Abstract
Problem statement
During especially the past two decades many discoveries in biological sciences, and in particular at the molecular and genetic level, have greatly impacted on our knowledge and understanding of drug action and have helped to develop new drugs and therapeutic strategies. Furthermore, many exciting new drugs acting via novel pharmacological mechanisms are expected to be in clinical use in the not too distant future.
Scope and contents of review
In this educational review, these concepts are explained and their relevance illustrated by examples of drugs used commonly in the clinical setting, with special reference to the pharmacology of G-protein-coupled receptors. The review also addresses the basic theoretical concepts of full and partial agonism, neutral antagonism, inverse agonism and protean and ligand-selective agonism, and the relevance of these concepts in current rational drug therapy. Moreover, the mechanisms whereby receptor signalling (and eventually response to drugs) is fine-tuned, such as receptor promiscuity, agonist-directed trafficking of receptor signalling, receptor trafficking, receptor ‘cross-talk’ and regulators of G-protein signalling (RGSs) are discussed, from theory to proposed therapeutic implications.
Conclusions
It is concluded that the understanding of molecular receptor and signal transduction pharmacology enables clinicians to improve their effective implementation of current and future pharmacotherapy, ultimately enhancing the quality of life of their patients.
Keywords: drug action, therapeutics, G-protein-coupled receptors, signal transduction
Introduction
We are all aware of the positive and negative side of drugs, of the ways in which they can enhance or decrease our quality of life, or even save or take lives. Whenever the potential therapeutic benefit of a drug is considered to outweigh its potential hazards, optimal drug selection needs to be made and sound pharmacotherapy becomes the ideal of every good clinical therapist. Rational and optimal pharmacotherapy has to be based on strong pillars of appropriate knowledge, skills and values. We need appropriate knowledge of basic pharmacology and evidence-based medicine, adequate skills to diagnose, interpret and synthesize creative solutions and applicable values to realize and respect the fact that it is all about a human being with his/her own preferences and individual criteria for evaluating quality of life. In this review we will emphasize the importance of basic pharmacology, in particular of receptor pharmacology and subcellular signal transduction. This knowledge is not only important in the understanding of current therapeutics, but also to create innovative strategies when there is no clear standard solution and also to be geared to understand the medicine of tomorrow.
In this regard the General Medical Council in Great Britain recently called for an investigation into the knowledge and skills required to ensure current and future standards for medical practitioners. By using appropriate questionnaires Mucklow [1] obtained the opinion of representative specialists in clinical pharmacology and therapeutics on this issue. Interestingly, but maybe not totally surprisingly, Mucklow found that knowledge of basic molecular receptor pharmacology and signal transduction mechanisms were rated as essential by invariably 100% of the respective representative panels.
There are many examples of how knowledge of basic molecular and signal transduction pharmacology can be of value in the clinical setting. In the 1980s the introduction of the selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, into clinical medicine was heralded as the first class of antidepressants with a selective action on a key neurobiological target in depression, namely serotonin. These drugs were the antithesis of their ‘dirtier’ predecessors, the tricyclic antidepressants (TCAs), which acted on a wide range of neurotransmitter receptor systems not necessarily linked to the neuropathology of depression. Consequently, the SSRIs were marketed as pharmacologically ‘pure’ antidepressants with a minimal risk of adverse effects related to actions at ‘unwanted’ (untargeted) receptors in the brain and elsewhere. These predictions and marketing strategy was only in part true. As the SSRIs realized greater popularity, evidence for rare yet troublesome adverse events began to appear. Most notable were symptoms resembling that evoked by typical neuroleptic agents, such as dystonia, extrapyramidal effects and the potentially fatal serotonin syndrome [2–5]. This suggested an action in the basal ganglia, probably involving attenuation of neuro-motor dopamine pathways, which was not initially expected for SSRIs. The SSRIs act by inhibiting synaptic reuptake of extracellular serotonin (5HT), thereby stimulating 5HT pathways in the brain. However, 5HT receptors on dopamine projections in the striatum are also activated, resulting in a suppression of dopamine synthesis and dopamine release [6]. This action produces a hypodopaminergic state, which has been linked to the aforementioned side-effects [7]. This insight into the mechanism of action of the SSRIs provides just one example of how extracellular receptor selectivity may translate into intercellular receptor promiscuity. This example describes how one receptor type may communicate with another within the same cell, or across different cells. These communication mechanisms between receptors and other signal modulating mechanisms are the focus of the current review.
Molecular receptor and signal transduction pharmacology has advanced significantly in the second half of the previous century and particularly during the last two decades, with many emerging new and exiting concepts and models of drug action. There are many exciting new drugs acting via novel pharmacological mechanisms that are expected to be in clinical use in the not so distant future. More than 40% of all marketed drugs display activity via G-protein-coupled receptors and with current bio-informatics it is expected that several new drug targets related to the G-protein-coupled receptors will be discovered and their physiological and clinical significance explored in future [8]. The clinician needs to be equipped and prepared to understand the actions of these drugs and the rationale for their therapeutic uses. This review aims to assist clinicians to gain a deeper understanding of drug action and the latest developments in the basic pharmacological sciences and its relationship to clinical therapeutics. It will also point out the likely future trends in drug treatments, with special emphasis on the so-called G-protein-coupled receptors (a special family of drug receptors that will be explained in this review) and their signal transduction mechanisms.
Important classical and novel concepts
In order to understand the therapeutic implications of the pharmacology of G-protein-coupled receptors and their signal transduction systems one has to gain some knowledge of key concepts and terminology.
This review addresses the current understanding of G-proteins, G-protein-coupled receptors, full agonists, partial agonists, neutral antagonists, inverse agonists and even protean and ligand-selective agonists. Moreover, concepts such as receptor promiscuity (receptor heterogeneity), agonist-directed trafficking of receptor signalling, receptor trafficking, receptor desensitization, receptor down regulation and recycling of receptors, receptor ‘cross-talk’ and regulators of G-protein signalling (RGSs) will also be discussed, from theory to proposed therapeutic implications.
Pharmacological receptors
Many agents such as neurotransmitters, hormones and drugs (and even light photons and odourants) transfer their signals to cells by interaction with a membrane receptor at the cell surface [9], upon which the cell responds with a series of intracellular events (intermediate responses collectively referred to as the signal transduction system) that eventually lead to altered cellular function(s). The resulting altered cellular function may in turn affect the function of a tissue or organ or a system of the body. To understand new drug developments, a deeper understanding of this signalling process is required, starting from the drug–receptor interaction to the final cellular response. Several receptor families can be distinguished, of which the ligand-gated ion channels, receptor protein kinases, G-protein-coupled receptors and transcription factor receptors are typical examples [10].
Ligand-gated ion channel receptors
A subgroup of membrane receptors are proteins or protein complexes that form ion channels, known as ligand-gated ion channels (where the word ‘ligand’ here refers to the binding transmitter or drug) [10]. Examples of ligand-gated ion channels include the nicotinic acetylcholine receptors, GABAA receptors and serotonin-3 (5HT3) receptors. Binding of the ligand to the receptor opens (‘gates’) the ion channel to allow for the flow of particular ions across the membrane.
Receptor protein kinases
The receptor protein kinases are receptor enzyme molecules involved in the transfer of extracellular signals to the intracellular domain, where the kinases phosphorylate effector proteins to alter their function [10]. A typical example is the insulin receptor.
G-protein-coupled receptors
The G-protein-coupled receptors (commonly abbreviated GPCRs) are a large family of seven-transmembrane spanning receptors [11, 12], amongst others the adrenergic, dopaminergic, serotonergic, muscarinic acetycholine and histaminergic receptor types. The GPCRs are so named by their common ability to activate so called G-proteins (guanine-nucleotide- or GTP-binding proteins), whereby they alter cell function. Being large protein molecules situated in the cell membrane (spanning the membrane seven times with their coils or helixes), GPCRs are able to transfer the signal from a drug bound to the extracellular surface to the intracellular surface (see Figure 1). This is achieved by a change in conformation (spatial orientation) to activate the G-proteins at the intracellular surface of the membrane. The G-protein then transfers the signal further down in a cascade of intracellular processes. One could refer to three components in this signalling process, namely the receptor (GPCR), the transducer (G-protein) and the effector (e.g. adenylyl cyclase, phospholipase C, Ca2+ channels and K+ channels) [10].
