorazepam ranged from less than 10 to 588 ng ml−1 and the 494 measured plasma concentrations of midazolam ranged from less than 10 to 10 160 ng ml−1. 1-hydroxymidazolam concentrations ranged from less than 10 to 944 ng ml−1. In 204 samples the 1-hydroxymidazolam/midazolam ratio could be calculated, which was less than 0.1 in 50% of the samples, between 0.1 and 0.5 in 48% of the samples and more than 0.5 in 2% of the samples. The maximum value was 1.3, which was in a patient who died of multiple organ failure with both liver and renal insufficiency. In the evaluation group, 120 measured plasma concentrations of lorazepam ranged from less than 10 to 420 ng ml−1 and the midazolam concentrations ranged from less than 10 to 6037 ng ml−1.
Table 2.
Dosing regimens for the study patients (mean ± SD, range).
| Variable | Lorazepam Learning group (n = 28) | Lorazepam Evaluation group (n = 31) | Midazolam Learning group (n = 21) | Midazolam Evaluation group (n = 33) |
|---|---|---|---|---|
| Duration of administration (h) | 149 ± 157 (24–572) | 141 ± 117 (24–583) | 134 ± 172 (24–715) | 141 ± 101 (30–426) |
| Amount of medication (mg day−1) | 18 ± 13.9 (3.8–63.2) | 23.1 ± 14.4 (5.4–59.3) | 497 ± 417 (119–1682) | 372 ± 256 (102–1190) |
| Number of infusion rate adjustments | 5 ± 4 (0–21) | 4 ± 4 (0–18) | 6 ± 9 (0–34) | 4 ± 3 (0–13) |
Table 3 shows data on the covariates that were analysed in step (2).
Table 3.
Covariates included in the analysis (mean ± SD, range).
| Variable | Lorazepam Learning group (n = 28) | Lorazepam Evaluation group (n = 31) | Midazolam Learning group (n = 21) | Midazolam Evaluation group (n = 33) |
|---|---|---|---|---|
| PEEP (mmHg) | 5.3 ± 2.5 (0–17) | 4.6 ± 2.5 (0–14) | 6.2 ± 2.5 (0–20) | 5.6 ± 3.3 (0–20) |
| Creatinine (µmol l−1) | 144 ± 119 (40–727) | 192 ± 157 (47–795) | 136 ± 85 (41–445) | 207 ± 262 (44–1197) |
| Urea (mmol l−1) | 14.6 ± 12.2 (3.2–59.7) | 20.4 ± 15.7 (4–120.2) | 15.8 ± 10 (1.8–58.5) | 17.5 ± 11.5 (2.8–55.2) |
| Albumin (g l−1) | 18 ± 6 (5–36) | 21 ± 5 (11–34) | 15 ± 5 (5–29) | 20 ± 4 (12–28) |
| CK (U l−1 30 °C) | 428 ± 2204 (5–19730) | 236 ± 438 (6–2636) | 1602 ± 5235 (3–19730) | 1017 ± 2061 (10–11420) |
| ASAT (U l−1 30 °C) | 50 ± 180 (2–2560) | 27 ± 47 (3–511) | 192 ± 142 (2–910) | 105 ± 173 (6–997) |
| ALAT (U l−1 30 °C) | 38 ± 51 (4–594) | 30 ± 35 (4–289) | 64 ± 79 (3–638) | 72 ± 103 (9–743) |
| AF (U l−1 30 °C) | 100 ± 67 (24–347) | 98 ± 70 (20–462) | 112 ± 73 (22–371) | 101 ± 122 (7–449) |
| Gamma-GT (U l−1 30 °C) | 85 ± 90 (4–442) | 71 ± 99 (5–435) | 98 ± 96 (4–442) | 74 ± 51 (2–214) |
| Bilirubin (µmol l−1) | 36 ± 65 (4–445) | 52 ± 122 (4–773) | 34 ± 38 (4–225) | 57 ± 115 (6–660) |
| Bilirubin glucuronide (%) | 0.64 ± 0.24 (0.17–0.94) | 0.69 ± 0.21 (0.21–0.93) | 0.7 ± 0.17 (0.17–0.89) | 0.7 ± 0.18 (0.21–0.89) |
Model building for lorazepam
A two-compartment pharmacokinetic model described the data significantly (P < 10−12) better than the one-compartment model, whereas analysis on the basis of a three-compartment model did not improve the objective function any further. A wide array of both discrete and continuous covariates was considered. Of the discrete variables, alcohol abuse (defined as chronic use of more than 6 units per day) was a significant covariate for clearance, presumably reflecting liver dysfunction. However, a relationship with the results of biochemical laboratory tests was not observed.
