Abstract
Aims
Our aim was to investigate the effect of the CYP2C8 inhibitor trimethoprim on the pharmacokinetics and pharmacodynamics of the antidiabetic drug repaglinide, and to examine the influence of the former on the metabolism of the latter in vitro.
Methods
In a randomized, double-blind, crossover study with two phases, nine healthy volunteers took 160 mg trimethoprim or placebo orally twice daily for 3 days. On day 3, 1 h after the last dose of trimethoprim or placebo, they ingested a single 0.25 mg dose of repaglinide. Plasma repaglinide and blood glucose concentrations were measured for up to 7 h post-dose. In addition, the effect of trimethoprim on the metabolism of repaglinide by human liver microsomes was investigated.
Results
Trimethoprim raised the AUC(0,∞) and Cmax of repaglinide by 61% (range, 30–117%; P = 0.0008) and 41% (P = 0.005), respectively, and prolonged the t½ of repaglinide from 0.9 to 1.1 h (P = 0.001). Trimethoprim had no significant effect on the pharmacokinetics of its aromatic amine metabolite (M1), but decreased the M1 : repaglinide AUC(0,∞) ratio by 38% (P = 0.0005). No effect of trimethoprim on the blood glucose-lowering effect of repaglinide was detectable. In vitro, trimethoprim inhibited the metabolism of (220 nm) repaglinide in a concentration-dependent manner.
Conclusions
Trimethoprim raised the plasma concentrations of repaglinide probably by inhibiting its CYP2C8-mediated biotransformation. Although the interaction did not significantly enhance the effect of repaglinide on blood glucose concentration at the drug doses used, the possibility of an increased risk of hypoglycaemia should be considered during concomitant use of trimethoprim and repaglinide in patients with diabetes.
Keywords: CYP2C8, drug interaction, repaglinide, trimethoprim
Introduction
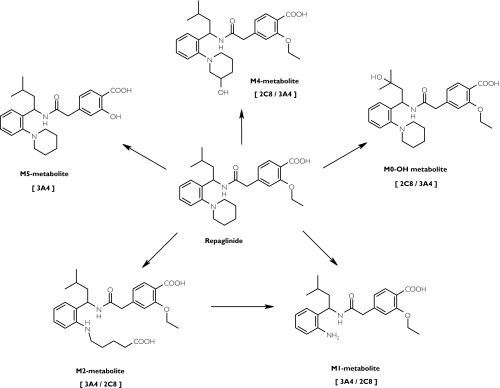
Repaglinide is a short-acting antidiabetic drug of the meglitinide analogue class [1]. It is completely metabolized in humans [2] and in vitro studies have suggested that cytochromes P450 (CYP) 2C8 and 3A4 mainly contribute to its oxidative biotransformation (Figure 1) [3]. Gemfibrozil, a fibric acid derivative hypolipidaemic drug and an inhibitor of CYP2C8 [4, 5] (but not of CYP3A4) [6], greatly increases (by about 8-fold) the plasma concentrations of repaglinide and prolongs its glucose-lowering effect [7].
Figure 1.
The main biotransformation pathways of repaglinide. The principal enzyme catalysing the reaction in vitro (22 µm) is given first, as described by Bidstrup et al.[3]
The widely used antimicrobial drug trimethoprim is a selective inhibitor of CYP2C8 in vitro[8]. It has been estimated that at normal clinical doses, trimethoprim would inhibit CYP2C8-mediated metabolic clearance by about 26% to 80%, with negligible effects on other CYP enzymes [8]. We have investigated the effects of trimethoprim on the pharmacokinetics and pharmacodynamics of repaglinide in healthy subjects and the influence of trimethoprim on the metabolism of repaglinide by human liver microsomes.
Methods
Human pharmacokinetic study
Subjects
Nine healthy volunteers (eight male, one female; age range 19–23 years; weight range 62–97 kg) participated in the study after they had given written informed consent. They were ascertained to be healthy by medical history, physical examination and routine laboratory tests. None was a tobacco smoker or used any continuous medication. Use of other drugs was prohibited 1 week before, during and 1 week after administration of repaglinide.
