Abstract
Aims
To evaluate the interindividual variability in the plasma concentrations of lopinavir in the context of routine monitoring with or without treatment with a non-nucleoside reverse transcriptase inhibitor and to assess the interaction between the coformulation of lopinavir/ritonavir and efavirenz or nevirapine.
Methods
Plasma trough and peak concentrations (Ctrough, Cmax) of lopinavir from 182 HIV-1-infected patients were analysed by high-performace liquid chromatography. Three lopinavir/ritonavir regimens were assessed, namely (A) 400 mg lopinavir/100 mg ritonavir twice daily given alone (n = 125), (B) 400/100 mg twice daily together with a non-nucleoside reverse transcriptase inhibitor (n = 25), and (C) 533/133 mg twice daily together with a non-nucleoside reverse transcriptase inhibitor (n = 32).
Results
Median (ng ml−1) Ctrough and Cmax lopinavir (interquartile range, CV) were: (A) 4852 (3198–6891, 56%) and 8501 (6333–11 584, 41%), (B) 2979 (1704–5186, 74%) and 5612 (3362–11 704, 76%) and (C) 5082 (2696–7226, 74%) and 9757 (4883–12 963, 60%). Median Ctrough of lopinavir was lower in patients taking both efavirenz [P = 0.01, 95% confidence interval (CI) for difference between medians 343, 2713] and nevirapine (P = 0.019, 95% CI for difference between medians 354, 3681) compared with those taking lopinavir/ritonavir alone. A higher interindividual variability was observed when lopinavir/ritonavir was given with a non-nucleoside reverse transcriptase inhibitor. The risk of achieving a ‘suboptimal’Ctrough of lopinavir (below a threshold of 3000 ng ml−1) was statistically higher in patients treated with a non-nucleoside reverse transcriptase inhibitor (P < 0.001, 95% CI for difference between percentages 8.8, 43.1%) compared with those receiving lopinavir/ritonavir alone.
Conclusions
Our results confirmed the interaction between lopinavir and efavirenz, and also demonstrated a significant interaction between the former drug and nevirapine, resulting in lower Ctrough of lopinavir. The wide interpatient variability in this interaction suggests that therapeutic drug monitoring may be useful in optimizing the dose of lopinavir.
Keywords: drug interaction, pharmacokinetic, protease inhibitors
Introduction
Lopinavir is a potent HIV protease inhibitor that is coformulated with ritonavir, which acts as an inhibitor of the cytochrome P450 3A4 (CYP3A4) metabolism of the former drug. Even at a low dose of ritonavir, there is a substantial increase in exposure to lopinavir [1]. The plasma trough (Ctrough) and peak (Cmax) concentrations (mean ± SD) of lopinavir at steady state and at stand-ard doses of 400 mg lopinavir/100 mg ritonavir twice daily are 5500 ± 4000 ng ml−1 and 9600 ± 4400 ng ml−1, respectively [2, 3]. These plasma drug concentrations widely exceed the inhibitory concentration (IC50) for the wild-type virus corrected for protein binding (70 ng ml−1). Consequently, the mean lopinavir trough concentration (Ctrough)/IC50 ratio or inhibitory quotient (IQ) is as high as 75 at the standard doses of the combination [4]. Based on this high IQ, lopinavir/ritonavir potentially provides a barrier to the emergence of viral resistance and activity against resistant virus.
The pharmacokinetics of protease inhibitors differ significantly between individuals, due to the variability in their absorption and metabolism. Moreover, a positive relationship between plasma concentrations of protease inhibitors and antiviral efficacy and/or toxicity has been clearly demonstrated [5, 6, 7, 8, 9, 10, 11, 12]. Therapeutic drug monitoring during therapy with protease inhibitors is recommended in certain circumstances and in several countries such as France, although its role in routine clinical practice remains to be established [13]. Recently, a prospective study showed the potential benefit of therapeutic drug monitoring on the virological outcome at 1 year, of indinavir and nelfinavir therapy in antiretroviral naive adult patients [14, 15].