The values of all pharmacokinetic model parameters are given in Table 4.
Table 4.
Pharmacokinetic parameter estimates for lorazepam using the two-compartment model in the learning group.
| Model without covariates | Model with covariates | |||
|---|---|---|---|---|
| Parameter | Estimate | CV (%) | Estimate | CV (%) |
| Estimated parameters | ||||
| CL no alcohol abuse (l h−1) | 3.90 | 102 | 4.13 − (PEEP − 5) × 0.417 | 63 |
| CL alcohol abuse (l h−1) | 1.20 | 606 | 0.74 | 389 |
| V (l) | 0.686 | 141 | 0.743 | 124 |
| Vss (l) | 264 | 209 | 156 − (age − 58) × 2.07 | 117 |
| Q (l h−1) | 32.7 | 79 | 36.3 | 75 |
| Proportional residual error (%) | 22.7 | 15.4 | ||
| Performance measures | ||||
| MDWR (%) | 3.35 | 1.51 | ||
| MDAWR (%) | 14.44 | 13.61 | ||
| Objective function | −2028.44 | −2082.15 | ||
CV coefficient of variation; MDWR median weighted residual; MDAWR median absolute weighted residual.
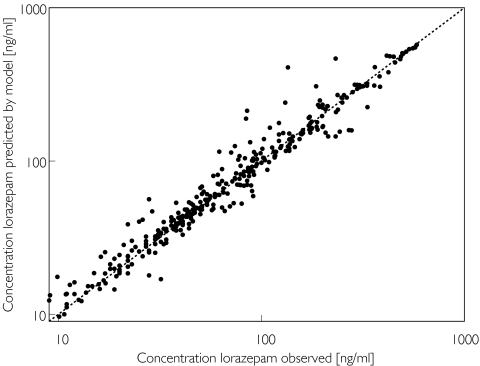
Alcohol abuse was not found to be a significant covariate for central (V) or steady state (Vss) volume of distribution. The graphical exploratory analysis showed no other obvious covariates. Therefore all possible covariates were entered sequentially into the population model. The positive end expiratory pressure (PEEP) and age were the only continuous covariates significantly improving the goodness of fit, the difference in the objective function from the initial model being −54 (P < 10−5) (Table 3). Sequentially deleting each factor from the final model resulted in an increase in objective function of 51 for PEEP as a covariate, and an increase of 6 for age. Alcohol abuse could not be deleted from the model without numerical failure. The plot of the observed plasma concentrations vs the plasma concentrations predicted by the individual Bayesian estimates of the final covariate-adjusted model is shown in Figure 1.
Figure 1.
Individual Bayesian lorazepam concentrations in the learning group predicted by the two-compartment covariate-adjusted model vs the corresponding observed concentrations, superimposed on the line of identity (broken lines)
A proportional model best described the intra-individual residual variability. The intra-individual residual variability was 23% in the simple model and 15% in the covariate-adjusted model.
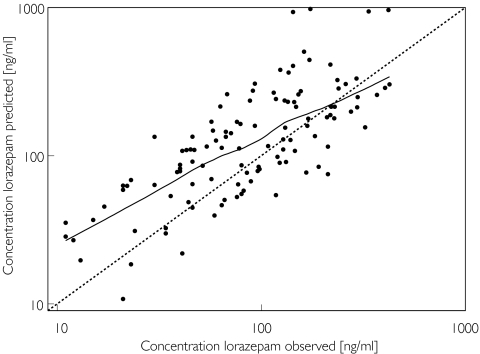
The model was validated in a separate group of patients, where only sparse data were collected, which had not been included in the data analysis. In these patients plasma concentrations of lorazepam were predicted on the basis of the population model, patient characteristics and the administered dose, and were compared with the observed concentrations (Figure 2). The nonparametric smooth fit of this graph shows that the predicted values are in accordance with the observed values, although over the lower concentration range the predicted concentrations are systematically higher than the observed concentrations.
Figure 2.