Study design
The study protocol was approved by the Ethics Committee for Studies in Healthy Subjects and Primary Care of the Helsinki and Uusimaa Hospital District and by the National Agency for Medicines. A randomized crossover study with two phases and a washout period of 2 weeks was carried out. The volunteers took 160 mg trimethoprim (one Trimetin 160 mg tablet, Vitabalans, Hämeenlinna, Finland) or placebo orally twice daily at 08.00 h and 20.00 h for 3 days. After an overnight fast on day 3 at 09.00 h and 1 h after the last trimethoprim dose, a single oral dose of 0.25 mg repaglinide (one half of a 0.5 mg Novonorm tablet, NovoNordisk, Bagsværd, Denmark) was administered with 150 ml water. Repaglinide tablets were weighed before and after halving. The mean weight of the original tablets was 95.0 mg (n = 18, coefficient of variation, CV, 1.9%) and that of the halved tablets was 48.4 mg (n = 18, CV 0.6%). The greatest percentage deviation from the mean weight of the halved tablets was less than 2%. Halved tablets with equal weight were selected for each subject for the two study phases, and the difference in weight between these two halved tablets was less than 1 mg (2%) for all subjects. A standardized light breakfast was served 15 min after the administration of repaglinide, a standardized snack at 1 and 2 h after repaglinide and a standardized warm meal after 3 h. The breakfast was eaten within 10 min and contained approximately 370 kcal energy, 70 g carbohydrates, 8 g protein and 6 g fat. The snacks were identical, were eaten within 5 min and contained about 200 kcal energy, 45 g carbohydrates, 2 g protein and 1 g fat each. Food intake was identical during both days of repaglinide administration. The subjects were under direct medical supervision during the period of administration of repaglinide and blood glucose concentrations were measured throughout. Glucose for intravenous use and glucagon for intramuscular use were available in case of severe hypoglycaemia, but they were not needed.
Blood sampling and determination of blood glucose concentrations
On the days of administration of repaglinide, a forearm vein of each subject was cannulated and kept patent with a stylet. Timed blood samples were drawn before the administration of repaglinide and 20, 40, 60, 80 and 100 min and 2, 2.5, 3, 4, 5 and 7 h later. The blood samples (10 ml each) were drawn to tubes that contained ethylenediaminetetraacetic acid (EDTA). Blood glucose concentration was measured immediately after each sample was drawn by the glucose oxidase method using the Precision G blood glucose testing system (Medisense, Bedford, MA). The between-day CV for the assay was 7.8% at 3.1 mmol l−1, 6.3% at 5.5 mmol l−1 and 6.2% at 16.9 mmol l−1 (n = 4). Plasma was separated within 30 min after sampling and the samples were stored at −70°C until analysis for repaglinide and trimethoprim.
Drug analysis
Plasma repaglinide and its M1 metabolite (an aromatic amine) concentrations were measured using a liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometer PE SCIEX API 3000 (Sciex Division of MDS Inc, Toronto, Ontario, Canada) and by a modification of a previously described method [9]. Nateglinide served as the internal standard, and solid-phase extraction was performed with C8 silica columns (Supelco, Bellefonte, PA, USA). The ion transitions monitored were m/z 453–230 for repaglinide, m/z 385–162 for M1 and m/z 318–69 for nateglinide. These transitions represent the product ions of the [M + H]+ ions. The quantification limit (peak to noise ratio 3 : 1) for repaglinide was 0.1 ng ml−1 and the between-day CV was 14.0% at 0.1 ng ml−1, 7.6% at 2.0 ng ml−1 and 8.8% at 20.0 ng ml−1 (n = 4). M1 is given in arbitrary units (U) relative to the peak height of M1 in the chromatogram of the [M + H]+ ions. Plasma trimethoprim concentrations on day 3 were measured by HPLC with ultraviolet detection [10, 11]. The between-day CV for trimethoprim was 4.9% at 0.8 mg ml−1, 2.5% at 2.1 mg ml−1 and 2.9% at 5.0 mg ml−1 (n = 6).