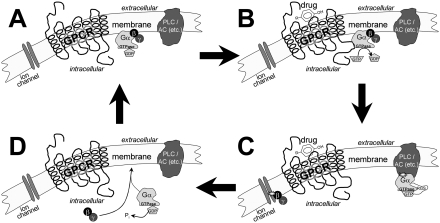
Figure 1.
A schematic representation of the G-protein ‘activation/deactivation cycle’, associated with the signalling mechanism of G-protein-coupled receptors (GPCRs). Heterotrimeric G-proteins consist of α- and βγ-subunits. Assume a case of no significant constitutive receptor activity. (A) In the resting (inactive) state the GPCR is not coupled to the G-protein. (B) As the agonist binds to the receptor, the equilibrium between the R and R* states is disturbed, so that a larger fraction of the GPCRs is in the R* conformation. The R* conformation couples efficiently with the G-protein, leading to the exchange of GDP for GTP on the Gα-subunit. (C) The Gβγ-subunit is released and both Gα and Gβγ interact with their respective effectors to continue the transduction of the signal. (D) After hydrolysis of GTP to GDP on the Gα-subunit (under influence of GTPase plus RGS) the Gα and Gβγ-subunits reunite. The system returns to its original state as presented in (A) and is ready for the next GPCR-mediated activation. PLC = phospholipase C; AC = adenylyl cyclase; GPCR = G-protein-coupled receptor; GDP = guanosine diphosphate; GTP = guanosine triphosphate
Transcription factor receptors
Several hormones (e.g. corticosteroidal hormones, thyroid hormone, vitamin D, etc.) bind to intracellular DNA-binding proteins that act as receptors to regulate the trancription of particular genes, with subsequent regulation of protein synthesis [10].
G-protein-coupled receptors
For the purpose of this review, the GPCRs and their function will be emphasized. A considerable degree of research has been focused on the pharmacology and signal transduction mechanisms of GPCRs, to such an extent that we may expect future new drugs to modulate GPCR function in novel ways. GPCRs are involved in the regulation of an array of diverse physiological functions after activation by neurotransmitters, hormones, lipids, photons, odourants, taste ligands, nucleotides and calcium ions [12, 13].
Classification of GPCRs
Mammalian GPCRs can be classified into three super families, namely Families A, B and C (or Classes I, II and III, respectively), based on sequence similarities, where these sequences are typified by the adrenergic, secretin and metabotropic glutamate receptors, respectively [14–16]. Little or no sequence similarities exist between these super families, although they all have seven membrane-spanning domains [14]. An estimated 1% of the mammalian genome codes for GPCRs and, as estimated from the human genome, about 450 of the approximately 950 predicted GPCRs are expected to be receptors for endogenous ligands [12, 16, 17].
Classically, pharmacological receptors, including the GPCRs, have been classified according to the endogenous agonist for the receptor and further subclassified according to the relative binding selectivity of ligands (receptor-binding substances) to these receptors. Already in 1948 Ahlquist [18] pioneered this field when he subclassified adrenergic receptors (with (−)-noradrenaline and (−)-adrenaline as endogenous agonists) into α- and β-adrenergic receptors, by showing that the order of potency (relative binding affinities) of a series of ligands differed for these two subtypes of adrenergic receptors. Experimental techniques employed for the classification of receptors have since become more sophisticated with radioligand binding techniques (e.g. saturation and competition binding studies) and eventually biochemical receptor purification techniques. However, with the emergence of molecular biology, and in particular the development of genomics, the identification of genomic DNA segments or mRNA (or cDNA) segments encoding for the expression of receptors have been a very successful approach to identify new receptors. From the mRNA sequence, the receptor protein encoded can be deduced. Not only can the structures of known receptors be confirmed, but new receptor subtypes (or isoforms where ligand selectivity has not been demonstrated), and even receptors without known endogenous ligands (i.e. so-called ‘orphan receptors’), have now been discovered. Due to the enormous number of receptor types and subtypes discovered and also the complexity of the classification of receptors, the International Union of Pharmacology (IUPHAR) has appointed the IUPHAR Committee on Receptor Nomenclature and Drug Classification (NC-IUPHAR) to provide guidelines for an optimal approach to receptor classification, as described in the online IUPHAR Receptor Database (see http://iuphar-db.org/iuphar-rd/index.html).
The classification of GPCRs (and other receptors) has proved to be a complex task. This is not only because of the enormous number of identified receptors and uncertainties about the endogenous ligand and the biophysiological significance of some of these receptors, but also due to the complexity of receptor regulation. Examples of such complexities include the obligatory dimeric GABAB receptors (i.e. two receptor proteins, associated as a dimer, necessary for biophysiological function) or where so-called RAMP proteins may associate with the calcitonin receptor, where after it is not only a receptor for calcitonin, but also for amylin (thereby altering the pharmacological profile of the receptor). The NC-IUPHAR concluded that, in such cases, one should classify not only the monomeric receptor protein, but also the functional protein complex [19].
GPCRs and orphan receptors
For about 160 of the GPCRs discovered by 2002, the endogenous ligands are still unknown [14]. These GPCRs have been termed ‘orphan receptors’, awaiting ‘de-orphanization’ by the discovery of their endogenous ligands. Orphan receptors are indeed viewed as potential new drug targets and are currently being exploited for their potential in treating debilitating diseases, including obesity, cardiovascular disease, inflammation and cancer [20–24]. Many ‘orphan receptors’ have now been paired with endogenous peptides, proposed as ligands (sometimes multiple ligands) and the challenge is to identify those of physiological and pathophysiological importance to motivate drug development [22, 24]. This in itself holds many challenges, as it is important to find selective ligands that modulate receptor function and also since investigation into relevant in vivo pharmacology is needed [23]. In addition, there are species differences, so that the pharmacology of ‘orphan receptors’, as studied in mice, for example, may be different to that in humans [22]. It is expected that the functional analysis of most ‘orphan receptors’ may be completed within the next decade [24], which may reveal many new drug targets and open exiting new therapeutic strategies.
GPCRs and theories of drug action
Current theories of GPCR function have greatly impacted our understanding of drug action and opened up new ways of searching for new drugs. The GPCR is a large protein that is in equilibrium between (presumably) several possible conformational states (spatial orientations) [13, 25, 26, 27]. Some of these conformational states are energetically more favourable than others. It is important to note that some of these conformations are assumed to be inactive (i.e. they do not activate G-proteins) while others are active (i.e. they activate G-proteins). We refer to models building on this concept as models of ‘multiple activation states of receptor activity’. As illustrated in Figure 2, the simplest theoretical model of these would assume only two conformations (two-state model), namely one inactive and one active conformation (usually designated the R and R* receptor states, respectively) [28]. There is also a three-state model of receptor activation, with one inactive state (R) and two active states (R* and R**), where the one active state will couple to one type of G-protein and the second to another type of G-protein [27, 29]. The significance of these models for drug therapy will be discussed further below.
Figure 2.