Individual Bayesian lorazepam concentrations in the evaluation group predicted by the two-compartment covariate-adjusted model vs the corresponding observed concentrations, superimposed on the line of identity (broken lines) and a nonparametric smooth fit with a Loess spline function (solid line).
More than 95% of the observed concentrations from the evaluation group were between −2 and +2 SDs of the mean population predicted values, indicating that they were in accordance with the range of variability described by the model derived from the learning group.
When data from the lorazepam evaluation group were fitted by the final simple and covariate-adjusted models, the coefficients of variation of all pharmacokinetic parameters were comparable with those found in the learning group during model building.
Model building for midazolam
The two-compartment pharmacokinetic model described the data significantly (P < 10−12) better than the one-compartment model, whereas analysis on the basis of a three-compartment model did not improve the objective function any further. The values of all model parameters are given in Table 5.
Table 5.
Pharmacokinetic parameter estimates for midazolam using the two-compartment model in the learning group.
| Model without covariates | Model with covariates | |||
|---|---|---|---|---|
| Parameter | Estimate | CV (%) | Estimate | CV (%) |
| Estimated parameters | ||||
| CL (l h−1) | 9.53 | 104 | ||
| CL no alcohol abuse (l h−1) | 11.3 − (age − 57) × 0.145 | 77 | ||
| CL alcohol abuse (l h−1) | 7.3 − (age − 57) × 0.145 | 77 | ||
| V (l) | 2.04 | 179 | 7.15 | 225 |
| Vss (l) | 364 | 282 | 431 | 355 |
| Q (l h−1) | 47.6 | 66 | 40.8 − (APACHE − 26) × 2.75 | 54 |
| Proportional residual error (%) | 31.9 | 30.9 | ||
| Residual error (ng ml−1) | 46 | 32 | ||
| Performance measures | ||||
| MDWR (%) | 1.22 | 1.22 | ||
| MDAWR (%) | 12.37 | 12.87 | ||
| Objective function | −336.191 | −422.668 | ||
CV coefficient of variation; MDWR median weighted residual; MDAWR median absolute weighted residual.
Alcohol abuse, APACHE score on admission, and age, but not PEEP, were identified as covariates that significantly (P < 10−5) improved the goodness of fit.
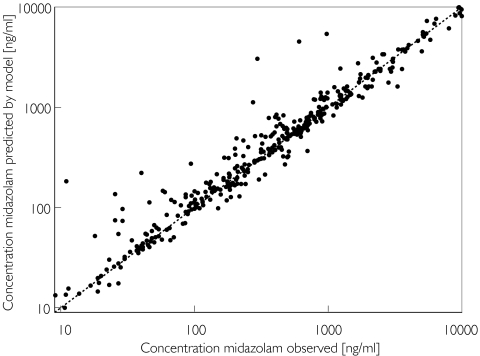
Inclusion of all covariates in a final covariate-adjusted model resulted in a difference in the objective function from the initial model of −86 (P < 10−10). Deleting each factor individually from the final model resulted in an increase in objective function of 19 for alcohol abuse, an increase of 26 for APACHE score and of 36 for age. The plot of the observed plasma concentrations vs the plasma concentrations predicted by the individual Bayesian estimates of the final covariate-adjusted model is shown in Figure 3.
Figure 3.
Individual Bayesian midazolam concentrations in the learning group predicted by the two-compartment covariate-adjusted model vs the corresponding observed concentrations, superimposed on the line of identity (broken lines)
An additive plus proportional model best described the intraindividual residual variability, values of which were 32% plus 46 ng ml−1 in the simple model and 31% plus 32 ng ml−1 in the covariate-adjusted model.
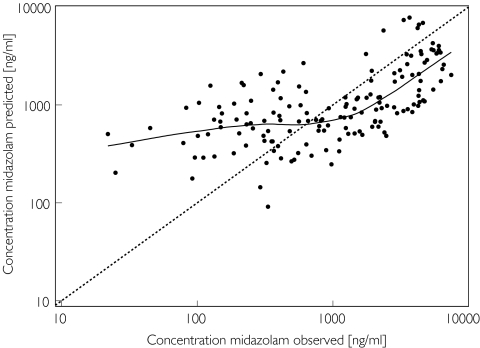
The model was also validated in a separate group of patients. The predicted and actual observed concentrations are shown in Figure 4. The nonparametric smooth fit shows that the predicted concentrations are in accordance with the observed concentrations, but that again over the lower concentration range the predictions are systematically higher than the observed concentrations.
