Abstract
The hazards of prescribing many drugs, including side-effects, drug interactions, and difficulties of compliance, have long been recognized as particular problems when prescribing for elderly people. The need for appropriate and rational prescribing for elderly patients has been prioritized in the National Service Framework for Older People. This review addresses the research evidence on epidemiology of prescribing in elderly patients, methods of measuring the quality, and the role of the prescriber and the multidisciplinary team in the day-to-day optimization of drug therapy.
Keywords: ageing, drug prescribing, elderly patients
Introduction
The art of prescribing for elderly people is balancing the potentially conflicting demands of research evidence, practical considerations and patients’ wishes. The old term ‘polypharmacy’, defined as the prescription, administration or use of more medications than are clinically indicated [1], is no longer useful as it fails to encompass the omission of treatments that are clinically indicated. The risk–benefit analysis for drug treatment is only one of a number of more complex factors that influence prescribing in older patients (Figure 1). The term ‘appropriateness’ has been used to describe a variety of aspects of medicines such as formulation and packaging as well as their prescription [2]. As far as prescribing is concerned, the term should be used to cover overuse, underuse, and misuse of treatments.
Figure 1.
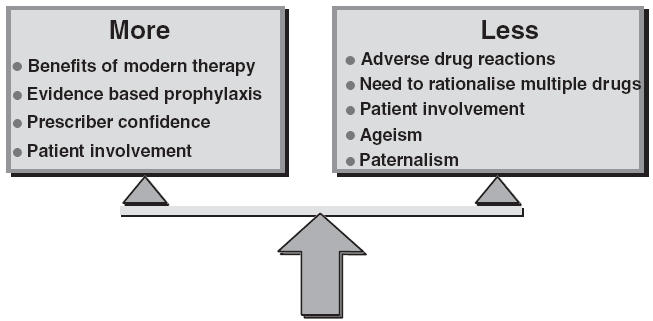
Conflicting pressures to prescribe more or less drugs to older patients
The term ‘optimizing prescribing’ has also crept into use to describe changes in prescribing reflecting an increase in appropriateness, however defined. Thus, a suboptimal treatment regimen is one that is not clinically appropriate. In some contexts, however, it is important to clarify who is benefiting from optimizing drug therapy. Enhanced appropriateness results in benefit to patients, whereas optimizing treatment costs, for example, may benefit others.
This review will address the research evidence on the epidemiology of prescribing in older patients, methods of measuring the quality of prescribing and methods of enhancing that quality. It will also examine the role of the prescriber and the multidisciplinary team in the day-to-day optimization of drug therapy.
Age-related patterns of prescribing
Within the UK the proportion of the population over the age of 65 years is 18% and rising, yet this group accounts for 45% of the NHS drug dispensing [3]. Elderly patients also consume more medications than younger patients during hospital admissions. This is so whether the calculation is based on cumulative consumption during a hospital stay or purely on cross-sectional data on a single day [4, 5]. Nielsen et al. found the average number of regular drugs prescribed increased almost linearly from two drugs per patient in patients aged 11–30 years to five drugs per patient in patients aged >71 years [4]. Houston et al. also found an almost linear increase in the number of drugs prescribed per patient during hospitalization in the UK, ranging from 1.8 in patients aged 10–19 years to 5.3 in those aged 80–89 years [5]. Studies of elderly hospital in-patients in a variety of settings have shown the mean number of drugs prescribed per patient of between 3.2 and 3.8, whereas studies reporting cumulative prescribing inevitably show rather higher figures [6–10]. These and other studies were purely descriptive and because clinical details were not collected, no conclusions on quality of prescribing could be drawn. Despite their failure to collect any clinical data, some of these authors concluded that prescribing was not excessive [6, 7, 10] or that drug utilization seemed appropriate [9]. These studies demonstrate that observations of appropriate use of drugs in elderly in-patients are often based on assumption and not evidence.