A schematic representation of the two-state receptor model. R, R*, DR and DR* are in constant equilibrium, where D is the drug, R is the receptor in the inactive state, R* is the receptor in the active state, and DR and DR* are the respective drug-receptor complexes (drug-bound receptor). KD, KD*, L and L(D) are kinetic constants describing the equilibrium between the respective states. In particular, KD and KD* describe the affinity (binding power) of the drug for the receptor in its inactive and active states, respectively
Usually, in the two-state model, most of the GPCRs, when not bound to a drug, will exist in the inactive R conformation, with only a small fraction in the active R* conformation. However, some GPCRs have a significant fraction of the receptors in the R* conformation. The receptors in the R* conformation will give rise to a basal response (also known as constitutive activity) of which the magnitude depends on the fraction of receptors in the R* conformation (i.e. the R : R* ratio). For any drug to influence the function of a receptor it has to be able to bind to the receptor, that is, it must foremost have affinity (binding power) for the receptor. When a drug has significant affinity for the receptor, it is predicted that drugs may bind selectively to either R or R* or with equal affinity for both R and R*. Receptor-binding drugs can therefore influence receptor function in one of three ways:
Full and partial agonists
Some endogenous substances and drugs preferentially bind to R*and will then drive the equilibrium between R and R* towards more R*. This will increase receptor signalling and eventually pharmacological response. These endogenous substances and drugs are known as agonists, such as (−)-noradrenaline, serotonin, dopamine and drugs such as dobutamine on β1-adrenergic receptors, isoproterenol on β1/2-adrenergic receptors, fenoldopam on D1 receptors and clonidine on α2-adrenergic receptors, to name but a few. The selectivity of agonists for R or R* may vary so that an agonist with great selectivity for R* over R will behave as a full (strong) agonist and one with only a small selectivity for R* over R, will behave as a partial (weak) agonist (see Figure 3). Buspirone and oxymetazoline are typical examples of partial agonists on 5HT1A and α2-adrenergic receptors, respectively. Several β-adrenoceptor blockers, e.g. pindolol and acebutolol, act as partial agonists at β-adrenoceptors and exhibit therefore intrinsic sympathomimetic activity (ISA). This is in contrast to a drug like propranolol that processes no ISA. Theoretically the presence of ISA in β-adrenoceptor blockers suggests that these drugs may be less hazardous in asthma patients. Nevertheless, these drugs should be used with caution in these patients. Other advantages claimed by some investigators include protection against myocardial depression, adverse lipid changes and peripheral vascular complications [30]. The ability of an agonist to decrease the R : R* ratio (i.e. increase R*, thereby ‘activating’ or ‘stimulating’ the receptor), is referred to as its ‘efficacy’. The greater the ability to increase R*, the higher the efficacy of the agonist. In molecular pharmacology therefore the term ‘efficacy’ has a different meaning from how it is used in clinical pharmacology, where ‘efficacy’ would refer to how effectively the drug treats the disease or symptoms [26]. An agonist is also described as having ‘intrinsic activity’, meaning that it is able to give rise to a pharmacological response. A drug with higher efficacy (receptor activating property) also has a higher intrinsic activity (ability to elicit response), except when there is a ceiling to the maximal response in which case higher efficacy does not necessarily increase the intrinsic activity of the agonist in that particular biological system.
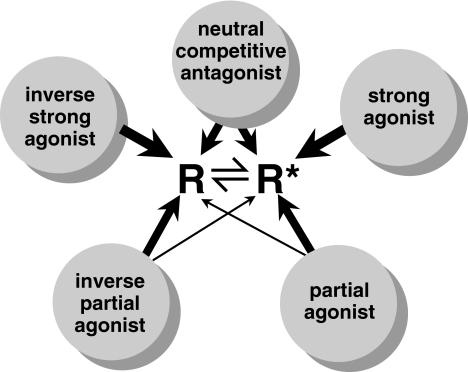
Figure 3.
A schematic representation of how the two-state receptor model relates to the action of drugs as strong agonists, partial agonists, neutral competitive antagonists, inverse agonists, and inverse partial agonists. The inactive and active receptor conformations (R and R*, respectively) are in constant equilibrium. A strong agonist binds selectively to R*, driving the equilibrium between R and R* in favour of R*, resulting in enhanced response. A partial agonist has higher affinity for R* than for R, but with less selectivity than the strong agonist. The neutral competitive antagonist binds with equal affinity to both R and R*, so that it does not disturb the resting equilibrium and therefore does not alter basal response. An inverse strong agonist binds selectively to R, driving the equilibrium between R and R* in favour of R, resulting in decreased response, that is, when there is significant constitutive activity (basal response). An inverse partial agonist has higher affinity for R than for R*, but with less selectivity than the strong inverse agonist
Inverse agonists
Some drugs preferentially bind to R over R* and will drive the equilibrium between R and R* towards R (decreasing the number of receptors in the R* state). If there is a significant level of constitutive receptor activity (basal response), these drugs will decrease receptor signalling and thereby decrease response. This kind of drug is known as an inverse agonist (see Figure 3), giving a response opposite to that of an agonist [26], i.e. a drug with negative efficacy. If, however, there is not significant constitutive receptor activity, the inverse response will not be noticeable and the inverse agonists will act as neutral competitive antagonists (see below). Examples of inverse agonists include cimetidine and ranitidine on H2 receptors, haloperidol on D2 receptors, prazosin on α1-adrenergic receptors, timolol on β2-adrenergic receptors, clozapine on D2 and 5HT2C receptors [31] and many experimental drugs such as the benzodiazepine inverse agonist β-CCB and yohimbine on α2A-adrenergic receptors [32]. Indeed, many drugs previously believed to be competitive antagonists have recently been shown to act as inverse agonists and it can be expected that several more inverse agonists will be discovered.
Neutral competitive antagonists
Drugs that do not differentiate between R and R* (i.e. they bind with equal affinity to both conformations) are known as neutral competitive antagonists (see Figure 3), i.e. a drug with no efficacy at its receptor. Neutral competitive antagonists do not alter basal receptor activity on their own, but they compete with agonists and inverse agonists for receptor binding, thereby competitively antagonizing the responses elicited by agonists and inverse agonists. A typical example of these drugs is propranolol on β-adrenergic receptors.
The clinical significance of the differentiation between neutral competitive antagonists and inverse agonists is not always clear. Much research is currently being done to look into this question. It has been argued that the chronic use of inverse agonists may lead to so called receptor up-regulation, as opposed to agonists that usually down-regulate receptors, but data on this are not conclusive. To illustrate this principle, the histamine H2-receptor inverse agonists cimetidine and ranitidine, but not the neutral antagonist burimamide, up-regulate H2-receptors after chronic exposure. The mechanism of receptor up- and down-regulation will be discussed further below under the concept of membrane trafficking of receptors. In addition to the example of H2-receptors, Kenakin [26] also refers to several examples of disease where constitutive activity of GPCRs have been observed and where inverse agonists may in future research be proven to have advantages over neutral antagonists, e.g. VIP receptor inverse agonists in the treatment of cancer and also appropriate inverse agonists in the treatment of retinitis pigmentosa, hyperthyroidism, autoimmune disease and certain types of viral infection.
Presynaptic receptors
In the nervous systems we distinguish pre- and postsynaptic receptors according to their location. Although postsynaptic receptors have been studied more extensively, a variety of presynaptic receptors have been identified that are of clinical significance. These receptors are important because of their ability to control the release of neurotransmitters [33–35]. Presynaptic receptors facilitate a feedback mechanism whereby they influence (inhibit or promote) the subsequent release of neurotransmitters from the same neurone (autoreceptors) or they may influence the release from neighbouring neurones (heteroreceptors).
Presynaptic inhibitory autoreceptors have been identified on both adrenergic and cholinergic nerve terminals. Activation of these receptors by released noradrenaline or by exogenously administered α2-adrenoceptor agonists such as clonidine decreases the further release of the neurotransmitter. The same subtype of α2-adrenoceptors inhibits the release of acetylcholine from cholinergic neurones [36]. Other inhibitory autoreceptors are also described, for example for dopamine (D2/D3 receptors), acetylcholine (M2 receptors), GABA (GABAB receptors), histamine (H3-receptors) and serotonin (5HT1D receptors) [33]. In addition to the presynaptic inhibitory autoreceptors, there are also presynaptic autoreceptors that enhance the release of the neurotransmitter, including acetylcholine (nicotinic) and noradrenaline (β2) presynaptic receptors.
Presynaptic heteroreceptors are receptors that modulate neurotransmitter release when they are stimulated by neurotransmitters other than the neurone's own transmitter that are present in the synaptic cleft. For example, noradrenaline nerve terminals possess presynaptic facilitatory angiotensin II receptors and presynaptic inhibitory opiate receptors [37–39].