Figure 4.
Individual Bayesian midazolam concentrations in the evaluation group predicted by the two-compartment covariate-adjusted model vs the corresponding observed concentrations, superimposed on the line of identity (broken line) and a nonparametric smooth fit with a Loess spline function (solid line)
When data from the midazolam evaluation group were fitted by the final simple and covariate-adjusted models, the coefficients of variation of all pharmacokinetic parameters were comparable with those found in the learning group during model building.
No model could be identified to describe the pharmacokinetics of 1-hydroxymidazolam, because only a few data points were available and the concentrations of this metabolite were low.
Discussion
The data collected in this study for both lorazepam and midazolam were best described by a two-compartment model, which is in agreement with previous observations by Barr et al.[9] The pharmacokinetic parameters derived were also comparable. For lorazepam, the clearances were somewhat higher than we found (CL 6.4 vs 4.1 l h−1, Q 112 vs 36 l h−1 but the volume of distribution was approximately the same (Vss143 vs 156 l). For midazolam clearance values were comparable (CL 15 vs 11 l h−1, Q 36 vs 40 l h−1, but the volume of distribution was smaller (159 vs 431 l). The differences in the estimates of the pharmacokinetic parameters and the covariates improving the models may be explained by the differences in patient selection and the duration of the study. Barr et al.[9] included relatively healthy postoperative patients for a study period of 12–72 h, whereas our patients were critically ill ICU patients with sepsis and cardiorespiratory insufficiency due to several underlying diseases. Furthermore our study period was 24–715 h. In addition 23 of the 24 patients in Barr et al.'s study were male and those with renal or liver insufficiency were excluded. Finally, patients in the latter study were less severely ill, as indicated by the APACHE II scores.
For both midazolam and lorazepam, the pharmacokinetic parameters showed a considerable degree of interindividual variability with relatively large coefficients of variation for each of the model parameters. However, a large interindividual variation is expected in critically ill patients with large differences in patient characteristics, pathology and duration of therapy. In order to explain at least part of the observed variation, several covariates were tested for their ability to improve the model. For both midazolam and lorazepam, the inclusion of alcohol abuse and age as covariates resulted in the most notable improvement in the performance of the model. For lorazepam, PEEP was an additional factor improving the model, whereas for midazolam, APACHE score had the same effect.
Patients with alcohol abuse showed a lower clearance for both drugs compared with patients without alcohol abuse. For lorazepam, this was unexpected. In a study by Kraus et al.[12] no changes in lorazepam systemic plasma clearance were found in patients with alcoholic liver cirrhosis compared with normal subjects. Although cirrhosis was associated with a doubling of the mean elimination half-life, this was shown to be due to a similar increase in the volume of distribution, which was explained by a reduction in the degree of plasma protein binding. We did not determine the latter in our patients, but in all cases albumin concentrations were far below normal. The average albumin concentration of the lorazepam learning group was 19 g l−1 during their stay at the ICU, which is equal to the lowest albumin concentrations of the patients with cirrhosis studied by Kraus et al.[12] (average 31 g l−1). However, alcohol abuse was not a significant factor contributing to the volume of distribution in our patients, nor were albumin concentrations. The observation of a reduction in midazolam clearance in patients with alcohol abuse is consistent with previous observations of a reduced clearance in patients with alcoholic cirrhosis [13]. Although in patients with alcohol abuse there was a tendency towards elevated values of the liver function parameters ALAT, ASAT, AF and bilirubin, these differences did not reach statistical significance. Furthermore, the liver function parameters were not identified as significant covariates for midazolam clearance.
Adding age as a covariate on volume of distribution and clearance resulted in a decrease in objective function for both lorazepam and midazolam, respectively, although the contribution of age is relatively small. This is in accordance with the results of several investigations on the influence of age on the pharmacokinetics of midazolam and lorazepam [6, 13–15].