Pharmacokinetics
The pharmacokinetics of repaglinide were characterized by peak concentration in plasma (Cmax), time to Cmax (tmax), areas under the concentration-time curve [AUC(0,7 h) and AUC(0,∞)] and elimination half-life (t1/2). The pharmacokinetics of trimethoprim were characterized by AUC(0,8 h) and Cmax. The terminal log-linear part of the concentration-time curve was identified visually for each subject. The elimination rate constant (kel) was determined by linear regression analysis of the log-linear part of the plasma drug concentration-time curve. The t1/2 was calculated by the equation t1/2= ln2/kel. The AUC values were calculated by use of the linear trapezoidal rule for the rising phase of the plasma drug concentration-time curve and the log-linear trapezoidal rule for the descending phase, with extrapolation to infinity, when appropriate, by dividing the last measured concentration by kel.
Pharmacodynamics
The pharmacodynamics of repaglinide were characterized by the mean change, maximum increase and maximum decrease in blood glucose concentration. The mean change was calculated by dividing the net area under the blood glucose concentration-time curve from 0 to 3 h and 0 to 7 h by the corresponding time interval.
Statistical analysis
Results are expressed as mean values ± SD in the text and tables and, for clarity, as mean values ± SEM in the figures. For all variables except tmax, 95% confidence intervals (CI) were calculated on the mean differences between placebo and trimethoprim phases. The pharmacokinetic and pharmacodynamic variables for repaglinide after the two pretreatments were compared using a paired t-test or, in the case of tmax, by the Wilcoxon signed-rank test. All the data were analysed using the statistical program Systat for Windows, version 6.0.1 (SPSS Inc, Chicago, Ill). The differences were considered statistically significant at a P value of less than 0.05.
In vitro study
Microsomes
Pooled human hepatic microsomes containing representative activities of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 and CYP4A were purchased from Gentest Corp. (Woburn, MA). Human liver tissue had been collected in accordance with all pertinent regulations, and permissions from the donors’ families had been obtained prior to organ collection. The procedures of organ collection had been reviewed and accepted by the respective institutional Human Subjects Committee.
Inhibition of the microsomal metabolism of repaglinide by trimethoprim
The effect of trimethoprim (0–200 µmol l−1) on the rate of repaglinide metabolism was studied by measuring the concentration of unchanged repaglinide remaining after incubation of drug with microsomes. The stock solutions of trimethoprim were prepared in methanol (final concentration 1% v/v). The concentration of repaglinide (molecular weight 452.58) used in the experiments was 220 nm, since this is comparable with its peak plasma concentrations after therapeutic doses in humans [1]. All incubations were conducted in duplicate in a shaking water bath at 37 °C. The incubations were carried out in 0.1 mol l−1 sodium phosphate buffer (pH 7.4), containing 5.0 mm MgCl2 and the microsomal protein concentration was 0.25 mg ml−1. Repaglinide (stock solution dissolved in methanol), buffer and β-NADPH (1.0 mm) were premixed and incubations were commenced by addition of microsomes. Aliquots (400 µl) were removed at 0, 15 and 30 min and then immediately added to 200 µl 2-propanol and cooled on ice. After addition of 400 µl 0.1 m potassium phosphate buffer (pH 5.9), the samples were extracted with 4.0 ml diethyl ether. After centrifugation, the supernatant fraction was evaporated to dryness under nitrogen, the residue was reconstituted with 100 µl of the mobile phase and 65 µl was subjected to HPLC analysis with UV-detection at the wavelength 243 nm. The intra-assay CV was less than 9% at the concentrations used in the incubations.
At each trimethoprim concentration, pseudo-first order rate constants (k) for repaglinide metabolism were determined by linear regression analysis of the logarithm of repaglinide concentration vs incubation time data. The initial reaction velocity (V) was calculated by using the equation V = C0×k, where C0 = initial concentration of repaglinide and the IC50 value (concentration of inhibitor to cause 50% inhibition of original enzyme activity) for trimethoprim was estimated from reaction velocity data using nonlinear regression analysis.