Presynaptic receptors are suitable targets for exogenous drugs such as agonists or antagonists. Consequently these receptors are targets of action for a new generation of drugs that may intervene selectively at the level of presynaptic release-modulating receptors [33]. Examples of the latter are the antidepressant mitazepine that antagonizes α2-adrenoceptors and modulates the release of noradrenaline and serotonin [40, 41], and the neuroleptic amisulpride that is a selective antagonist at D2/D3 dopamine autoreceptors that modulate the release of dopamine [33]. The improved efficacy of the atypical antipsychotics, such as clozapine and olanzepine, in the treatment of schizophrenia, and their lower incidence of motor side-effects, has been linked to their ability to promote dopamine release via actions at auto- and heteroreceptors in the limbic and striatal regions of the brain [2].
G-proteins and signalling
As discussed above, transmembrane GPCRs may activate G-proteins on the inner surface of the cell membrane to continue the signal transduction initiated by the drug binding to the GPCR [11]. The G-proteins are composed of three subunits, namely the α-, β and γ subunits (hence the heterotrimeric character of G proteins), where the β- and γ-subunits function as a unit (see Figure 1). The G-proteins can be subdivided into four families (namely Gs, Gi/o, Gq/11 and G12/13 proteins) [42] where GPCRs show selectivity for coupling to these respective G-protein families. It has been found that Gs proteins primarily activate adenylyl cyclase, Gi proteins primarily inhibit adenylyl cyclase, Gq proteins primarily activate phospholipase C and G12/13 proteins primarily regulate small GTP binding proteins (not mentioning, of course, other G-protein effectors). Within these G-protein families, researchers have identified several different subtypes of α-, β and γ-subunits and combinations thereof to even further complicate this classification, although there are still many investigations and discussions about their relevance and function [12, 43, 44, 45]. The same subtypes of subunits may also be further subdivided into splice variants that may be expressed differently in different tissue or with age [46].
To understand how potential future drugs, such as the RGS-modulating drugs discussed below, may modulate G protein function (and consequently signal transduction), it is important to understand more about the mechanism by which G-proteins function. In the inactive state, the α-subunit of the G-protein is bound to GDP. The complex formation between the active receptor state and the G protein is followed by the release of GDP from the α-subunit of the G protein, subsequently allowing for a GTP molecule to bind. The G protein is now activated and the α- and βγ-subunits become separated (see Figure 1). The active GTP-bound α-subunit is sometimes referred to as Gα(GTP) and the βγ-subunit as Gβγ. These presumably mobile subunits are known to influence cell function by, for example, altering enzyme function (e.g. adenylyl cyclase or phospholipase C) and the consequent production of second messengers (e.g. cAMP or inositol-1,4,5-triphosphate (IP3) and diacylglycerol) or to alter ion channel function [45].
Within a fraction of a second the GTP on the Gα(GTP) is hydrolysed to GDP by GTPase on the Gα and this allows for the α- and βγ-subunits to re-unite and to form inactive G-protein complexes again.
RGS modulating drugs
Regulators of G-protein signalling (RGSs) are a family of proteins that can modify (regulate) the signal transduction by G-proteins. Because of their important physiological function combined with several other special properties, they are important potential drug targets. Their proposed clinical significance will be discussed further below. RGSs regulate G-protein signalling in any of the following ways [45, 47, 48].
GAP function
As mentioned earlier, the α-subunits of G-proteins have inherent GTPase activity that hydrolyses GTP to GDP, thereby self-inactivating Gα(GTP) back to Gα(GDP). The GTPase activity is inherently too low for normal physiological functioning, but can be regulated (greatly enhanced) by the RGSs, so that they accelerate the inactivation of Gα(GTP) to Gα(GDP) and thereby accelerate the G-protein cycle. As a result RGSs will also shorten any R* and agonist-R* mediated signalling duration and thereby weaken agonist action, as is illustrated in Figure 1. Since this function of the RGSs relates to enhanced GTPase activity, RGSs with this function are also referred to as GTPase activating proteins (GAPs). The GTPase activating function of RGSs is therefore sometimes referred to as their GAP function. It has been found that the majority of RGSs function as GAPs.
Non-GAP functions
However, not all RGSs function as GAPs. RGSs may also directly antagonize Gα effectors, thereby preventing Gα from signalling to its effector. Other functions and mechanisms (of lesser importance for the purpose of this review) have also been proposed. For example, RGSs may antagonize Gβγ-subunits by binding to, e.g. the β-subunit, thereby preventing the unit from performing its physiological role. RGSs may also directly bind to receptors or act as Gα effectors to modulate signal transduction pathways.
RGSs are now regarded as important drug targets. Currently there are no drugs registered as RGS modulators, but active research is being conducted and the following arguments support RGS modulating agents as potential drugs of the future.
Several families of RGSs (each with subfamily members) have been identified so far. RGS subtypes may show selectivity for various G protein subtypes, so that it may be possible to target specific G proteins by targeting specific RGSs with modulating drugs [47].
Importantly these various types of RGSs have been suggested to be expressed selectively and differentially in various tissues. RGS7 is expressed at higher levels in the neocortex, hippocampus and certain nuclei than in other brain regions [47]. RGS4 is abundant in many brain tissues, but its expression can be regulated differently in different brain regions following stress or corticosterone administration (specifically a decrease in paraventricular nucleus and pituitary and increase in locus coeruleus) [47].
Altered RGS expression in disease has been reported. In schizophrenia, for example, RGS4 expression is decreased in the prefrontal cortex (as determined by microarray analysis of mRNA in postmortem brain). It is believed that this may account for typical symptoms of schizophrenics in situations of stress [49]. Moreover, in Parkinson's disease it has been shown that the levels of RGS9 are increased in both the caudate and putamen, relating Parkinson's disease with decreased D2-receptor signalling [50]. It has also been proposed that RGS proteins associated with Gq and Gi proteins may be linked to embryonic cardiomyocyte proliferation, as well as cardiac hypertrophy and cardiovascular adaptation to pressure overload and physiological stress in adults [42].
Any selective inhibitor of a particular RGS type will enhance agonist activity, making this an attractive approach for drug discovery. The above mentioned data on Parkinson's disease and RGS9 suggests that a RGS9 antagonist may be of therapeutic benefit in patients with Parkinson's disease. Likewise a RGS4 agonist (or potentiating agent) may be of therapeutic benefit in schizophrenic patients. This therapeutic potential underscores why current research is focusing on the development of selective RGS modulating agents as novel drugs of the future [48]. Whether this strategy will enable us to modify GPCR-mediated signalling without affecting associated problems such as receptor sensitization or desensitization (see membrane trafficking below), however, still needs to be addressed.
Fine-tuning GPCR signalling
Although the human body expresses probably thousands of different types and subtypes of GPCRs, the number of GPCR effector types (e.g. second messenger systems) are far less [12]. It is therefore obvious that the body needs extraordinary and delicate mechanisms to differentially regulate the various diverse cellular functions with only a limited number of signalling pathways [51]. Researchers have put forward explanations such as the specificity of GPCR coupling with G proteins [9, 11, 43], membrane organization [9, 44, 52, 53, 54] and signalling cross-talk mechanisms [12, 55] to explain this phenomenon.