Appropriateness of prescribing
Harvey defined appropriate care in terms of an evaluation of available choices about alternative uses of resources [11] and Welsh and Grover [12] defined it as ‘the medical necessity of and the correct process of care’. However, a ‘risk–benefit’ approach to appropriate care as defined by the RAND Corporation is the most widely used definition [13]. They define appropriate care as that where ‘the expected health benefit (e.g. increased life expectancy, relief of pain, reduction in anxiety, improved functional capacity) exceeds the expected negative consequences (e.g. mortality, morbidity, anxiety of anticipating the procedure, pain produced by the procedure, misleading or false diagnoses, time lost from work) by a sufficiently wide margin that it is worth providing’[13]. However, Hopkins made the point that many clinicians will view examinations of appropriateness as ‘cost-cutting’ exercises [14] and subsequently added two further dimensions to the definition of appropriateness: the individuality of the patient under consideration, and the availability of healthcare resources [15]. Studies assessing appropriateness should bring to bear the evidence base relevant to the patient population under study in order to quantify the health gain and the negative consequences rather than to rely on subjective opinions.
Beers et al. developed a comprehensive set of explicit criteria based solely on prescribing data with no clinical data required [16]. Some of the criteria covered dosage, frequency, and duration of therapy, which, even with clinical data, would be hard to relate to an evidence base. Similarly, Chrischilles et al. used a different set of explicit consensus criteria based on daily dosage, duplication of therapy and interacting drug combinations using medication profiles from prescriptions and the medical records [17]. Over 40% of patients appeared to have been given inappropriate medication using their criteria. The inclusion of interacting combinations of drugs but without evidence of resulting harm cannot be used to identify suboptimal prescribing in patients who take several medications. Lipton et al. combined explicit criteria with implicit criteria via which different experts (panel members) could bring to bear their own experience, again using summaries of the clinical records [18]. These approaches, along with others [19–21], have the major weakness in research terms that they are consensus based although they all use what evidence base there is. This weakness outweighs their main strength that they are comprehensive, covering a wide range of treatments.
Prescribing indicators reflecting desirable or undesirable prescribing have been developed from the literature and used to assess the appropriateness of prescribing for patients over the age of 65 in hospital [22, 23]. The indicators incorporate some clinical information, and were effective in evaluating the performance of 102 hospitals in the UK. Prescribing to elderly medical in-patients was found to be suboptimal, but targets were achieved by some hospitals [24].
Legal and ethical framework
Adults are presumed to be capable of giving and withholding consent to medical treatment and such consent must be obtained before treatment is given. To treat a person without their consent is to commit the civil wrong of trespass to the person and may constitute a crime (Re T. 1992) [25]. A patient's capacity may be impaired temporarily or permanently for a variety of reasons including physical illness, mental illness, brain pathology, drugs, and fatigue. A patient who lacks capacity can neither consent to nor refuse treatment. If a patient wholly lacks capacity, the doctor has a duty to act in the patient's best interests (Re F. 1989) [26]. This must take into account any Advance Directive (Re C. 1994) [27] clearly made by the patient at a time when he or she had capacity and that the circumstances that now apply were clearly understood at the time when the Advance Directive was made. No advance directive permits the doctor to break the law.
A patient may have impaired capacity rather than a total lack. In this case the decision to go ahead with treatment against the patient's wish or without their consent should take into account both the immediacy of the need for treatment and the severity of the consequences of failing to treat. Discussions with relatives are always helpful and are part of good clinical practice, but no adult can consent on behalf of another. In order to test for capacity it must be established that the patient understands the information given (about the treatment, its wanted effects, adverse effects and the consequences of not having the treatment), can retain the information and believes it. The decision does not have to be reasonable, nor does the patient have to explain the reasons for their decision. It is important to note that lack of capacity in, for example, activities of daily living does not imply lack of capacity with regard to a specific medical decision. If a patient is suffering from a mental disorder, consideration must be given to the use of the Mental Health Act 1983, although whether this covers patients with dementia is not yet clear [28].
Quite apart from the legal requirements, it is important to provide all patients with information appropriate to their needs prior to prescribing treatments and to provide a balanced view of the advantages and disadvantages of any particular course of action. The discussion that follows assumes competency and consent.