PEEP was a significant covariate only for lorazepam. Hepatic blood flow is one of the physiological determinants of drug elimination, along with enzyme activity and protein binding. Alterations in one or more of these processes may result in variable effects on hepatic metabolism. Alterations in hepatic blood flow occur frequently in critically ill patients by several mechanisms. Examples of drugs that may affect hepatic blood flow are vasoactive compunds like α-adrenergic receptor agonists, causing hepatic arterial and portal vein vasoconstriction [16]. Mechanical ventilation also affects cardiac output and hepatic blood flow. Increasing PEEP would be expected to compromise hepatic blood flow even more by increasing the intrathoracic pressure [16, 17]. In patients with trauma and those in the surgical ICU, increasing PEEP has been shown to decrease total hepatic blood flow by 4%, 12%, and 32% at PEEP values of 10,15 and 20 mmHg, respectively [18]. The drugs most susceptible to hepatic blood flow alterations are those that are highly extracted by the liver [16, 17]. However, lorazepam has a low extraction ratio of approximately 0.06 and midazolam an intermediate extraction ratio of approximately 0.4 [19–21]. Thus PEEP would not be expected to affect lorazepam clearance, which is largely independent of hepatic blood flow. Midazolam clearance may vary with both hepatic blood flow and hepatic enzyme activity, but the variability in the latter may be much more important, because midazolam is eliminated through oxidation by hepatic enzymes, with a restricted capacity. The results from a previous small study are in agreement with the observation that midazolam clearance is decreased in critically ill patients with a variety of organ dysfunctions presumably secondary to decreased hepatic perfusion [20]. For midazolam, introducing PEEP as a covariate did not improve the model, probably because of its intermediate extraction ratio and the variability in other factors like enzyme activity and comedication that affect hepatic metabolism. It is possible, however, that the severity of illness is a main factor, because high PEEP values and high doses of vasoactive drugs given to these patients are generally associated with more severe illness.
The Acute Physiology and Chronic Health Evaluation (APACHE II) is a scoring system for severity of patient illness and outcome in the ICU. An increasing score indicates more severe illness [10]. Patients with organ failure have a higher APACHE score, which may be an explanation for their observed lower intercompartmental clearance for midazolam.
Both the simple and the covariate-adjusted model were tested for their predictive value in the evaluation group. The advantage over a cross-validation method is that real data are used from a comparable group of critically ill patients, thus enabling the estimation of the prospective performance of both models in new patients. The results of the validation analysis confirmed the better performance of the covariate-adjusted model over the simple model for lorazepam, although both models tended to overestimate especially at the lower plasma concentrations.
For midazolam the covariate-adjusted model did not perform better in the evaluation group. The covariates included did not appear to explain the interindividual and intraindividual variability. One explanation for this might be that there were many more patients receiving comedication that inhibits the metabolism of midazolam in the evaluation group than in the learning group. The effect of other drugs on the pharmacokinetics of midazolam can be of major importance [22–26]. There were three patients in the learning group who received medication known to interact with midazolam (two received fluconazole, one verapamil). However, the number of patients was too low to incorporate medication into the pharmacokinetic model. In the evaluation group there were significantly more patients with interacting medication. Twelve patients had treatment with either erythromycin (n = 10), cisapride (n = 4), itraconazole (n = 2) or verapamil (n = 1) during midazolam administration. Furthermore, five patients received two of these drugs in combination. The reason for this difference is that in the evaluation group the medication was blinded. Because the medication in the evaluation group was blinded, interacting comedication could not be avoided, as it could be in the learning group. At present lorazepam is preferred in ICU patients who receive potentially interacting comedication, as was the case for the learning group. Posthoc analysis showed that the clearance of midazolam in patients in the evaluation group who received interacting comedication tended to be lower, although the difference was not statistically significant. There may be a large variation in the influence of these drugs on the metabolism of midazolam.
The results of the current study demonstrate significant pharmacokinetic differences between lorazepam and midazolam. The pharmacokinetics of both drugs showed a considerable degree of interindividual variability. Medical ICU patients are a highly heterogeneous group, and during long-term treatment with sedatives, both the physical condition of the patient and their requirements for sedatives and additional therapies may vary considerably. On average, lorazepam has a smaller Vss than midazolam, which may be explained by the greater lipid solubility of the latter, with greater distribution into peripheral tissues compared with lorazepam. The estimated CL for midazolam is approximately 2.5 times that for lorazepam. This may be explained by differences in the metabolism of these two drugs; the hepatic glucuronidation of lorazepam is slower than the oxidative hydroxylation of midazolam. The mean elimination half-lives of lorazepam and midazolam are almost the same (30 h and 22.7 h, respectively). Variability in the elimination half-life was much larger for midazolam (CV 149%) than for lorazepam (CV 61%), making the effect of a change in infusion rate less predictable.
