Abstract
Aims
To investigate the influence of St. John's wort (SJW) on CYP3A4, CYP1A2, CYP2D6, N-acetyltransferase 2 (NAT2), and xanthine oxidase (XO) activities in healthy males and females.
Methods
Eight males and eight females were treated with SJW extract (3 × 300 mg day−1) for 14 days. Assessment of CYP1A2, NAT2, XO, CYP2D6, and CYP3A4 activities was performed before and at the end of the study period, using caffeine, dextromethorphan, and endogenous cortisol as probes. The corresponding metabolic ratios measured were 17MX/137MX in saliva and (AFMU+1MX+1MU)/17MU in urine for CYP1A2, AFMU/1MX for NAT2, 1MU/1MX for XO, DOR/DMO for CYP2D6, 3MM/DMO and 6OHC/C for CYP3A4, all determined in urine.
Results
The ratios of the treatment to baseline values for CYP3A4 using cortisol as the probe were 1.5 [95% confidence interval (CI) 1.3, 1.9] for males, and 1.9 (1.1, 3.0) for females. The corresponding ratios using dextromethorphan as the probe for CYP2D6 were 0.9 (95% CI 0.5, 2.1) for males and 1.9 (1.3, 3.2) for females. For CYP1A2, a significant increase in the metabolic ratios was found only for females (ratio of values 1.2; 95% CI 1.1, 1.4). No influence of SJW on CYP2D6, NAT2, and XO activities was observed.
Conclusions
An induction of CYP3A4 by SJW was confirmed. CYP1A2 appears to be induced by SJW only in females. The activities of CYP2D6, NAT2, and XO were not affected by SJW.
Keywords: CYP1A2, CYP2D6, CYP3A4, N-acetyltransferase 2, St. John's wort, xanthine oxidase
Introduction
Pharmacokinetic interactions between St. John's wort (Hypericum perforatum, SJW) and other drugs, such as cyclosporin, indinavir, simvastatin, fexofenadine, and digoxin have been observed in humans [1]. These interactions can be explained by the induction of cytochrome P450 3A4 (CYP3A4) and/or P-glycoprotein [2, 3]. In addition, an apparent sexual dimorphism in CYP3A4 inducibility has been observed [4]. In a single case study treatment with SJW was associated with substantially decreased theophylline plasma concentrations, suggesting induction of CYP1A2 [5], although this has not been confirmed [6]. Induction of the CYP1A2 has been observed in vitro in a LS180 intestinal cell model [7]. However, other in vitro experiments have demonstrated that SJW constituents can have inhibitory effects on CYP1A2 [8]. Furthermore, flavonoids, some of which are constituents of SJW (e.g. quercetin), showed a potent inhibitory effect on XO in vitro[9]. The effect of SJW on XO has not been investigated in vivo.
The primary aim of this study was to investigate the influence of Hypericum on the activities of CYP1A2, CYP2D6, CYP3A4, NAT2 and XO.
Methods
Subjects
Sixteen nonsmoking healthy subjects (eight males and eight females, aged between 25 and 58 years) were recruited. Before commencing the study, all subjects gave written informed consent. The protocol was approved by the University Hospital, Basel, Ethics Committee. The subjects were asked to take no concomitant medication (including oral contraceptives) 1 week prior to and throughout the study.
Study design
In this open study all subjects abstained from methylxanthine-containing food and beverages 12 h prior to and 24 h post drug intake. In addition, subjects were asked to abstain from grapefruit products, charcoal grilled meat, and broccoli vegetable during the entire study period, which lasted 16 days. Assessment of CYP1A2, CYP3A4, CYP2D6, NAT2 and XO activities was carried out on day 1, before SJW intake (baseline), and on day 15 of the study. After fasting overnight, the subjects received a capsule of caffeine (200 mg) p.o., prepared by the Institute of Hospital Pharmacy, University Hospital (Basel, Switzerland), and a tablet of 25 mg of dextromethorphan (Bexin®; Spirig Corp., Egerkingen, Switzerland). Urine samples were collected from 0 h to 8 h, and from 8 h to 24 h and stored at − 70 °C until assayed. A saliva sample of 2-5 ml was collected without stimulation 6 h after drug intake. From day 2 onwards, the participants received 300 mg SJW extract (Jarsin® 300; Medichemie, Ettingen, Switzerland, containing 900 µg hypericin), three times a day for 14 days. On day 15 all phenotyping tests were repeated as described before.