Methods of enhancing prescribing
Avorn and colleagues assessed a structured parenteral antibiotic order form designed to guide physicians towards correct therapeutic decisions without restricting their clinical options [29]. The use of the form was supplemented with verbal educational communication and dissemination of printed material. There was no control group. A significant reduction in pharmacokinetically incorrect prescriptions of antibiotics was observed after introduction of the programme. A similar study relating to the prescribing of a single antibiotic, tobramycin, using cost as well as specific patient recommendations, also showed a significant reduction in prescribing outside the consensus protocol compared with baseline in the absence of a control group [30]. A study from Hong Kong of sultamicillin and co-amoxiclav prescribing used the technique of immediate concurrent feedback, whereby prescribers were presented with a reminder of the agreed protocol attached to the prescription chart [31]. This technique achieved a significant improvement in adherence to the consensus protocols. Other studies from the UK in nursing homes [32] and secondary care [33] have demonstrated a statistically significant improvement in the appropriateness of prescribing when intervention/feedback is multifaceted. The use of bulletins containing educational material and data on potential financial savings alone is ineffective [34, 35].
Prescribing practice
Poor prescribing practice has been ascribed to difficulty prescribers had in understanding clinical pharmacology (the scientific basis of treatment) and therapeutics (the practical aspects of treatment) [36]. Whilst this is undoubtedly an important factor, there are many others (Table 1).
Table 1.
Causes of suboptimal prescribing
| Nonclinical skills |
| • Failure of grasp of clinical pharmacology and therapeutics |
| • Failure to keep up to date with published work |
| • Prescriber's opinion outweighing scientific data |
| • Medical ageism |
| • Pressure to cut drug costs |
| Clinical skills |
| • Inadequate clinical assessment leading to incorrect diagnosis |
| • Failure to obtain history/document previous adverse drug reactions |
| • Failure to record current medication including over the counter drugs |
| • Failure to monitor response to treatment |
| • Failure to recognize potentially serious drug interactions |
| • Failure to recognize subtle adverse drug reactions |
| • Failure to review repeat medication |
| • Failure to take account of altered pharmacokinetics and pharmacodynamics |
| • Simple error |
Modified from Royal College of Physicians, 1997 [42].
The use of a prescribing checklist (Table 2) can prompt prescribers to think through the rationale, choice of treatment and dosing regimen that is best suited to a particular patient. Of particular importance is the need to discuss treatment options with the patient. This will also help to maintain good compliance with the agreed regimen. In some cases compliance aids or carer support may be needed. Many drugs or regimens may be judged good practice (or otherwise) only on the basis of clinical data from an individual patient. Some drugs, however, should very rarely, if ever, be prescribed regardless of the clinical scenario (Table 3).
Table 2.
Prescription checklist
| • What are the patient's views? |
| • What is the diagnosis (or diagnoses) you are treating? |
| • What is the aim of treatment? |
| • What are the treatment possibilities in this patient? |
| Nonpharmacological |
| Pharmacological |
| • How is your preferred drug (and its metabolites if relevant) cleared? |
| • Will other disease states affect your choice? |
| • Will physiological states affect your choice? |
| • Could one drug treat more than one problem? |
| • What is the best route and starting dosage? |
| • How will treatment be monitored? |
| • When will you increase the dose? |
| • What will its duration be? |
| • What are the potential adverse effects? |
| • What potential drug interactions are relevant? |
| • Would discontinuing another drug help? |
| • What information should you discuss with the patient? |
Table 3.
Drugs that should rarely, if ever, be prescribed to older patients
| Drug class | Reason |
|---|---|
| Long-acting oral hypoglycaemics | Unacceptably high risk of hypoglycaemia |
| Benzodiazepines (particularly long acting drugs) | Increased risk of falls and nocturnal disorientation |
| Anticholinergics where alternatives exist | Increased risk of cognitive impairment and peripheral anticholinergic effects |
| Cerebral vasodilators | No evidence of efficacy |
It is important to recognize that however good prescribing practice is, adverse drug reactions will still occur and, indeed, will occur more commonly amongst patients receiving more drugs (e.g. appropriate prophylactic treatments) than those inappropriately not receiving such treatments. The balance of benefit, however, will be in favour of treatment.