Cross-talk suggests that signalling pathways may be interlinked (can merge at some point), so that one type of GPCR may influence the signalling of another (for example the SSRI-induced 5HT receptor-dopamine cross-talk described in the introduction of this article). Cross-talk is believed to be a general phenomenon, since most cell systems have a large number of different GPCR types with a much more limited number of effector (e.g. second messenger) systems [12]. A particular GPCR type may couple to more than one G protein type (receptor heterogeneity/promiscuity) or, conversely, different GPCR types may couple to the same metabolic pathway of even the same G protein type [25, 55]. A particular receptor (Rx) may therefore be able to couple to both G protein types G1 and G2 in a cell, or conversely, if both receptor 1 (R1) and receptor 2 (R2) are able to couple to a particular G protein (Gx), it may be possible that both R1 and R2 activate Gx in the cell (see Figure 4). To illustrate this phenomenon, α2A-adrenoceptors can couple to Gi, Gs and Gq proteins (although with different strength) [53, 56, 57] and conversely both serotonergic 5HT2A receptors and muscarinic acetylcholine M5 receptors are known to couple to Gq proteins [36, 58]. Thereby one cellular response may result from a fine balance of several substances, and not merely by the fluctuation of the concentration of one substance. In the clinical setting, cross-talk is not a new concept. It has been used to explain, for example, why antidepressants of different neurotransmitter selectivity (e.g. (-)-noradrenaline or serotonin) ultimately evoke the same neuronal response regardless of receptor selectivity. Examples of this form of unpredictable post-receptor cross-talk include similar antidepressant-induced changes in biogenic amine metabolites and down regulation of β-adrenoceptors in limbic regions of the brain that correlate with improvement in depressive symptoms [2, 59]. The implications are that these far-reaching inter-regulatory elements allow a particular underactive/overactive pathway to be modulated, despite the drug's having a ‘select’ action on another extracellular receptor system. Figure 5 illustrates various cell-surface receptor-linked pathways utilizing G-protein-coupled second messenger systems and how these pathways may interact at the postreceptor level, e.g. at second messenger level (e.g. cAMP-phospholipase C), at enzyme level (e.g. cGMP-phosphodiesterase), at protein kinase level and at the G-protein level (e.g. Gs–Gq interactions). Activation, or inhibition, of one particular extracellular receptor may modulate events set in motion by separate extracellular receptor responses [2].
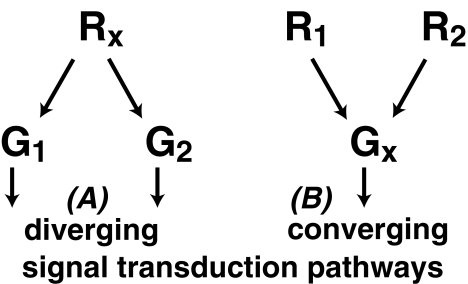
Figure 4.
A schematic representation of how receptor promiscuity may lead to either the divergence of one signal transduction pathway into several downstream pathways or the convergence of signal transduction pathways into one pathway. (A) Rx, represents a single GPCR type that couples to two different G protein types G1 and G2, thereby diverging the signal into two independent signal transduction pathways. (B) R1 and R2 are two different GPCR types that both couple to a particular G protein type Gx, so that their signals converge into one signal transduction pathway
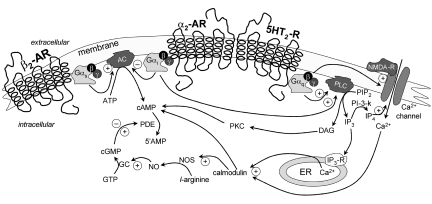
Figure 5.
A schematic representation of receptor cross-talk, illustrating various examples of GPCR signal transduction pathways, where β2-AR = β2-adrenergic receptor; α2-AR = α2-adrenergic receptor; 5HT2-R = serotonin type 2 receptor; NMDA-R = N-methyl-d-aspartate receptor; ER = endoplasmic reticulum; AC = adenylyl cyclase; PLC = phospholipase Cβ; PDE = phosphodiesterase; PKC = protein kinase C; ATP/GTP = adenosine/guanosine triphosphate; cAMP/cGMP = cyclic adenosine/guanosine monophosphate; PIP2 = phosphatidyl inositol biphosphate; IP3/IP4 = inositol tri/tetra-phosphate; NO = nitric oxide; NOS = nitric oxide synthase; ! = stimulating effect; @ = inhibitory effect
Another example of where future therapy may benefit from our understanding of cross-talk, is in the treatment of Parkinsonism. This severely debilitating disease is caused by the progressive degeneration of dopaminergic neurotransmission from the mesencephalon to the striatum. Current therapy involves the replacement of central dopamine, but is often associated with a progressive decrease in efficacy and increase in dyskinesias. Recent research indicates cross-talk between serotonergic 5HT1B, dopaminergic D2 and cannabinoid CB1 receptors. Since these receptors are shown to be colocalized, it was suggested that their signal transduction systems may converge [60]. In this regard, it has also been shown that D1-receptor-mediated activation of adenylyl cyclase can be completely blocked by CB1 stimulation and, conversely, that dopamine receptors regulate the release of endocannabinoids. In addition, it has been suggested that in Parkinsonism, the brain might normalize striatal function by elevating striatal endocannabinoids and CB1 receptors (receptor up-regulation). This has led to the proposal that new cannabinoid-based drugs and inhibitors that reduce the enzymatic breakdown of these derivates might be useful in treating Parkinsonism [61]. One such plant alkaloid, a main psychoactive component of Cannabis sativa (dagga), exerts its effects by interacting with cannabinoid receptors [60].
Membrane organization, on the other hand, would suggest that different GPCRs and G proteins are concentrated in specialized and distinct microdomains on the cell membrane, as opposed to random distribution or freely diffusible systems [12, 44]. For example, if R1 and R2 are able to couple to a Gx, it may be possible that only R1 activates Gx in the cell, simply because R1 and Gx are colocalized, whereas R2 is located in a different microdomain. In a second cell type, R2 and Gx may be colocalized and therefore R2 can activate Gx. Membrane organization would therefore provide us with an explanation for differences in the regulation of the same signal transduction pathway in different cells and also explains why the signals of two receptors linked to the same G protein do not necessarily merge. The significance of the close association of signalling proteins in cellular microdomains is not yet fully understood, but it may serve to recruit important components, thereby enhancing efficiency and rapidity of coupling, or to hold signalling molecules inactive until needed or even to attract signalling components to terminate the signal [12].
Agonist-directed trafficking of receptor signalling (ADTRS) is a recent concept in molecular pharmacology that also may impact on clinical therapeutics in the future [26, 62, 63, 64]. GPCRs are known for their heterogeneous coupling to G-proteins, meaning that a particular GPCR type may be able to couple to more than one type of G-protein. As an example, α2A-adrenergic receptors are able to couple to Gi, Gs and Gq proteins [53, 56, 57], but with different affinity. The following cases can be distinguished:
In cells containing all three of these G-proteins, it is predicted by classical theory that a maximal concentration of a strong agonist will be able to cause the activation of all three G-protein types to the maximum (100%), while a maximal concentration of a partial agonist will cause equal but partial activation of all three G proteins (i.e. < 100%). Therefore, if a particular agonist is able to cause activation of one type of G protein type (e.g. Gi proteins) to a certain percentage of its maximum, it will also cause the activation of the other types of G proteins (e.g. Gq and Gs proteins) to exactly same percentage of their maximum [62].
According to the concept of ADTRS, however, there may be agonists that activate the GPCR in such a way that it selectively activates only one G-protein type. An example of such action is to be found in the weak α-adrenoceptor agonist, (-)-isoproterenol, that activates α2-adrenergic receptors in such a way that it selectively activate Gs, as opposed to Gi proteins [63]. Similar findings were reported for serotonergic 5HT2 receptors [62]. This can be explained by assuming a model where different active receptor states (R* and R**) are responsible for activating the different G proteins (see Figure 6). Agonists that selectively promote only one active receptor state (i.e. R* or R**), have been referred to as ligand-selective agonists or receptor active-state selective agonists. Since different G-proteins activate different metabolic pathways (or activate the same pathway differently), ADTRS allows selective activation of only one metabolic pathway by a receptor. If, for example, one metabolic pathway accounts for unwanted side-effects and the other for the beneficial therapeutic pathway of most agonists of a receptor type, then an agonist that selectively activates the therapeutic pathway will be beneficial. In the search for new drugs, it is therefore important not only to search for drugs that are more selective for particular receptor subtypes, but also for drugs that may work on the same receptor, but preferentially activates appropriate metabolic pathways or that selectively inhibits the inappropriate metabolic pathway. As a relatively novel concept, ADTRS has not found place in the clinical therapeutic setting as yet.
Figure 6.