Results
Pharmacokinetic study
The plasma concentrations of repaglinide were significantly increased by trimethoprim compared with placebo (Table 1 and Figure 2). Trimethoprim raised the AUC(0,∞) and Cmax of repaglinide by 61% (P = 0.0008) and 41%(P = 0.005), respectively, and prolonged the t1/2 of repaglinide from 0.9 to 1.1 h (P = 0.001). An increase in the AUC(0,∞) of repaglinide was seen in every subject (range 30–117%). Trimethoprim had no significant effect on the pharmacokinetics of the M1 metabolite of repaglinide, but decreased the M1 : repaglinide AUC(0,∞) ratio by 38% (P = 0.0005).
Table 1.
The pharmacokinetics of repaglinide (0.25 mg single dose) in nine healthy subjects after treatment for 3 days with 160 mg trimethoprim or placebo twice daily
| Variable | Placebo phase (control) | Trimethoprim phase | Mean difference between placeboand trimethoprim (95% CI) | P value |
|---|---|---|---|---|
| Repaglinide | ||||
| Cmax (ng ml−1) | 4.7 ± 2.6 | 6.6 ± 3.0 | 1.9 (0.8, 3.0) | 0.005 |
| % of control (range) | 100% | 141% (99–197%) | ||
| tmax (min) | 40 (40–60) | 40 (20–40) | 0.18 | |
| t1/2 (h) | 0.9 ± 0.2 | 1.1 ± 0.2 | 0.2 (0.1, 0.3) | 0.001 |
| % of control (range) | 100% | 120% (102–143%) | ||
| AUC(0,7 h) (ng ml−1 h) | 5.9 ± 3.5 | 9.4 ± 5.2 | 3.5 (2.0, 5.0) | 0.0007 |
| % of control (range) | 100% | 160% (129–216%) | ||
| AUC(0,∞) (ng ml−1 h) | 5.9 ± 3.6 | 9.6 ± 5.4 | 3.6 (2.0, 5.3) | 0.0008 |
| % of control (range) | 100% | 161% (130–217%) | ||
| M1 | ||||
| Cmax (U ml−1) | 0.38 ± 0.11 | 0.35 ± 0.10 | −0.03 (−0.08, 0.02) | 0.22 |
| % of control (range) | 100% | 92% (69–124%) | ||
| tmax (min) | 40 (40–80) | 40 (40–60) | 0.38 | |
| t1/2 (h) | 1.0 ± 0.2 | 1.2 ± 0.3 | 0.2 (−0.1, 0.4) | 0.23 |
| % of control (range) | 100% | 115% (80–174%) | ||
| AUC(0,7 h) (U ml−1 h) | 0.68 ± 0.20 | 0.66 ± 0.15 | −0.03 (−0.11, 0.05) | 0.45 |
| % of control (range) | 100% | 96% (76–130%) | ||
| AUC(0,∞) (U ml−1 h) | 0.75 ± 0.20 | 0.74 ± 0.14 | −0.01 (−0.10, 0.08) | 0.86 |
| % of control (range) | 100% | 99% (82–147%) | ||
| AUC(0,∞) ratio (U ng−1) | 0.15 ± 0.054 | 0.093 ± 0.035 | −0.058 (−0.082, −0.034) | 0.0005 |
| (M1 : repaglinide) | ||||
| % of control (range) | 100% | 62% (47–79%) |
Data are mean values (± SD); tmax data are given as median (range).
Figure 2.
Mean (± SEM) plasma concentrations of repaglinide (A) and its aromatic amine metabolite (M1) (B) in nine healthy volunteers after a single oral dose of 0.25 mg repaglinide and following treatment for 3 days with placebo or 160 mg trimethoprim twice daily. Placebo phase (○); trimethoprim phase (•)
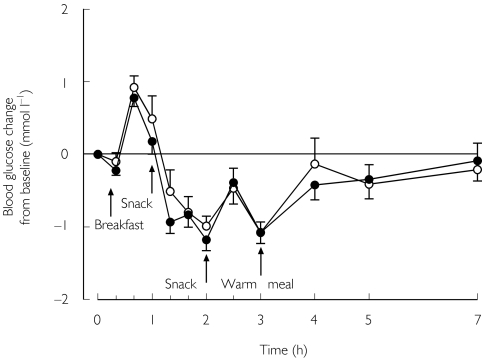
No significant differences were seen in the blood glucose response between the placebo and trimethoprim phases (Table 2 and Figure 3). None of the subjects had symptomatic hypoglycaemia.