Intra-individual variability, expressed as the residual error, was much lower in the models for lorazepam. The introduction of covariates lowered this variability and they serve as an explanation for at least part of the vanation in pharmacokinetics. For midazolam there was a larger intra-individual heterogeneity, which is not explained by any factors identified in this study. The predictive value of the lorazepam covariate-adjusted model also seems to be better than that of the midazolam model, as indicated by the plots of predicted vs observed concentrations in the evaluation groups. Another factor of major importance is the elimination half-life of both drugs. A short elimination half-life would facilitate rapid dose adjustments because a new steady state will be quickly obtained. The average elimination half-life of lorazepam and midazolam in our group is almost equal.
The findings of the present investigation indicate that lorazepam may be preferred over midazolam in critically ill patients for long-term sedation, because the variability in its pharmacokinetics is smaller. In the study with the evaluation group, we found that it was significantly easier to reach a predetermined level of sedation with lorazepam than with midazolam. Thus, a smaller intra-individual variability in pharmacokinetics may result in a more predictable effect, but also the pharmacodynamics have to be taken into account. Combining the pharmacokinetics and pharmacodynamics of both drugs will provide a rational basis for the individualization of dosages between and within individuals.
References
- 1.Burns AM, Shelly MP, Park GR. The use of sedative agents in critically ill patients. Drugs. 1992;43:507–15. doi: 10.2165/00003495-199243040-00007. [DOI] [PubMed] [Google Scholar]
- 2.Aitkenhead AR. Analgesia and sedation in intensive care. Br J Anaesth. 1989;63:196–206. doi: 10.1093/bja/63.2.196. [DOI] [PubMed] [Google Scholar]
- 3.Swart EL, Strack van Schijndel RJM, van Loenen AC, Thijs LG. Continuous infusion of lorazepam vs midazolam in patients in the intensive care unit: sedation with lorazepam is easier to manage and is more cost-effective. Crit Care Med. 1999;27:263–9. doi: 10.1097/00003246-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Mandema JW, Tuk B, van Steveninck AL, Breimer DD, Cohen AF, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite α-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther. 1992;51:715–28. doi: 10.1038/clpt.1992.84. [DOI] [PubMed] [Google Scholar]
- 5.Bauer TM, Ritz R, Haberthür C, Riem Ha H, Hunkeler W, Sleight AJ, Scollo-Lavizzari G, Haefeli WE. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet. 1995;346:145–7. doi: 10.1016/s0140-6736(95)91209-6. [DOI] [PubMed] [Google Scholar]
- 6.Boulieu R, Lehmann B, Salord F, Fisher C, Morlet D. Pharmacokinetics of midazolam and its main metabolite 1-hydroxymidazolam in intensive care patients. Eur J Drug Metab Pharmacokinet. 1998;23:255–8. doi: 10.1007/BF03189348. [DOI] [PubMed] [Google Scholar]
- 7.Verbeeck RK, Branch RA, Wilkinson GR. Drug metabolites in renal failure. Clin Pharmacokinet. 1981;6:329–45. doi: 10.2165/00003088-198106050-00001. [DOI] [PubMed] [Google Scholar]
- 8.Park GR. Molecular mechanisms of drug metabolism in the critically ill. Br J Anaesth. 1996;77:32–49. doi: 10.1093/bja/77.1.32. [DOI] [PubMed] [Google Scholar]
- 9.Barr J, Zomorodi K, Bertaccini EJ, Shafer SL, Geller E. A double-blind, randomized comparison of IV lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiology. 2001;95:286–98. doi: 10.1097/00000542-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Knaus WA, Draoer EA, Wagner DP, Zimmerman JE. APACHE II, a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 11.Vletter AA, Burm AGL, Breimer LTM, Spierdijk J. High performance liquid chromatographic assay of midazolam and flumazenil simultaneously in human plasma. J Chromatogr Biomed Appl. 1990;530:177–85. doi: 10.1016/s0378-4347(00)82319-1. [DOI] [PubMed] [Google Scholar]