Assessment of enzyme activities
CYP3A4 activity was estimated from the 6OHC/C molar concentration ratio in urine collected for 24 h, and from the 3MM/DMO ratio in urine 8 h after ingestion of 25 mg of DMO. The ratio DOR/DMO was used as the index of CYP2D6 activity. CYP1A2 was estimated either from the 17MX/137MX ratio in saliva 6 h after caffeine intake, and from the (AFMU+1MX+1MU)/17MU ratio in urine obtained 8 h after dosing. The AFMU/1MX and 1MU/1MX ratios were used as indices of NAT2 and XO activities, respectively.
Analysis of caffeine metabolites in urine
Caffeine metabolites in urine were analysed by high-performance liquid chromatography (HPLC) as described previously [10]. The within-assay coefficients of variation (CVs) for 5-acetylamino-6-formylamino-3-methyluracil (AFMU), 1-methyluric acid (1MU), 1-methylxanthine (1MX), 1,7-dimethyluric acid (17MU), and 1,7-dimethylxanthine (17MX) in the concentration range 60-70 µm were 1.4, 1.0, 1.5, 2.0, and 2.2%, respectively. The corresponding between-assay CVs were 5.2, 5.4, 4.0, 3.5, and 3.5%, respectively.
Analysis of caffeine and 17MX in saliva
Fifty microlitres of trichloroacetic acid (20%, v/v) were added to saliva (200 µl), and after centrifugatiion 25 µl of the supernatant were injected onto the HPLC column. Proxyphylline (50 µl, 0.1 mg ml−1) was used as internal standard. The HPLC conditions were similar to those described for urine analysis [10]. The within-assay CVs for 1,3,7-trimethylxanthine (137MX, caffeine) and 17MX in the concentration range 10-30 µm were 2.4 and 1.9%, respectively (n = 10). The corresponding between-assay CVs were 2.4 and 2.5%, respectively (n = 7). The limit of quantification was 0.4 µm for both compounds.
Analysis of cortisol and 6β-hydroxycortisol in urine
Urine samples were extracted on Oasis HBL®, 60 mg, solid-phase cartridges (Waters Corp., Milford, MA, USA), using a previously described method [11] after modification. 6α-hydroxy-methyl-prednisolone was used as internal standard instead of dexamethasone. The HPLC separation was performed on a Luna® phenyl-hexyl, 3µ, 150 × 4.6-mm column (Phenomenex, Torrance, CA. USA) operating at 36 °C. A binary gradient of 15% acetonitrile (phase A) and 52% acetonitrile and 13% methanol (phase B), all in 0.05 m potassium dihydrogen phosphate buffer pH 4.0, was used. The within-assay CVs of cortisol (C) and 6β-hydroxycortisol (6OHC) were 2.8 and 1.2%, respectively. The between-assay CVs for C and 6OHC were 7.9 and 3.9%, respectively.
Analysis of dextromethorphan and metabolites in urine
The determination of dextromethorphan (DMO), dextrorphan (DOR), 3-methoxymorphinan (3MM), and 3-hydroxy-morphinan (3HM) was performed by HPLC with ion pair separation on a Zorbax® SB-Phenyl, 5µ, 4.6 × 250-mm column (Agilent Technologies, Palo Alto, CA, USA) with fluorescence detection, using a previously published method [12] after fluid-fluid extraction [13]. Because the concentrations of DOR and 3HM in urine are substantially higher in extensive metabolizers than those of DMO and 3MM, two different internal standards were used. For the determination of DMO and 3MM in the concentration range 5-500 ng ml−1, levallorphan was used as an internal standard. DOR and 3HM (concentration range 0.25-80 µg ml−1) were analysed after a 26-fold dilution of the solvent extracts, using codeine as internal standard. The within-assay CVs for all four compounds were between 2.1 and 4.7%. The corresponding between-assay CVs ranged from 5.2 to 7.5%.
Data analysis
Statistical analysis was performed using the SPSS software package (SPSS, Chicago, IL, USA). The nonparametric Wilcoxon test for paired data was used to analyse changes in the metabolic ratios before and after ingestion of SJW. A two-sided P-value < 0.05 was considered to be significant. Mean data from males and females were compared using anova. The Pearson coefficient of correlation was used to determine the relationship between the two measures of CYP3A4 activity. Data are presented as mean ± standard deviation (± SD) and median and range, where appropriate.