Table 2.
Blood glucose response to repaglinide (0.25 mg single oral dose) followed by meals in nine healthy subjects after treatment for 3 days with placebo or 160 mg trimethoprim twice daily
| Change in blood glucoseconcentration | Placebo phase (control) | Trimethoprim phase | Mean difference between placebo andtrimethoprim (95% CI) | P value |
|---|---|---|---|---|
| Mean change 0–3 h (mm) | −0.9 ± 1.2 | −1.3 ± 0.9 | −0.4 (−1.3, 0.6) | 0.38 |
| Mean change 0–7 h (mm) | −2.4 ± 2.7 | −2.9 ± 2.3 | −0.5 (−3.2, 2.3) | 0.72 |
| Maximum increase (mm) | 1.1 ± 0.7 | 0.8 ± 0.4 | −0.3 (−0.9, 0.3) | 0.26 |
| Maximum decrease (mm) | 1.3 ± 0.3 | 1.4 ± 0.3 | 0.1 (−0.3, 0.5) | 0.59 |
Data are mean values (±SD).
Figure 3.
Mean (± SEM) blood glucose concentrations in nine healthy subjects after a single oral dose of 0.25 mg repaglinide and following treatment for 3 days with placebo or 160 mg trimethoprim twice daily. Placebo phase (○); trimethoprim phase (•)
The AUC(0,8 h) and Cmax of trimethoprim on day 3 were 18.7 ± 3.8 mg l −1h (range 13.9–26.8) and 2.9 ± 0.6 mg l−1 (range 2.1–4.1), respectively. There was a linear relationship between the AUC(0,8 h) of trimethoprim and the increase in the AUC(0,7 h) of repaglinide caused by trimethoprim (Pearson r = 0.68, P = 0.04).
In vitrometabolism study
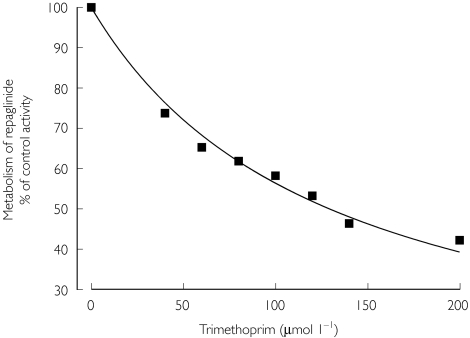
Incubation of repaglinide (220 nm) with human liver microsomes resulted in a time- and NADPH-dependent substrate consumption. The decline in repaglinide concentration was log-linear for 30 min with an in vitro t1/2 of 22 min and an estimated initial reaction velocity of 28 pmol min−1 mg−1 protein. Trimethoprim inhibited repaglinide metabolism with an estimated IC50 value of 129 µm(Figure 4).
Figure 4.
Effect of trimethoprim on the metabolism of repaglinide by pooled human liver microsomes. Repaglinide (220 nm) was incubated with increasing concentrations of trimethoprim. Values represent the mean of duplicate determinations
Discussion
Trimethoprim, given in therapeutic doses, was found to elevate the plasma concentrations of repaglinide and prolong its half-life. In addition, trimethoprim significantly decreased the M1 : repaglinide AUC(0,∞) ratio, indicating that it had inhibited the metabolism of repaglinide. This conclusion was supported by the finding that trimethoprim inhibited the metabolism of repaglinide by human liver microsomes. There was marked variation in the extent of the pharmacokinetic interaction between trimethoprim and repaglinide, with the increase in the AUC(0,∞) of the latter ranging from 30% to 117% even in this homogeneous group of young healthy subjects. Trimethoprim had no significant effect on the blood glucose-lowering effect of repaglinide, which might be explained, at least partially, by the repeated intake of food as well as by the use of a subtherapeutic dose of repaglinide (for safety reasons).