Lopinavir is metabolized almost entirely by CYP3A4. Lopinavir is also an inhibitor of this enzyme, although it is less potent than ritonavir [16]. Lopinavir is now frequently given with non-nucleoside reverse transcriptase inhibitors, such as efavirenz or nevirapine, both of which are metabolized by and induce CYP3A4. The interaction has been reported to cause a 30% decrease in the Ctrough of lopinavir [17]. The interaction between lopinavir and nevirapine in adult patients has not been investigated. However, in a paediatric population, nevirapine significantly decreased the plasma Ctrough of lopinavir. Thus, a higher dose of the latter should be considered when the two drugs are given together [18], although the manufacturers of both lopinavir and nevirapine do not recommend any dose adjustment except for patients with a suspected decreased response to lopinavir. Thus, the role of therapeutic drug monitoring when these drugs are given in combination needs further investigation.
In the present study, we have examined the interindividual variability in plasma lopinavir concentrations measured in samples taken for routine monitoring in adult patients receiving lopinavir/ritonavir alone or together with non-nucleoside reverse transcriptase inhibitors. We have also assessed the interaction between lopinavir and efavirenz or nevirapine to evaluate the benefit of therapeutic drug monitoring in these patients.
Methods
Patients
During routine monitoring for clinical purposes, we assessed plasma lopinavir Ctrough and Cmax concentrations from 182 HIV-1-infected patients followed up between January 2000 and April 2002. The study was observational, both retrospective and prospective, and carried out in eight clinical care units. Patients included in the study were treated with lopinavir/ritonavir with or without efavirenz or nevirapine with or without one or two nucleoside reverse transcriptase inhibitors for at least 1 month (allowing time to reach steady-state pharmacokinetics). The regimens assessed were lopinavir/ritonavir 400/100 mg twice daily without non-nucleoside reverse transcriptase inhibitor (group A), lopinavir/ritonavir 400/100 mg twice daily with a non-nucleoside reverse transcriptase inhibitor (group B), and lopinavir/ritonavir 533/133 mg twice daily with a non-nucleoside reverse transcriptase inhibitor (group C). Data were transferred from carers to researchers in a completely anonymized, nontraceable fashion.
Pharmacokinetic sampling and analysis
Plasma drug concentrations of lopinavir and ritonavir were measured by a sensitive and validated high-performance liquid chromatography method with ultraviolet detection [19]. The limit of quantification was 100 ng ml−1. Inter- and intra-assay variability were 6.9–13.8% and 2.9–7.2 % for lopinavir and 3.3–10.5% and 1.6–9.5% for ritonavir. Blood samples were drawn at steady state, 10–12 h post-dose for the determination of Ctrough and 3–5 h post-dose for the determination of Cmax. The time of last lopinavir/ritonavir dose was ascertained by patient report. No other specific measure of adherence was used. None of the patients had been prescribed inhibitors or inducers of CYP3A4 activity.
Drug analysis
Interindividual variability in lopinavir concentrations was estimated using the coefficient of variation ex-pressed as a percentage (CV%). The proportion of patients with a lopinavir Ctrough below the expected range was estimated for each lopinavir/ritonavir regimen, to evaluate the magnitude of any drug–drug interaction. Because the target range of lopinavir Ctrough has not yet been defined, we first used a threshold value of 3000 ng ml−1 based on a previously proposed therapeutic range [13]. Therefore, a lopinavir Ctrough below this value was defined as ‘suboptimal’. We also used a threshold value of 1500 ng ml−1, since the mean ± SD lopinavir Ctrough in the population has been estimated to be 5500 ± 4000 ng ml−1[3]. Thus, we took account of the interindividual variability in LPV concentrations.
Nonparametric test (Mann–Whitney U-test) was used to compare lopinavir and ritonavir concentrations between the different regimens. Categorical variables were compared using the χ2 test. Statistical analysis was performed using the computer software program SPSS® PC for Windows, version 10.1 (SPSS Inc., Chicago, IL, USA). A P-value ≤0.05 was considered statistically significant.