Results
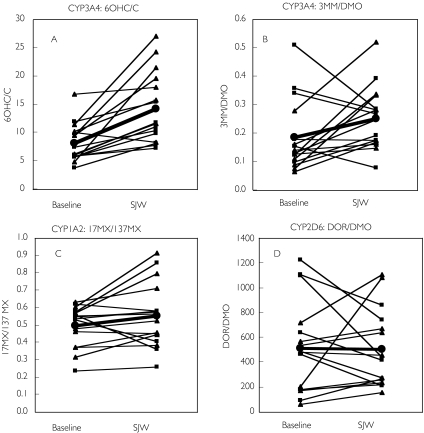
All 16 subjects completed the treatment period without any adverse effects. The metabolic ratios used for the assessment of CYP1A2, CYP3A4, CYP2D6, NAT2, and XO activities before and after treatment with hypericum extract are shown in Table 1 and Figure 1. With respect to CYP3A4, the 6OHC/C ratio increased significantly in both females and males. In addition, there was a significant difference between males and females for baseline 6OHC/C ratios (P < 0.05) and after treatment with SJW (P < 0.005). In contrast, the 3MM/DMO ratio increased only in females but not in males. The changes associated with ingestion of SJW in the two ratios correlated significantly (r = 0.825, P < 0.01).
Table 1.
Indices of CYP1A2, CYP3A4, CYP2D6, NAT2, and XO activites before and after ingestion of St. John's wort extract (3 × 300 mg day−1) for 14 days
| Enzyme | Ratio | Volunteers | Baseline | After treatment | Ratio (95% CI) |
|---|---|---|---|---|---|
| CYP1A2 | 17MX/137MX (saliva) | All (n = 16) | 0.54 (0.24-0.63) | 0.54 (0.26-0.91) | 1.09 (0.99, 1.25) |
| Males (n = 8) | 0.57 (0.23-0.62) | 0.57 (0.26-0.86) | 1.03 (0.80, 1.22) | ||
| Females (n = 8) | 0.47 (0.32-0.63) | 0.49 (0.39-0.91)* | 1.18 (1.1, 1.4) | ||
| CYP1A2 | (AFMU+1MX+1MU)/17MU (urine) | All | 3.5 (1.8.0-5.3) | 3.8 (2.0-6.5) | 1.14 (0.99, 1.23) |
| Males | 3.5 (1.8-5.3) | 3.7 (2.0-6.5) | 1.12 (0.85, 1.23) | ||
| Females | 3.5 (3.1-5.0) | 4.1 (3.6-5.9)* | 1.19 (1.0,1.34) | ||
| CYP3A4 | 6OHC/C (urine) | All | 6.8 (3.9-19.6) | 11.6 (7.3-27.1)*** | 1.78 (1.41, 2.30) |
| Males | 5.8 (3.9-11.9) | 10.1 (7.8-15.5)** | 1.55 (1.33, 1.90) | ||
| Females | 9.7 (6.2-16.9) | 18.8 (8.4-27.1)** | 1.95 (1.1, 3.0) | ||
| CYP3A4 | 3MM/DMO (urine) | All | 0.14 (0.06-0.51) | 0.26 (0.08-0.52)* | 1.82 (1.20, 2.37) |
| Males | 0.17 (0.10-0.51) | 0.26 (0.08-0.39) | 0.89 (0.54, 2.11) | ||
| Females | 0.12 (0.06-0.28) | 0.23 (0.15-0.52)** | 1.92 (1.3, 3.2) | ||
| CYP2D6 | DOR/DMO (urine) | All | 485 (66-1226) | 432 (157-1110) | 1.18 (0.77, 2.09) |
| Males | 553 (96-1226) | 344 (210-868) | 0.72 (0.38, 1.72) | ||
| Females | 485 (66-716) | 549 (157-1110) | 1.27 (0.54, 3.1) | ||
| NAT2 | AFMU/1MX (urine) | All | 0.82 (0.17-2.06) | 0.77 (0.13-2.2) | 0.95 (0.88,1.08) |
| Males | 0.86 (0.17-1.70) | 0.77 (0.13-2.27) | 0.90 (0.76, 1.21) | ||
| Females | 0.82 (0.18-2.06) | 0.74 (0.17-2.0) | 0.98 (0.91, 1.04) | ||
| XO | 1MU/1MX (urine) | All | 1.15 (0.46-1.59) | 1.20 (0.79-1.64) | 1.05 (0.95, 1.36) |
| Males | 1.04 (0.53-1.56 | 1.20 (0.79-1.47) | 1.13 (0.86, 1.40) | ||
| Females | 1.24 (0.46-1.59) | 1.20 (0.95-1.64) | 0.99 (0.79,1.56) |
Data are given as median, range, and 95% confidence intervals (CI) of the ratio of treatment vs. baseline.