What can be done locally?
Application of research evidence
The strict application to clinical practice of the limited research evidence is problematic. This is because the types of intervention that have been used lend themselves to the research environment rather than to routine clinical practice. The collection of prescribing data, at any rate at the present time, is not part of routine clinical practice and could not therefore be used to inform prescribers. It has been suggested that a system similar to the Prescribing Analysis and Cost (PACT) system used in general practice could be implemented in hospital. This system has proved very useful for monitoring some aspects of prescribing, including costs, but is useless for monitoring appropriateness of prescribing.
Nevertheless, although not part of routine practice, prescribing data can readily be collected together with relevant clinical data and fed back to prescribers verbally with key educational points. This approach can easily be developed by the clinical audit team. It is not clear from the literature how frequently such interventions should be performed, but in the UK, with heavy dependence on junior medical staff for hospital-based prescribing, it would seem sensible to carry out such feedback at least as frequently as staff rotate.
Educational approaches
Although not proven by randomized controlled trials, consultant physicians and clinical pharmacists can educate prescribers in the rational use of drugs in older patients. This education should be patient based on and cover the basic principles of age-related changes in pharmacokinetics and pharmacodynamics as well as factors responsible for the excess prevalence and common causes of adverse drug reactions and drug interactions. Beyond these basic principles the practice of evidence-based medicine as it applies to prescribing is also an essential part of the educational process. This involves both the updating of personal knowledge and the application to clinical medicine of the findings of randomized controlled trials.
Educationalists maintain that repeated exposure in a practical setting results in enhanced learning compared with didactic teaching. The ward round is the obvious regular teaching/learning opportunity for clinical teams and provides an opportunity for reinforcement.
The role of the multidisciplinary team
The role of ward pharmacists in enhancing the quality of prescribing is well documented [37] and, in routine clinical practice, is usually unrecorded [38]. It is usual for pharmacists to be involved in the collection and presentation of prescribing data as part of clinical audit. In specialized units, e.g. stroke units or coronary care units, nurses are often involved in more than merely dispensing medication. Such extended roles may now include prescribing certain drugs or other treatments, involvement in the prescribing decision making, or merely the prompting of medical staff. A study of 16 hospitals throughout England and Wales found the three hospitals recording the highest percentage of completed allergy/sensitivity boxes on drug charts were those where the responsibility for this was multidisciplinary rather than purely a medical one [39].
Future developments
Participation in clinical effectiveness projects is high on the Governments agenda [40] and national comparative audits can be used to improve patient care. The National Service Framework for Older People [41] has identified the need for linking prescribing and clinical data, and reducing adverse drug reactions. Many hospitals in the USA and a few in the UK have implemented electronic prescribing. These systems offer important advances: (i) the routine collection of prescribing data that is linked to patient details enables feedback of prescribing information to prescribers with no additional data collection; (ii) the opportunity to direct the prescriber's thinking by issuing ‘alerts’ in real time – so-called decision support. These can prompt the prescriber for required information (e.g. penicillin allergy, serum creatinine) or ask the prescriber to respond to queries.
Conclusions
At the sharp end of clinical practice all attempts to improve awareness of the problems and enhance the quality of prescribing should be facilitated and positively encouraged. Operational research designed to evaluate methods of achieving enhanced prescribing quality inevitably lags behind the need for such data. The way forward, however, must be to implement what little research evidence there is. Second, although unproven, education of staff of all disciplines remains crucial.
References
- 1.Montamat SC, Cusak B. Overcoming problems with polypharmacy and drug misuse in the elderly. Clin Geriatr Med. 1992;8:143–58. [PubMed] [Google Scholar]
- 2.Parish PA. Drug prescribing – the concern of all. R Soc Health J. 1973;93:213–17. doi: 10.1177/146642407309300414. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health Statistics of prescriptions dispensed in the Family Health Service Authorities. Statistical Bulletin 2000/2002. England 1989–1999.