A schematic representation of how the three-state receptor model for GPCRs explains the phenomenon of agonist-directed trafficking of receptor signalling (ADTRS). R is the inactive receptor state, R* the active receptor state coupling to and activating G-protein type 1 (G1) and R** is a second active receptor state coupling to and activating G-protein type 2 (G2). R, R* and R** are in constant equilibrium. Agonists that binds equally well to R* and R** will not display ADTRS, whereas agonists with selective binding to either R* or R** will favour coupling of the GPCR to either G1 or G2, respectively, thereby selectively activating one signal transduction pathway and therefore displaying ADTRS
Protean agonism is another interesting concept in molecular pharmacology that is related to ADTRS. This unique type of agonism describes a situation where a particular agonist is able to present itself as an agonist in one system and as an inverse agonist in another system, but at the same receptor [26, 65, 66]. Protean agonism is currently being researched and there are several examples of drugs that have been suggested to act via this mechanism. The mechanism behind protean agonism is rather complex and falls beyond the scope of the review. As with ADTRS, protean agonists may, at least in theory, hold potential therapeutic benefits in that it may reveal agonism selective to one tissue, while acting as inverse agonist or antagonist in another.
Receptor function may further be regulated by receptor trafficking, whereby the cell regulates the number of available receptors in the membrane. The body uses this mechanism to prevent continuous over stimulation of a particular receptor. GPCR trafficking is also believed to play an important role in drug abuse, for example with the opioids (morphine and related drugs) and hallucinogens (e.g. lysergic acid diethylamide or LSD), where it causes tolerance associated with typical drug seeking behaviour [67]. The chronic use of antidepressants (including several tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors and atypical antidepressants) and antipsychotics have also been associated with the down-regulation of serotonergic 5-HT2A receptors. Paradoxically, both agonists and antagonists at 5-HT2A receptors have been shown to down regulate these receptors [68]. It has also been suggested that future drugs that regulate the trafficking of dopaminergic receptors may have therapeutic value in Parkinson's disease [69].
Receptor trafficking typically includes the processes described below [20, 68, 70] (for an animation of agonist-induced activation of GPCRs, receptor desensitization, internalization and eventual recycling, see http://archive.bmn.com/supp/tips/tips2210a.html) [20]:
Receptor desensitization
Receptor desensitization is the initial process whereby an agonist-bound receptor is phosphorylated by G-protein-coupled receptor kinases (GRKs). The phosphorylated receptor subsequently binds to so called arrestins to potentiate the desensitization, rendering the receptor nonfunctional. This is a rapid process that may occur within seconds after agonist stimulation and can be viewed as a negative feedback mechanism whereby the body prevents the excessive stimulation of a particular receptor. The process whereby an agonist desensitizes its own receptor is known as homologous receptor desensitization, such as is found with the overuse of β2-adrenoceptor agonists (bronchodilators such as salbutamol) in asthma. It is, however, also possible that stimulation of one receptor may desensitize another, known as heterologous receptor desensitization, where generally protein kinase A or C (and not GRK) is be responsible for the receptor phosphorylation.
Receptor sequestration and internalization
Once desensitized, GPCRs are scaffolded to specific membrane regions where the membrane folds inward (invagination) to form vesicles (so-called clathrin-coated pits), enclosing the GPCRs. These GPCR containing vesicles are released into the cellular cytoplasm by a process called internalization. Some GPCRs (e.g. muscarinic acetylcholine receptors) are internalized via caveolae (a smooth nonclathrin vesicle, containing caveolin), but this alternative endocytosis pathway for GPCRs needs more thorough investigation.
Receptor degradation or recycling
Once internalized, the GPCR can be either metabolized by lysosomes (down-regulation) or dephosphorylated and recycled to the cell membrane to restore function (resensitization).
Research data suggest that desensitization and down-regulation can be dissociated, implying that a drug may, for example, cause desensitization without down-regulation. Also, since it is assumed that receptor internalization may be necessary for dephosphorylation and recycling (resensitization) of desensitized receptors, drugs that inhibit receptor internalization but not receptor desensitization may actually lead to an increased number of receptors remaining in the desensitized state [68]. Studies suggest that an increase in the number of desensitized µ-opioid receptors may be associated with treatment with morphine [71] and fentanyl [72]. This increase in the number of desensitized receptors is presumably responsible for the tolerance and associated drug seeking behaviour. Although both morphine and etorphine induce tolerance after seven days treatment in mice, only etorphine produces µ-opioid receptor down-regulation [73]. Similar results have been obtained in cell cultures [74]. These results suggest that opioid agonists may regulate trafficking proteins differentially. It has also been shown that l-type calcium channels may be involved in µ-opioid receptor trafficking, since calcium channel blockers, such as nimodipine, are able to prevent µ-opioid receptor down-regulation by agonists [75]. In addition, it has been shown that the body controls chronic inflammatory pain by increasing δ-opioid receptor expression (receptor up-regulation) and the recruitment of intracellular receptors to the plasma membrane, thereby decreasing pain. This finding has initiated the challenge to develop endogenous enkephalin-like peptides for controlling inflammatory pain [76]. All of the above allows us to appreciate not only the complexity of this phenomenon, but also illustrates the importance thereof for drug interactions in therapeutics.
Dimerization and oligomerization of GPCRs is another mechanism whereby the fine-tuning of the signal transduction systems is accomplished. Dimerization implies structural complex formation between two GPCRs to operate/function together, whereas oligomerization would imply more than two GPCRs in such a complex. We also distinguish between homodimerization and heterodimerization, where the former would imply that two identical GPCRs would form a complex, whereas the latter imply that two different types of GPCRs form a complex. Dimerization may be necessary for efficient agonist binding and signalling or may even generate new drug binding sites [8, 20]. It has been shown that adenosine A1 and dopaminergic D1 receptors form dimers, suggesting that future drug treatments for Parkinson's disease may be targeted at adenosine receptors rather than at dopaminergic receptors [8].
Different isoforms of GPCRs (differences in amino acid sequence) have been implicated in specific disease states, resulting from alternative splicing or mRNA editing. These substitutions of one or more amino acids within the GPCR protein may lead to altered activity or ligand-binding properties of the receptor. It is now recognized that interindividual or interpopulation differences in drug responses may result from genetic polymorphisms. Best studied are polymorphisms that influence drug metabolism, but there are also numerous studies suggesting polymorphisms of receptors, notably β1- and β2-adrenergic receptors [77, 78]. For example, β2-adrenergic receptor desensitization following continuous agonist exposure is less profound in individuals with a homozygous Arg-to-Gly amino acid change at codon 16 of the β2-adrenergic receptor (ADRB2 gene) [79], whereas this polymorphism has also been shown to be associated with a lesser immediate response to a single dose bronchodilator [80]. Therefore, prior knowledge of β2-adrenergic receptor polymorphism may predict patient response and direct therapeutic approach in asthmatic patients. For example, it may be advisable to start earlier and more aggressively with anti-inflammatory therapy in asthmatic patients with homozygous β2-adrenergic receptor codon 16 Arg/Arg [81]. However, since multiple, distinct single-nucleotide polymorphisms are usually possible for a receptor gene, it may be important to study patterns of the altered functional properties resulting from various combinations of the possible amino acid changes (haplotype structures). Such combinations of amino acid changes may in concert be different from the sum total of the individual functional changes induced by the single-nucleotide polymorphisms. Technology may soon make haplotype analyses generally accessible. Another example of the potential of exploiting polymorphism in the clinical setting has been found for the serotonergic 5HT2C receptor in the human prefrontal cortex of depressed suicide victims, where premRNA editing has been shown to exist. Strikingly, fluoxetine has also been shown to cause mRNA editing in mice exactly opposite to that seen in the depressed suicide victims, suggesting that it may reverse such abnormalities in humans [82]. Furthermore, G protein mutations, with resulting altered signalling function, have been observed in hypertensive patients, patients with testotoxicosis and patients with type I pseudohypoparathyroidism [83].
Conclusions
Understanding molecular receptor and signal transduction pharmacology enables practitioners to improve their understanding and effective implementation of current and future pharmacotherapy. It helps practitioners to understand and predict possible drug interactions, develop and improve therapeutic strategies and with subsequent enhancement of the quality of life of their patients. According to Kenakin [26] the challenge for the next millennium in drug discovery and receptor pharmacology will be to exploit the complex pharmacological properties of the drugs acting on GPCRs for therapeutic advantage. New findings in the near future in the field of GPCRs will indeed lead to novel therapeutic approaches aiming at the optimization of drug therapies.