P < 0.05;
P < 0.01;
P < 0.001 vs. baseline values (Wilcoxon paired t-test).
Figure 1.
Ratios of 6OHC/C (A) and 3MM/DMO (B) (indices of CYP3A4 activity), 17MX/137MX in saliva (an index of activity of CYP1A2) (C), and DOR/DMO (an index of activity of CYP2D6) (D) before and after 14 days of treatment with St. John's wort extract (SJW). Males (▪); females (▴); mean ratios (•)
Neither of the metabolic ratios used as indices of CYP1A2 changed after treatment with SJW in the sujects overall. However, both ratios were increased significantly (P < 0.01) in females. We also genotyped the subjects in this study for the two mutations CYP1A2*1C and CYP1A2*1F[14], but no relationship between the allelic variants and CYP1A2 activity was detected (data not shown). SJW had no detectable effect on the activity of CYP2D6, NAT2 and xanthine oxidase (Table 1).
Discussion
In the present study, after 2 weeks of treatment with SJW the mean increase in the 6OHC/C ratio, used as an index of activity of CYP3A4, was 85%, which is comparable to published data [15, 16]. Induction of CYP3A4 in humans by SJW has been demonstrated using endogenous cortisol [15, 16], intravenous and/or oral midazolam [3, 4], and erythromycin [2]. Like Gurley et al.[4], we also found a trend for a sex difference in CYP3A4 inducibility (mean ratio 6OHC/C 1.95 for females vs. 1.55 for males), but this trend did not reach significance. Gender differences in hepatic CYP3A4 activity have been described previously [17, 18], but studies investigating the influence of sex on the inducibility of CYP3A4 and other drug metabolizing enzymes are uncommon. The metabolism of DMO to 3MM is also used to assess CYP3A4 activity. In the present study the changes in the 3MM/DMO ratio after SJW were significant for females but not for males. Thus, the different probes used to assess CYP3A4 activity appear to give different results for the effect of gender on induction of this enzyme. A recent study [18] has shown that intestinal and hepatic induction of CYP3A4 by rifampicin are inversely related and that induction is dependent on sex. These differences in induction of hepatic and intestinal CYP3A4 might explain the discrepancy between our results obtained with oral DMO and endogenous cortisol.
In contrast to CYP3A4, no influence of SJW on CYP2D6 activity was found, which is in agreement with Wang et al.[6], who also used DMO as the probe drug. In contrast, Gurley et al.[4] described a modest induction of CYP2D6 by SJW using debrisoquine as the probe drug.
The influence of SJW treatment on CYP1A2 activity is less clear. In the present study we found a slight (but not significant) increase in the 17MX/137MX ratio in saliva after SJW. However, most of the subjects exhibiting an apparent induction of CYP1A2 were females. Similar results were found with the (AFMU+1MX+ 1MU)/17MU ratio in urine, an alternative index of CYP1A2 activity. Thus, differences in CYP1A2 induction by SJW observed by others [4, 6] might be due to the different proportions of males and females studied.
In contrast to CYP3A4 and CYP1A2, NAT2 activity was not affected by SJW. Similarly, the hypothesis that XO might be inhibited by flavonoids present in SJW [9] could not be confirmed in the present study.
In conclusion, induction of CYP1A2 after treatment with SJW for 14 days was demonstrated, but only in females. Induction of CYP3A4 by SJW was observed using two different metabolic probes (DMO and cortisol), but differences between males and females were evident.
Acknowledgments
The authors thank Dr Paul Roberts for reviewing the manuscript.