- 4.Nielsen IK, Osterlind AW, Christiansen LV, et al. Drug consumption and age in a department of internal medicine. Dan Med Bull. 1981;28:71–73. [PubMed] [Google Scholar]
- 5.Houston AH. Drug use in Southampton hospitals. In: O'Malley J, editor. Clinical pharmacology and drug treatments in the elderly. Edinburgh: Churchill Livingstone; 1984. p. 72. [Google Scholar]
- 6.Christopher LJ, Ballinger BR, Shepherd AMM, Ramsay A, Crooks G. Drug prescribing patterns in the elderly: a cross-sectional study of in-patients. Age Ageing. 1978;7:74–82. doi: 10.1093/ageing/7.2.74. [DOI] [PubMed] [Google Scholar]
- 7.Moir DC, Davidson JF, Gallon SC, Dingwall-Fordyce I, Weir RD. The extent of drug prescribing for the older hospital patient. J Clin Exp Gerontol. 1979;1:159–71. [Google Scholar]
- 8.Gosney M, Tallis R. Prescription of contraindicated and interacting drugs in elderly patients admitted to hospital. Lancet. 1984;2:564–67. doi: 10.1016/s0140-6736(84)90775-x. [DOI] [PubMed] [Google Scholar]
- 9.Passmore AP, Crawford VLS, Beringer TRO, Gilmore DH, Montgomery A. Determinants of drug utilisation in an elderly population in North and West Belfast. Pharmacoepidemiol Drug Safety. 1995;4:147–60. [Google Scholar]
- 10.Primrose WR, Capewell AE, Simpson GK, Smith RG. Prescribing patterns observed in registered nursing homes and long-stay geriatric wards. Age Ageing. 1987;16:25–28. doi: 10.1093/ageing/16.1.25. [DOI] [PubMed] [Google Scholar]
- 11.Harvey R. Making it better: strategies for improving the effectiveness and quality of health services in Australia. Melbourne: Treble Press; 1991. National Health Strategy, Background paper number 8. [Google Scholar]
- 12.Welsh CE, Grover PL. An overview of quality assurance. Med Care. 1991;29:S8–S28. [Google Scholar]
- 13.Chassin MR, Park RE, Fink A. Indications for selected medical and surgical procedures – a literature review and ratings appropriateness: coronary artery bypass graft surgery. Santa Monica: RAND Corporation; 1986. 3204/2-CWF HF/HCFA/PMT/RWJ. [Google Scholar]
- 14.Hopkins A. Appropriate investigation and treatment in clinical practice. London: Royal College of Physicians; 1989. [Google Scholar]
- 15.Hopkins A. Report of a working group prepared for the Director of Research and Development of the NHS Management Executive. What do we mean by appropriate health care? Qual Health Care. 1993;2:117–23. doi: 10.1136/qshc.2.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151:1825–32. [PubMed] [Google Scholar]
- 17.Chrischilles EA, Helling DK, Booth BM, Lemke JH, Mustion AL. Documentation and appropriateness of prescribing for veterans administration ambulatory-care patients. Am J Hosp Pharm. 1988;45:2345–51. [PubMed] [Google Scholar]
- 18.Lipton HL, Bero LA, Bird JA, McPhee SJ. The impact of clinical pharmacists’ consultation on physicians’ geriatric drug prescribing. Med Care. 1992;30:646–58. doi: 10.1097/00005650-199207000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Cantrill JA, Sibbald B, Buetow S. Indicators of appropriateness of long term prescribing in general practice in the United Kingdom: consensus development, face and content validity, feasibility, and reliability. Qual Health Care. 1998;7:130–5. doi: 10.1136/qshc.7.3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045–51. doi: 10.1016/0895-4356(92)90144-c. [DOI] [PubMed] [Google Scholar]
- 21.McLeod PJ, Huang AR, Tamblyn RM, Gayton DC. Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ. 1997;156:385–91. [PMC free article] [PubMed] [Google Scholar]
- 22.Batty GM. MD Thesis Prifysgol Cymru 1999. The development of indicators and the investigation of methods to promote quality prescribing in elderly hospital patients. [Google Scholar]
- 23.Grant RL, Aggarwal R, Lowe D, et al. National Sentinel Clinical Audit of Evidence-Based Prescribing for Older People: methodology and development. J Eval Clin Pract. 2001;8:189–98. doi: 10.1046/j.1365-2753.2002.00309.x. [DOI] [PubMed] [Google Scholar]
- 24.Batty GM, Grant RI, Aggarwal R, et al. Using prescribing indicators to measure the quality of prescribing to elderly medical in-patients. Age Ageing. 2003;32:292–8. doi: 10.1093/ageing/32.3.292. [DOI] [PubMed] [Google Scholar]
- 25.Re T. (An Adult) (Consent to Medical Treatment) [1992] 2 FLR 458.