Results
A total of 182 patients [135 men and 47 women with a median (range) age of 40 years (16–63)] were enrolled during the observation period. There were 125 in group A, 25 in group B (16 taking efavirenz and nine taking nevirapine), and 32 in group C (29 taking efavirenz and three taking nevirapine). Only 13% of the patients had not received a protease inhibitor previously. Five patients had impaired liver function (two in group A, two in group B and one in group C). Overall, 229 plasma Ctrough and 57 plasma Cmax of lopinavir and ritonavir were determined.
At a standard dose of 400 mg lopinavir/100 mg ritonavir twice daily, a 39% decrease in the median Ctrough of lopinavir was found when the combination was given with a non-nucleoside reverse transcriptase inhibitor [P = 0.001, difference between medians 1718, 95% confidence interval (CI) 719, 2692], whereas no significant change was observed in Cmax (P = 0.35, difference between medians 1909, 95% CI −2667, 6470). There was no statistical difference in the median Ctrough of lopinavir between group A (400 mg lopinavir/100 mg ritomavir twice daily) and group C (533 mg lopinavir/133 mg ritomavir twice daily). At 400 mg lopinavir/100 mg ritomavir twice daily, no statistical difference was found for the Ctrough of lopinavir {median [interquartile range (IQR)] ng ml−1, n samples} between patients taking efavirenz [3733 (2097–4570), 24] and those taking nevirapine [2658 (1502–5199), 12] (P = 0.61, difference between medians 587, 95% CI −1403, 2387). The Ctrough of lopinavir was significantly de-creased both for patients taking efavirenz (P = 0.01, difference between medians 1549, 95% CI 343, 2713) and nevirapine (P = 0.019, difference between medians 2053, 95% CI 354, 3681) compared with those not taking a non-nucleoside reverse transcriptase inhibitor. Interindividual variability in both the Ctrough and Cmax of lopinavir was increased from 56% to 74% when a non-nucleoside reverse transcriptase inhibitor was given with lopinavir/ritonavir.
The median Ctrough and Cmax[(IQR), CV%] of ritonavir were 389 ng ml−1[(254–624), 71%] and 709 ng ml−1[(519–911), 95%]for regimen A, 311 ng ml−1[(181–553), 76%] and 622 ng ml−1[(226–999), 91%] for regimen B and 586 ng ml−1[(195–873), 74%] and 1166 ng ml−1[(570–1749), 74%] for regimen C. No statistical difference was observed in the Ctrough and Cmax of ritonavir at the standard dose of 400 mg liponavir/100 mg ritonavir twice daily given alone or with a non-nucleoside reverse transcriptase inhibitor. In contrast, higher Ctrough values for ritonavir were noted in group C compared with both group A (P = 0.033, difference between medians 163, 95% CI 9, 322) and B (P = 0.021, difference between medians 235, 95% CI 35, 439). Significant interindividual variability in ritonavir concentrations was observed in all of the regimens.
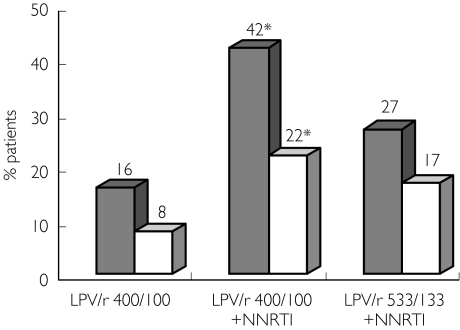
On a standard dose of lopinavir/ritonavir alone, only 16% of the patients had a Ctrough of lopinavir below the 3000-ng ml−1 threshold (Figure 1). The proportion of patients with Ctroughs below this value was statistically lower in group A (16%, 95% CI 10.2, 21.8) compared with group B (42%, 95% CI 26, 58) who were also taking a non-nucleoside reverse transcriptase inhibitor (P ≤ 0.001, 95% CI for difference between percentages 8.8, 43.1) but was comparable to group C (27%, 95 CI 13.4, 40.5) who were receiving the higher dose of lopinavir/ritonavir (P = 0.12, 95% CI for difference between percentages 3.7, 25.7). Patients treated with lopinavir/ritonavir 400/100 mg twice daily and a non-nucleoside reverse transcriptase inhibitor had a higher risk of having a ‘suboptimal’Ctrough of lopinavir [odds ratio (OR) 3.91, 95% CI 1.63, 9.37]. Similar results were found when using a threshold value of 1500 ng ml−1 (Figure 1).