Of the CYP enzymes investigated, only CYP2C8 and CYP3A4 have been shown to contribute to the metabolism of repaglinide to any significant extent, with both enzymes catalyzing the formation of the aromatic amine (M1) and dicarboxylic acid (M2) metabolites [3]. CYP2C8 and CYP3A4 have distinct, but often overlapping substrate and inhibitor specificities. For example, ketoconazole has previously been considered to be a selective inhibitor of CYP3A4 at 1 µm, but was recently shown to inhibit CYP2C8 activity at this concentration [12].
Trimethoprim inhibits the CYP2C8-mediated 6α-hydroxylation of paclitaxel in vitro with an inhibition constant (Ki) of 32 µm, but does not inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 or CYP3A4 at concentrations below 100 µm[8]. In the present study, trimethoprim, at concentrations similar to this Ki value, inhibited the total metabolism of repaglinide suggesting that CYP2C8 plays a role in the metabolism of repaglinide.
In previous studies in healthy volunteers, the potent CYP3A4 inhibitors clarithromycin [13, 14] (250 mg twice daily for 3 days) and itraconazole [13, 15] (100 mg twice daily for 3 days, first dose 200 mg) both raised the AUC(0,∞) of repaglinide by about 40%[7, 9]. In these studies, clarithromycin also significantly enhanced the insulin response to repaglinide [9]. In one in vitro study, the Ki of itraconazole for the CYP3A4-mediated hydroxylation of midazolam was 0.27 µm[16]. The finding that trimethoprim, with a Ki for CYP2C8 inhibition two orders of magnitude greater than that of itraconazole for CYP3A4 inhibition, had a greater effect on the pharmacokinetics of repaglinide than itraconazole again suggests that CYP2C8 catalyzes the biotransformation of repaglinide in vivo.
Because the blood glucose-lowering effect of repaglinide is dose- and concentration-dependent [1], concomitant use of trimethroprim may increase the blood glucose-lowering effect of repaglinide and thus increase the risk of hypoglycaemia. Therefore, it is advisable to monitor blood glucose concentrations closely if trimethoprim is started in a patient using repaglinide, and to adjust the dosage of the latter as necessary. In a previous study, the combination of the CYP2C8 inhibitor gemfibrozil and the CYP3A4 inhibitor itraconazole increased synergistically the plasma concentrations and effects of repaglinide [7]. Such synergism may also occur if trimethoprim is combined with a CYP3A4 inhibitor, for example, the antimycotics itraconazole, ketoconazole, voriconazole and fluconazole, the macrolide antibiotics clarithromycin, erythromycin and telithromycin, the calcium-channel blocking agents diltiazem and verapamil, the antidepressive drug nefazodone and most of the HIV protease inhibitors [17–20].
In conclusion, trimethoprim raises the plasma concentrations of repaglinide in healthy subjects probably by inhibiting its CYP2C8-mediated biotransformation. Although there was no detectable increase in the effect of repaglinide on blood glucose at the doses used, an enhanced risk of hypoglycaemia during concomitant use of trimethoprim and repaglinide is a possibility.
Acknowledgments
We would like to thank Mr Jouko Laitila, Mrs Kerttu Mårtensson, Mrs Eija Mäkinen-Pulli and Mrs Lisbet Partanen for skilful technical assistance. This study was supported by grants from the Helsinki University Central Hospital Research Fund and the National Technology Agency (Tekes).