References
- 1.Hammerness P, Basch E, Ulbricht C, et al. St John's wort: a systematic review of adverse effects and drug interactions for the consultation psychiatrist. Psychosomatics. 2003;44:271–82. doi: 10.1176/appi.psy.44.4.271. [DOI] [PubMed] [Google Scholar]
- 2.Durr D, Stieger B, Kullak-Ublick GA, et al. St John's Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- 3.Dresser GK, Schwarz UI, Wilkinson GR, Kim RB. Coordinate induction of both cytochrome P4503A and MDR1 by St John's wort in healthy subjects. Clin Pharmacol Ther. 2003;73:41–50. doi: 10.1067/mcp.2003.10. [DOI] [PubMed] [Google Scholar]
- 4.Gurley BJ, Gardner SF, Hubbard MA, et al. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–87. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 5.Nebel A, Schneider BJ, Baker RK, Kroll DJ. Potential metabolic interaction between St. John's wort and theophylline. Ann Pharmacother. 1999;33:502. doi: 10.1345/aph.18252. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Gorski JC, Hamman MA, Huang SM, Lesko LJ, Hall SD. The effects of St John's wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther. 2001;70:317–26. [PubMed] [Google Scholar]
- 7.Karyekar CS, Eddington ND, Dowling TC. Effect of St. John's Wort extract on intestinal expression of cytochrome P4501A2: studies in LS180 cells. J Postgrad Med. 2002;48:97–100. [PubMed] [Google Scholar]
- 8.Obach RS. Inhibition of human cytochrome P450 enzymes by constituents of St. John's Wort, an herbal preparation used in the treatment of depression. J Pharmacol Exp Ther. 2000;294:88–95. [PubMed] [Google Scholar]
- 9.Nagao A, Seki M, Kobayashi H. Inhibition of xanthine oxidase by flavonoids. Biosci Biotechnol Biochem. 1999;63:1787–90. doi: 10.1271/bbb.63.1787. [DOI] [PubMed] [Google Scholar]
- 10.Vrtic F, Haefeli WE, Drewe J, Krahenbuhl S, Wenk M. Interaction of ibuprofen and probenecid with drug metabolizing enzyme phenotyping procedures using caffeine as the probe drug. Br J Clin Pharmacol. 2003;55:191–8. doi: 10.1046/j.1365-2125.2003.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lykkesfeldt J, Loft S, Poulsen HE. Simultaneous determination of urinary free cortisol and 6 beta-hydroxycortisol by high-performance liquid chromatography to measure human CYP3A activity. J Chromatogr B Biomed Appl. 1994;660:23–9. doi: 10.1016/0378-4347(94)00265-7. [DOI] [PubMed] [Google Scholar]
- 12.Wenk M, Todesco L, Keller B, Follath F. Determination of dextromethorphan and dextrorphan in urine by high-performance liquid chromatography after solid-phase extraction. J Pharm Biomed Anal. 1991;9:341–4. doi: 10.1016/0731-7085(91)80203-l. [DOI] [PubMed] [Google Scholar]
- 13.Wieling J, Tamminga WJ, Sakiman EP, Oosterhuis B, Wemer J, Jonkman JH. Evaluation of analytical and clinical performance of a dual-probe phenotyping method for CYP2D6 polymorphism and CYP3A4 activity screening. Ther Drug Monit. 2000;22:486–91. doi: 10.1097/00007691-200008000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Todesco L, Török M, Krähenbühl S, Wenk M. Determination of −3858G→A and −164C→A genetic polymorphisms of CYP1A2 in blood and saliva by rapid allelic discrimination: large difference in the prevalence of the −3858G→A mutation between Caucasians and Asians. Eur J Clin Pharmacol. 2003;59:343–6. doi: 10.1007/s00228-003-0623-1. [DOI] [PubMed] [Google Scholar]
- 15.Bauer S, Stormer E, Kerb R, Johne A, Brockmoller J, Roots I. Differential effects of Saint John's Wort (Hypericum perforatum) on the urinary excretion of D-glucaric acid and 6 beta-hydroxycortisol in healthy volunteers. Eur J Clin Pharmacol. 2002;58:581–5. doi: 10.1007/s00228-002-0527-5. [DOI] [PubMed] [Google Scholar]
- 16.Roby CA, Anderson GD, Kantor E, Dryer DA, Burstein AH. St John's Wort: effect on CYP3A4 activity. Clin Pharmacol Ther. 2000;67:451–7. doi: 10.1067/mcp.2000.106793. [DOI] [PubMed] [Google Scholar]
- 17.Hunt CM, Westerkam WR, Stave GM. Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol. 1992;44:275–83. doi: 10.1016/0006-2952(92)90010-g. [DOI] [PubMed] [Google Scholar]
- 18.Gorski JC, Vannaprasaht S, Hamman MA, et al. The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin Pharmacol Ther. 2003;74:275–87. doi: 10.1016/S0009-9236(03)00187-5. [DOI] [PubMed] [Google Scholar]