Figure 1.
Proportion of patients with suboptimal Ctrough of lopinavir with respect to lopinavir/ritonavir dose and co-treatment with a non-nucleoside reverse transcriptase inhibitor. The results are expressed as percentages *Significant increase (P < 0.001) in the percentage of patients with Ctrough of lopinavir below the threshold value between groups treated with lopinavir/ritonavir 400/100 and a non-nucleoside reverse transcriptase inhibitor and those treated with lopinavir/ritonavir 400/100 alone. LPV Ctrough <3000 ng/ml (▪), LPV Ctrough <1500 ng/ml (□)
Discussion
In the present work median Ctrough and Cmax values for lopinavir were comparable to previously reported data [2, 3] irrespective of the dosing regimen. However, we demonstrated that the median Ctrough of lopinavir was decreased from 39%by coadministration of efavirenz or nevirapine. When the lopinavir/ritonavir dose was increased to 533 mg lopinavir/133 mg ritonavir twice daily, median Ctrough values for lopinavir were comparable to those observed at the standard dose in patients not receiving a non-nucleoside reverse transcriptase inhibitor. Nevertheless, the higher dose of lopinavir/ritonavir led to elevated concentrations (above the expected values) for some patients, emphasizing the potential risk of toxicity.
In contrast, treatment with a non-nucleoside reverse transcriptase inhibitor did not affect the concentrations of ritonavir. Moreover, the Ctrough of ritonavir was statistically higher in patients taking 533 mg lopinavir/133 mg ritonavir twice daily compared with patients from both regimen A and B taking 400/100 mg twice daily.
A lower interindividual variability in the Ctrough of lopinavir at the standard dose of 400/100 mg twice daily (CV = 56%) was observed compared with other ritonavir-boosted regimens. For example, values of 92% and 75% have been reported for indinavir/ritonavir 800/100 mg twice daily and saquinavir/ritonavir 400/400 mg twice daily, respectively [20, 21]. The interindividual variability in the Ctrough of lopinavir greatly increased when lopinavir/ritonavir was given with a non-nucleoside reverse transcriptase inhibitor (CV = 74%) even at the higher dose of lopinavir/ritonavir. Thus, non-nucleoside reverse transcriptase inhibitor therapy enhances the pharmacokinetic variability of lopinavir and decrease its Ctrough, probably through induction of CYP3A4. Unpredictable lopinavir concentrations may then be obtained when lopinavir/ritonavir is given with efavirenz or nevirapine. Even if the mean Ctrough of lopinavir remains above the proposed therapeutic threshold, concentrations in a percentage of patients may fall below this value. Indeed, we showed that the proportion of patients with a ‘suboptimal’Ctrough value was significantly higher in the group taking lopinavir/ritonavir 400/100 mg twice daily with a non-nucleoside reverse transcriptase inhibitor. However, the term ‘subopti-mal’ should be used with caution. A Ctrough value for lopinavir below 3000 ng ml−1 or 1500 ng ml−1 may be ‘suboptimal’ but, since this drug has a high inhibitory quotient, the in vivo minimal effective concentration may be even lower.Table 1
Table 1.