References
- 1.Mucklow JC. What knowledge and skills are essential for specialists in clinical pharmacology and therapeutics? Results of a Delphi study. Br J Clin Pharmacol. 2002;53:341–6. doi: 10.1046/j.1365-2125.2002.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey BH. The neurobiology and pharmacology of depression: a comparative overview of serotonin selective antidepressants. S Afr Med J. 1997;87:540–52. [PubMed] [Google Scholar]
- 3.Lane RM. SSRI-induced extrapyramidal side-effects and atathisia: Implications for treatment. J Psychopharmacol. 1998;12:192–214. doi: 10.1177/026988119801200212. [DOI] [PubMed] [Google Scholar]
- 4.Settle EC. Antidepressant drugs: Disturbing and potentially dangerous adverse effects. J Clin Psychiatry. 1998;59:25–30. [PubMed] [Google Scholar]
- 5.Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- 6.Kapur S, Remington G. Serotonin–dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153:466–76. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- 7.Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705–13. doi: 10.1176/ajp.148.6.705. [DOI] [PubMed] [Google Scholar]
- 8.Marshall FH. Heterodimerization of G-protein-coupled receptors in CNS. Curr Opin Pharmacol. 2001;1:40–4. doi: 10.1016/s1471-4892(01)00001-7. [DOI] [PubMed] [Google Scholar]
- 9.Neer EJ. Heterotrimeric G proteins organizers of transmembrane signals. Cell. 1995;80:249–57. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 10.Ross EM, Kenakin TP. Pharmacodynamics: Mechanisms of drug action and the relationship between drug concentration and effect. In: Hardman JG, Limbird LE, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 10. New York: McGraw-Hill; 2001. pp. 31–43. [Google Scholar]
- 11.Brady AE, Limbird LE. G protein-coupled receptor interacting proteins: Emerging roles in localization and signal transduction. Cell Signal. 2002;14:297–309. doi: 10.1016/s0898-6568(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 12.Hur E, Kim K. G protein-coupled receptor signalling and cross-talk. Achieving rapidity and specificity. Cell Signal. 2002;14:397–405. doi: 10.1016/s0898-6568(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 13.Gether U, Kobilka BK. G protein-coupled receptors. II. Mechanism of agonist activation. J Biol Chem. 1998;273:17979–82. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- 14.Foord SM. Receptor classification: post genome. Curr Opin Pharmacol. 2002;2:561–6. doi: 10.1016/s1471-4892(02)00214-x. [DOI] [PubMed] [Google Scholar]
- 15.Gaulton A, Attwood TK. Bioinformatics approaches for the classification of G-protein-coupled receptors. Curr Opin Pharmacol. 2003;3:114–20. doi: 10.1016/s1471-4892(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 16.Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/s0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- 17.Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22:532–7. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- 18.Ahlquist RP. A study of adrenotropic receptors. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- 19.Spedding M, Bonner TI, Watson SP, International Union of Pharmacology XXXI. Recommendations for the Nomenclature of Multimeric G Protein-Coupled Receptors. Pharmacol Rev. 2002;54:231–2. doi: 10.1124/pr.54.2.231. [DOI] [PubMed] [Google Scholar]
- 20.Nothacker H, Reinscheid RK, Civelli O. From receptor to endogenous ligand. In: Stanford C, Horton R, editors. Receptors: Structure and Function. 2. New York: Oxford University Press; 2001. pp. 41–64. [Google Scholar]
- 21.Shaaban S, Benton B. Orphan G protein-coupled receptors: from DNA to drug targets. Curr Opin Drug Discov Devel. 2001;4:535–47. [PubMed] [Google Scholar]
- 22.Katugampola S, Davenport A. Emerging roles for orphan G-protein-coupled receptors in the cardiovascular system. Trends Pharmacol Sci. 2003;24:30–5. doi: 10.1016/s0165-6147(02)00007-x. [DOI] [PubMed] [Google Scholar]
- 23.Bennett T, Gardiner SM. Fostering orphan receptors: an indispensable role for integrative, in vivo, haemodynamic studies. Curr Opin Pharmacol. 2003;3:140–5. doi: 10.1016/s1471-4892(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 24.Willson TM, Moore JT. Genomics versus orphan nuclear receptors – a half-time report. Mol Endocrinol. 2002;16:1135–44. doi: 10.1210/mend.16.6.0849. [DOI] [PubMed] [Google Scholar]
- 25.Kenakin T. Agonist-receptor efficacy I. mechanisms of efficacy and receptor promiscuity. Trends Pharmacol Sci. 1995;16:188–92. doi: 10.1016/s0165-6147(00)89020-3. [DOI] [PubMed] [Google Scholar]
- 26.Kenakin T. Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- 27.Strange PG. Three-state and two-state models: Recent models of receptor function raises issues about the conformations of their coupling. Trends Pharmacol Sci. 1998;19:85–6. doi: 10.1016/s0165-6147(98)01175-4. [DOI] [PubMed] [Google Scholar]
- 28.Leff P. The two-state model of receptor activation. Trends Pharmacol Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- 29.Leff P, Scaramellini C, Law C, McKechnie K. A three-state receptor model of agonist action. Trends Pharmacol Sci. 1997;18:355–62. doi: 10.1016/s0165-6147(97)01105-x. [DOI] [PubMed] [Google Scholar]
- 30.Sonnenblick EH, Frishman WH. Cardiovascular Pharmacoptherapeutics: a Companion Handbook. New York: McGraw-Hill; 1998. p. 37. [Google Scholar]
- 31.Strange PG. Mechanisms of inverse agonism at G-protein-coupled receptors. Trends Pharmacol Sci. 2002;23:89–95. doi: 10.1016/s0165-6147(02)01993-4. [DOI] [PubMed] [Google Scholar]
- 32.Wade SM, Lan K, Moore DJ, Neubig RR. Inverse agonist activity at the α2a-adrenergic receptor. Mol Pharmacol. 2001;59:532–42. doi: 10.1124/mol.59.3.532. [DOI] [PubMed] [Google Scholar]
- 33.Langer SZ. 25 Years since the discovery of presynaptic receptors: present knowledge and future perspectives. Trends Pharmacol Sci. 1997;18:95–9. doi: 10.1016/s0165-6147(96)01034-6. [DOI] [PubMed] [Google Scholar]
- 34.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–85. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 35.Von Kugelgen I, Norenberg W, Koch H, Meyer A, Illes P, Starke K. P2-receptors controlling neurotransmitter release from postganglionic sympathetic neurones. Prog Brain Res. 1999;120:173–82. doi: 10.1016/s0079-6123(08)63554-2. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman BB, Taylor P. Neurotransmission: The autonomic and somatic motor nervous systems. In: Hardman JG, Limbird LE, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 10. New York: McGraw-Hill; 2001. pp. 115–53. [Google Scholar]
- 37.Langer SZ. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980;32:337–62. [PubMed] [Google Scholar]
- 38.Langer SZ, Lehmann J, Trendelenburg U, Weiner N. Handbook of Experimental Pharmacology: Catecholamine I. Vol. 85. Berlin: Springer-Verlag; 1988. pp. 429–507. [Google Scholar]
- 39.Starke K. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1981;21:7–30. doi: 10.1146/annurev.pa.21.040181.000255. [DOI] [PubMed] [Google Scholar]
- 40.Baldessarini RJ. Drugs and the treatment of psychiatric disorders. In: Hardman JG, Limbird LE, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 10. New York: McGraw-Hill; 2001. pp. 447–83. [Google Scholar]
- 41.Blier P. The pharmacology of putative early-onset antidepressant strategies. Eur Neuropsychopharmacol. 2003;13:57–66. doi: 10.1016/s0924-977x(02)00173-6. [DOI] [PubMed] [Google Scholar]
- 42.Sierra DA, Popov S, Wilkie TM. Regulators of G-protein Signaling in Receptor Complexes. Trends Cardiovasc Med. 2000;10:263–8. doi: 10.1016/s1050-1738(00)00072-4. [DOI] [PubMed] [Google Scholar]