References
- 1.Hatorp V. Clinical pharmacokinetics and pharmacodynamics of repaglinide. Clin Pharmacokinet. 2002;41:471–83. doi: 10.2165/00003088-200241070-00002. [DOI] [PubMed] [Google Scholar]
- 2.van Heiningen PN, Hatorp V, Kramer Nielsen K, et al. Absorption, metabolism and excretion of a single oral dose of 14C-repaglinide during repaglinide multiple dosing. Eur J Clin Pharmacol. 1999;55:521–5. doi: 10.1007/s002280050667. [DOI] [PubMed] [Google Scholar]
- 3.Bidstrup TB, Bjørnsdottir I, Sidelmann UG, Thomsen MS, Hansen KT. CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. Br J Clin Pharmacol. 2003;56:305–14. doi: 10.1046/j.0306-5251.2003.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JS, Neuvonen M, Wen X, Backman JT, Neuvonen PJ. Gemfibrozil inhibits CYP2C8-mediated cerivastatin metabolism in human liver microsomes. Drug Metab Dispos. 2002;30:1352–6. doi: 10.1124/dmd.30.12.1352. [DOI] [PubMed] [Google Scholar]
- 5.Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72:685–91. doi: 10.1067/mcp.2002.128469. [DOI] [PubMed] [Google Scholar]
- 6.Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68:122–9. doi: 10.1067/mcp.2000.108507. [DOI] [PubMed] [Google Scholar]
- 7.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia. 2003;46:347–51. doi: 10.1007/s00125-003-1034-7. [DOI] [PubMed] [Google Scholar]
- 8.Wen X, Wang JS, Backman JT, Laitila J, Neuvonen PJ. Trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Drug Metab Dispos. 2002;30:631–5. doi: 10.1124/dmd.30.6.631. [DOI] [PubMed] [Google Scholar]
- 9.Niemi M, Neuvonen PJ, Kivistö KT. The cytochrome P4503A4 inhibitor clarithromycin increases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2001;70:58–65. doi: 10.1067/mcp.2001.116511. [DOI] [PubMed] [Google Scholar]
- 10.Weber A, Opheim KE, Siber GR, Ericson JF, Smith AL. High-performance liquid chromatographic quantitation of trimethoprim, sulfamethoxazole and N4-acetylsulfamethoxazole in body fluids. J Chromatogr. 1983;278:337–45. doi: 10.1016/s0378-4347(00)84793-3. [DOI] [PubMed] [Google Scholar]
- 11.Svirbely JE, Pesce AJ. A high performance liquid chromatography method for trimethoprim utilizing solid-phase column extraction. Ther Drug Monit. 1987;9:216–20. doi: 10.1097/00007691-198706000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Ong CE, Coulter S, Birkett DJ, Bhasker CR, Miners JO. The xenobiotic inhibitor profile of cytochrome P4502C8. Br J Clin Pharmacol. 2000;50:573–80. doi: 10.1046/j.1365-2125.2000.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jurima-Romet M, Crawford K, Cyr T, Inaba T. Terfenadine metabolism in human liver. In vitro inhibition by macrolide antibiotics and azole antifungals. Drug Metab Dispos. 1994;22:849–57. [PubMed] [Google Scholar]
- 14.Gorski JC, Jones DR, Haehner-Daniels BD, Hamman MA, O'Mara EM, Jr, Hall SD. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133–43. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 15.Olkkola KT, Backman JT, Neuvonen PJ. Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther. 1994;55:481–5. doi: 10.1038/clpt.1994.60. [DOI] [PubMed] [Google Scholar]
- 16.von Moltke LL, Greenblatt DJ, Schmider J, et al. Midazolam hydroxylation by human liver microsomes in vitro: inhibition by fluoxetine, norfluoxetine, and by azole antifungal agents. J Clin Pharmacol. 1996;36:783–91. doi: 10.1002/j.1552-4604.1996.tb04251.x. [DOI] [PubMed] [Google Scholar]
- 17.Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38:41–57. doi: 10.2165/00003088-200038010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Romero AJ, Le Pogamp P, Nilsson LG, Wood N. Effect of voriconazole on the pharmacokinetics of cyclosporine in renal transplant patients. Clin Pharmacol Ther. 2002;71:226–34. doi: 10.1067/mcp.2002.121911. [DOI] [PubMed] [Google Scholar]
- 19.Varhe A, Olkkola KT, Neuvonen PJ. Effect of fluconazole dose on the extent of fluconazole–triazolam interaction. Br J Clin Pharmacol. 1996;42:465–70. doi: 10.1046/j.1365-2125.1996.45111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balfour JA, Figgitt DP. Telithromycin. Drugs. 2001;61:815–29. doi: 10.2165/00003495-200161060-00016. [DOI] [PubMed] [Google Scholar]