Plasma concentrations of lopinavir at two doses of lopinavir/ritonavir taken alone or together with a non-nucleoside reverse transcriptase inhibitor
| Plasma concentrations of lopinavir (ng ml−1) | |||||||
|---|---|---|---|---|---|---|---|
| (A) 400/100 | (B) 400/100 + non-nucleoside reversetranscriptase inhibitor | (C) 533/133 + non-nucleoside reverse transcriptase inhibitor | |||||
| Ctrough | Cmax | Ctrough | Cmax | Ctrough | Cmax | ||
| Mean ± SD | 5293 ± 2959 | 8984 ± 3723 | 3555 ± 2646 | 7004 ± 5350 | 5582 ± 4123 | 9271 ± 5544 | |
| Median | 4852 | 8501 | 2979* | 5612 | 5082 | 9757 | |
| (IQR)† | (3198–6891) | (6333–11584) | (1704–5186) | (3362–11704) | (2696–7226) | (4883–12963) | |
| CV% | 56% | 41% | 74% | 76% | 74% | 60% | |
| n (samples) | 152 | 40 | 36 | 7 | 41 | 10 | |
P = 0.001 (Mann–Whitney test) compared with regimen A.
Interquartile range.
Because our study was observational, and partially retrospective being performed in a routine clinical setting, an accurate estimate of adherence to therapy was not possible. Therefore, prospective controlled trials assessing the relationship between plasma concentration and virological outcome and/or toxicity are required to determine the exact target range for the Ctrough of lopinavir and to provide guidelines for using therapeutic drug monitoring in routine clinical practice.
In conclusion, therapeutic drug monitoring may provide information that can help patients achieve adequate concentrations of anti-HIV drugs. Nevertheless, it seems that for patients treated with lopinavir/ritonavir alone and not receiving other drugs affecting CYP3A4 activity, a low degree of therapeutic drug monitoring is required. On contrast, when efavirenz or nevirapine are coadministered with lopinavir/ritonavir, therapeutic drug monitoring may be useful for dosage adjustment.
Acknowledgments
We thank Ségolene Duran and Anderson Loundou (INSERM U379) for statistical analysis assistance. This work was partly supported by Abbott Laboratories.
References
- 1.Sham H, Kempf D, Molla A, et al. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob Agents Chemother. 1998;42:3218–24. doi: 10.1128/aac.42.12.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy RL, Brun S, Hicks C, et al. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-weeks results. AIDS. 2001;15:F1–F9. doi: 10.1097/00002030-200101050-00002. [DOI] [PubMed] [Google Scholar]
- 3.US Prescribing information. Abbott Park, IL: Abbott Laboratories; 2000. Kaletra (lopinavir/ritonavir) capsules. [Google Scholar]
- 4.Hsu A, Granneman GR, Kempf DJ, et al. The Ctrough inhibitory quotient predicts virologic response to ABT-378/ritonavir (ABT378/R) therapy in treatment-experienced patients. 5th International Congress on Drug Therapy in HIV Infection, Glasgow. 2000 Abstract PL9.4. [Google Scholar]
- 5.Acosta EP, Henry K, Baken L, Page LM, Fletcher CV. Indinavir concentrations and antiviral effect. Pharmacotherapy. 1999;19:708–12. doi: 10.1592/phco.19.9.708.31544. [DOI] [PubMed] [Google Scholar]
- 6.Acosta EP, Havlir DV, Richman DD, et al. Pharmacodynamics (PD) of indinavir (IDV) in protease-naive HIV-infected patients receiving ZDV and 3TC. 7th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA. 2000 Abstract 455. [Google Scholar]
- 7.Dieleman JP, Gyssens IC, Van der Ende ME, de Marie S, Burger DM. Urologic complaints in relation to indinavir plasma concentrations in HIV-infected patients. AIDS. 1999;13:473–8. doi: 10.1097/00002030-199903110-00005. [DOI] [PubMed] [Google Scholar]