- 12.Kraus JW, Desmond PV, Marshall JP, Johnson RF, Schenker S, Wilkinson GR. Effects of aging and liver disease on disposition of lorazepam. Clin Pharmacol Ther. 1978;24:411–19. doi: 10.1002/cpt1978244411. [DOI] [PubMed] [Google Scholar]
- 13.Macgilchrist AJ, Birnie GG, Cook A, Scobie G, Murray T, Watkinson G, Brodie MJ. Pharmacokinetics and pharmacodynamics of intravenous midazolam in patients with severe alcoholic cirrhosis. Gut. 1986;27:190–5. doi: 10.1136/gut.27.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albrecht S, Ihmsen H, Hering W, Geisslinger G, Dingemanse J, Schwilden H, Schüttler J. The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin Pharmacol Ther. 1999;65:630–9. doi: 10.1016/S0009-9236(99)90084-X. [DOI] [PubMed] [Google Scholar]
- 15.Greenblatt DJ, Divoll Allen M, Locniskar A, Harmatz JS, Shader RI. Lorazepam kinetics in the elderly. Clin Pharmacol Ther. 1979;26:103–13. doi: 10.1002/cpt1979261103. [DOI] [PubMed] [Google Scholar]
- 16.McKindley DS, Hanes S, Boucher BA. Hepatic drug metabolism in critical illness. Pharmacotherapy. 1998;18:759–78. [PubMed] [Google Scholar]
- 17.Richard C, Berdeaux A, Delion F, Riou B, Rimailho A, Giudicelli JF, Auzzepy P. Effect of mechanical ventilation on hepatic drug pharmacokinetics. Chest. 1986;90:837–41. doi: 10.1378/chest.90.6.837. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet F, Richard C, Glaser P, Lafay M, Guesde R. Changes in hepatic blood flow induced by continuous positive pressure ventilation in critically ill patients. Crit Care Med. 1982;10:703–5. doi: 10.1097/00003246-198211000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Thummel KE, O'Shea D, Paine MF, Shen DD, Kunze KL, Perkins JD, Wilkinson GR. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 20.Shelly MP, Mendel L, Park GR. Failure of critically ill patients to metabolise midazolam. Anaesthesia. 1987;42:619–26. doi: 10.1111/j.1365-2044.1987.tb03086.x. [DOI] [PubMed] [Google Scholar]
- 21.Greenblatt DJ, Shader RI, Franke K, MacLauglin DS, Harmatz JS, Divoll Allen M, Werner A, Woo E. Pharmacokinetics and bioavailability of intravenous, intramuscular, and oral lorazepam in humans. J Pharm Sci. 1979;68:57–63. doi: 10.1002/jps.2600680119. [DOI] [PubMed] [Google Scholar]
- 22.Driessen JJ, Vree TB, Guelen PJM. The effects of acute changes in renal function on the pharmacokinetics of midazolam during long-term infusion in ICU patients. Acta Anaesth Belg. 1991;42:49–55. [PubMed] [Google Scholar]
- 23.von Moltke LL, Greenblatt DJ, Schmider J, Duan SX, Wright CE, Harmatz JS, Shader RI. Midazolam hydroxylation by human liver microsomes in vitro: inhibition by fluoxetine, norfluoxetine, and by azole antifungal agents. J Clin Pharmacol. 1996;36:783–91. doi: 10.1002/j.1552-4604.1996.tb04251.x. [DOI] [PubMed] [Google Scholar]
- 24.Ahonen J, Olkkola KT, Takala A, Neuvonen PJ. Interaction between fluconazole and midazolam in intensive care patients. Acta Anaesthesiol Scand. 1999;43:509–14. doi: 10.1034/j.1399-6576.1999.430504.x. [DOI] [PubMed] [Google Scholar]
- 25.Backman JT, Olkkola KT, Aranko K, Himberg JJ, Neuvonen PJ. Dose of midazolam should be reduced during diltiazem and verapamil treatments. Br J Clin Pharmacol. 1994;37:221–5. doi: 10.1111/j.1365-2125.1994.tb04266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olkkola KT, Aranko K, Luurila H, Hiller A, Saarnivaara L, Himberg JJ, Neuvonen PJ. A potentially hazardous interaction between erythromycin and midazolam. Clin Pharmacol Ther. 1993;53:298–305. doi: 10.1038/clpt.1993.25. [DOI] [PubMed] [Google Scholar]