- 26.Re F. (Mental Health Sterilisation v West Berkshire Health Authority [1989] 2 WLR 1025: [1989] All ER 673.
- 27.Re C. (Adult: Refusal of Treatment) [1994] 1 WLR 290. [PubMed]
- 28.Department of Health; [23/6/2003]. Mental Health Act 1983. http://www.doh.gov.uk/mhact1983/index.htm. [Google Scholar]
- 29.Avorn J, Soumerai SB, Taylor W, Wessels MR, Janousek J, Weiner M. Reduction of incorrect antibiotic dosing through a structured educational order form. Arch Intern Med. 1988;148:1720–24. [PubMed] [Google Scholar]
- 30.Soumerai SB, Avorn J, Taylor WC, Wessels M, Maher D, Hawley SL. Improving choice of prescribed antibiotics through concurrent reminders in an educational order form. Med Care. 1993;31:552–8. doi: 10.1097/00005650-199306000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Seto WH, Ching TY, Kou M, Chiang SC, Lauder IJ, Kumana CR. Hospital antibiotic prescribing successfully modified by ‘immediate concurrent feedback’. Br J Clin Pharmacol. 1996;41:229–34. doi: 10.1111/j.1365-2125.1996.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 32.Fahey T, Montgomery AA, Barnes J, Protheroe J. Quality of care for elderly residents in nursing homes and elderly people living at home: controlled observational study. Br Med J. 2003;326:580–3. doi: 10.1136/bmj.326.7389.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batty GM, Husk J, Swan J, Grant RL. What it takes to change prescribing. Age Ageing. 2001;30:79. [Google Scholar]
- 34.Avorn J, Soumerai SB. Improving drug-therapy decisions through educational outreach: a randomized controlled trial of academic based ‘detailing’. New Engl J Med. 1983;308:1457–63. doi: 10.1056/NEJM198306163082406. [DOI] [PubMed] [Google Scholar]
- 35.Schaffner W, Ray WA, Federspiel CF, Miller WO. Improving antibiotic prescribing in office practice: a controlled trial of three educational methods. JAMA. 1983;250:1728–32. [PubMed] [Google Scholar]
- 36.Jackson PR, Yeo WW, Bax NDS, Ramsay LE. Essentials of clinical Pharmacology and Therapeutics – 1. Prescribing. Student BMJ. 1995;3:13–16. [Google Scholar]
- 37.Lipton HL, Bird JA, Bero LA, McPhee SJ. Assessing the appropriateness of physician prescribing for geriatric outpatients. J Pharm Technol. 1993;9:107–13. [PubMed] [Google Scholar]
- 38.Cotter S, McKee M, Barber N. Pharmacists and prescribing: an unrecorded influence. Qual Health Care. 1993;2:75–6. doi: 10.1136/qshc.2.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oborne CA, Batty GM, Maskrey V, Swift CG, Jackson SHD. Development of prescribing indicators for elderly medical inpatients. Br J Clin Pharmacol. 1997;43:91–7. doi: 10.1111/j.1365-2125.1997.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 40.Department of Health. A first class service: quality in the new NHS. London: The Stationary Office; 1998. [Google Scholar]
- 41.Department of Health. National Service Framework for older people: medicines and older people. London: The Stationary Office; 2001. [Google Scholar]
- 42.Royal College of Physicians. Medications for older people. London: Royal College of Physicians; 1997. [Google Scholar]