- 8.Dumon C, Solas C, Thuret I, et al. Relationship between efficacy, tolerance and plasma drug concentration of ritonavir in children with advanced HIV infection. Ther Drug Monit. 2000;22:402–8. doi: 10.1097/00007691-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Gatti G, Di Biagio A, Casazza R, et al. The relationship between ritonavir plasma levels and side-effects: implications for therapeutic drug monitoring. AIDS. 1999;13:2083–9. doi: 10.1097/00002030-199910220-00011. [DOI] [PubMed] [Google Scholar]
- 10.Gieschke R, Fotteler B, Buss N, Steimer J. Relationships between exposure to saquinavir monotherapy and antiviral response in HIV-positive patients. Clin Pharmacokinet. 1999;37:75–86. doi: 10.2165/00003088-199937010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Sadler BM, Gillotin C, Lou Y, Stein DS. Pharmacokinetic and pharmacodynamic study of the human immunodeficiency virus protease inhibitor amprenavir after multiple oral dosing. Antimicrob Agents Chemother. 2001;45:30–7. doi: 10.1128/AAC.45.1.30-37.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schapiro JM, Winters MA, Stewart F, et al. The effect of high-dose saquinavir on viral load and CD4+ T-cell counts in HIV-infected patients. Ann Intern Med. 1996;124:1039–50. doi: 10.7326/0003-4819-124-12-199606150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Recommandations du Groupe d’Experts. Rapport 2000 sous la direction du Pr JF DelfraissyPrise en charge thérapeutique des personnes infectés par le VIH. Paris: Medecine-Sciences, Flammarion; 2000. [Google Scholar]
- 14.Burger DM, Hugen PWH, Droste J, et al. Therapeutic drug monitoring (TDM) of indinavir (IDV) and of nelfinavir (NFV) in treatment-naive patients improves therapeutic outcome after 1 year: results from ATHENA. 2nd International Workshop on Clinical Pharmacology of HIV Therapy, Noordwijk. 2001 Abstract 6.2. [Google Scholar]
- 15.Burger DM, Hugen PWH, Aarnoutse RE, et al. Treatment failure of nelfinavir-containing triple therapy can largely be explained by low nelfinavir plasma concentrations. Ther Drug Monit. 2003;25:73–80. doi: 10.1097/00007691-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Bertz R, Hsu A, Lam W, et al. Pharmacokinetic interactions between lopinavir/ritonavir (ABT-378/r) and other non-HIV drugs. 5th International Congress on Drug Therapy in HIV Infection, Glasgow. 2000 Abstract P291. [Google Scholar]
- 17.Rockstroh J, Brun S, Sylte J, et al. ABT-378/ritonavir (ABT-378/r) and efavirenz one year safety/efficacy evaluation in multiple PI experienced patients. 5th International Congress on Drug Therapy in HIV, Glasgow. 2000 Abstract P43. [Google Scholar]
- 18.Hsu A, Bertz R, Renz C, et al. Assessment of pharmacokinetic interactions between Kaletra™ (lopinavir/ritonavir or ABT-378/r) and nevirapine in pediatric subjects. 5th International Congress on Drug Therapy in HIV, Glasgow. 2000 Abstract 440. [Google Scholar]
- 19.Jayewardene AL, Zhu F, Aweeka FT, Gambertoglio JG. Simple high-performance liquid chromatographic determination of the protease inhibitor indinavir in human plasma. J Chromatogr B. 1998;707:203–11. doi: 10.1016/s0378-4347(97)00607-5. [DOI] [PubMed] [Google Scholar]
- 20.Solas C, Basso S, Poizot-Martin I, et al. High indinavir Ctrough is associated with higher toxicity in patients on indinavir-ritonavir 800/100 mg twice-daily regimen. J Acquir Immune Defic Syndr. 2002;29:374–7. doi: 10.1097/00126334-200204010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Cameron DW, Japour AJ, Xu Y, et al. Ritonavir and saquinavir combination therapy for the treatment of HIV infection. AIDS. 1999;13:213–24. doi: 10.1097/00002030-199902040-00009. [DOI] [PubMed] [Google Scholar]