- 43.Lim WK, Neubig RR. Selective inactivation of guanine-nucleotide-binding regulatory protein (G-protein) α and βγ subunits by urea. Biochem J. 2001;354:337–44. doi: 10.1042/0264-6021:3540337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neubig RR. Membrane organization in G-protein mechanisms. FASEB J. 1994;8:939–46. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- 45.Wieland T, Chen CK. Regulators of G-protein signalling: a novel protein family involved in timely deactivation and desensitization of signalling via heterotrimeric G proteins. Naunyn Schiedebergs Arch Pharmacol. 1999;360:14–26. doi: 10.1007/s002109900031. [DOI] [PubMed] [Google Scholar]
- 46.Yoo JH, Yang Y, Choi I, et al. Expression of novel splice variants of the G protein subunit, Goα, is tissue-specific and age-dependent in the rat. Gene. 2002;296:249–55. doi: 10.1016/s0378-1119(02)00866-1. [DOI] [PubMed] [Google Scholar]
- 47.Neubig RR, Siderovski DP. Regulators of G-protein signalling as new central nervous system drug targets. Nat Rev Drug Discov. 2002;1:187–97. [Google Scholar]
- 48.Zong H, Neubig RR. Regulator of G protein signaling proteins: Novel multifunctional drug targets. J Pharmacol Exp Ther. 2001;297:837–45. [PubMed] [Google Scholar]
- 49.Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001;24:479–86. doi: 10.1016/s0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- 50.Tekumalla PK, Calon F, Rahman Z, et al. Elevated levels of ΔFosB and RGS9 in striatum in Parkinson's disease. Biol Psychiatry. 2001;50:813–6. doi: 10.1016/s0006-3223(01)01234-3. [DOI] [PubMed] [Google Scholar]
- 51.Ji TH, Grossmann M, Ji I. G protein-coupled receptors. I. Diversity of receptor-ligand interactions. J Biol Chem. 1998;273:17299–302. doi: 10.1074/jbc.273.28.17299. [DOI] [PubMed] [Google Scholar]
- 52.Graeser D, Neubig RR. Compartmentation of receptors and guanine nucleotide-binding proteins in NG108-15 cells: Lack of cross-talk in agonist binding among the α2-adrenergic, muscarinic, and opiate receptors. Mol Pharmacol. 1992;43:434–43. [PubMed] [Google Scholar]
- 53.Saunders C, Limbird LE. Localization and trafficking of α2-adrenergic receptor suntypes in cells and tissues. Pharmacol Ther. 1999;84:193–205. doi: 10.1016/s0163-7258(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 54.Neubig RR. Specificity of receptor-G protein coupling: Protein structure and cellular determinants. Neuroscience. 1998;9:189–97. [Google Scholar]
- 55.Selbie LA, Hill SJ. G protein-coupled receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- 56.Chabre O, Conclin BR, Brandon S, Bourne HR, Limbird LE. Coupling of the α2A-adrenergic receptor to multiple G-proteins. J Biol Chem. 1994;269:5730–4. [PubMed] [Google Scholar]
- 57.Eason MG, Kurose H, Holt BD, Raymond JR, Liggett SB. Simultaneous coupling of α2A-adrenergic receptors s to two G proteins with opposing effects. J Biol Chem. 1992;267:15795–801. [PubMed] [Google Scholar]
- 58.Sanders-Bush E, Mayer SE. 5-hydroxytryptamine (serotonin). Receptor agonists and antagonists. In: Hardman JG, Limbird LE, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 10. New York: McGraw-Hill; 2001. pp. 269–90. [Google Scholar]
- 59.Leonard BE. The comparative pharmacology of new antidepressants. J Clin Psychiatry. 1993;54(Suppl):3–15. [PubMed] [Google Scholar]
- 60.Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–60. doi: 10.1016/s0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- 61.Brotchie JM. CB1 cannabinoid receptor signalling in Parkinson's disease. Curr Opin Pharmacol. 2003;3:1–8. doi: 10.1016/s1471-4892(02)00011-5. [DOI] [PubMed] [Google Scholar]
- 62.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent R. e. at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- 63.Brink CB, Wade SM, Neubig RR. Agonist-directed trafficking of porcine α2A-adrenergic receptor signaling in CHO cells. l-Isoproterenol selectively activates Gs. J Pharmacol Exp Ther. 2000;294:539–47. [PubMed] [Google Scholar]
- 64.Kenakin T. Agonist-receptor efficacy. II. agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995;16:232–8. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- 65.Brink CB. Protean behavior by agonists. Role of ‘agonist-directed trafficking of receptor signaling’. Trends Pharmacol Sci. 2002;23:454–5. doi: 10.1016/s0165-6147(02)02079-5. [DOI] [PubMed] [Google Scholar]
- 66.Kenakin T. Pharmacological proteus? Trends Pharmacol Sci. 1995;16:256–8. doi: 10.1016/s0165-6147(00)89037-9. [DOI] [PubMed] [Google Scholar]
- 67.Roth BL, Willins DL, Kroeze WK. G protein-coupled receptor (GPCR) trafficking in the central nervous system: relevance for drugs of abuse. Drug Alcohol Depend. 1998;51:73–85. doi: 10.1016/s0376-8716(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 68.Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT2A receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–51. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 69.Von Zastrow M. Endocytosis and downregulation of G protein-coupled receptors. Parkinsonism Relat Disord. 2001;7:265–71. doi: 10.1016/s1353-8020(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 70.Tsao PI, von Zastrow M. Diversity and specificity in the regulated endocytic membrane trafficking of G-protein-coupled receptors. Pharmacol Ther. 2001;89:139–47. doi: 10.1016/s0163-7258(00)00107-8. [DOI] [PubMed] [Google Scholar]
- 71.Polastron J, Meunier JC, Jauzac P. Chronic morphine induces tolerance and desensitization of mu-opioid receptor but not down-regulation in rabbit. Eur J Pharmacol. 1994;266:139–46. doi: 10.1016/0922-4106(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 72.Chen JJ, Dymshitz J, Vasko MR. Regulation of opioid receptors in rat sensory neurons in culture. Mol Pharmacol. 1997;51:666–73. doi: 10.1124/mol.51.4.666. [DOI] [PubMed] [Google Scholar]
- 73.Patel MB, Patel CN, Rajashekara V, Yoburn BC. Opioid agonists differentially regulate µ-opioid receptors and trafficking proteins in vivo. Mol Pharmacol. 2002;62:1464–70. doi: 10.1124/mol.62.6.1464. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Ferguson SG, Barrak LS, et al. Role for G protein-coupled receptor kinase in agonist-specific regulation of µ-opioid receptor responsiveness. Proc Natl Acad Sci USA. 1998;95:7157–62. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diaz A, Ruiz F, Florez J, Pazos A, Hurle MA. Regulation of dihydropyridine-sensitive Ca++ channels during opioid tolerance and supersensitivity in rats. J Pharmacol Exp Ther. 1995;274:1538–44. [PubMed] [Google Scholar]
- 76.Cahill CM, Morinville A, Hoffert C, O'Donnel D, Beaudet A. Up-regulation and trafficking of δ opioid receptor in a model of chronic inflammation: Implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- 77.Evans WE, McLeod HL. Pharmacogenomics – drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–49. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 78.Johnson JA, Terra SG. Beta-adrenergic receptor polymorphisms: cardiovascular disease associations and pharmacogenetics. Pharm Res. 2002;19:1779–87. doi: 10.1023/a:1021477021102. [DOI] [PubMed] [Google Scholar]
- 79.Dishy V, Sofowora GG, Xie HG, Kim RB, Byrne DW, Stein CM, Wood AJ. The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030–5. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- 80.Lima JJ, Thomason DB, Mohamed MH, Eberle LV, Self TH, Johnson JA. Impact of genetic polymorphisms of the beta2-adrenergic receptor on albuterol bronchodilator pharmacodynamics. Clin Pharmacol Ther. 1999;65:519–25. doi: 10.1016/S0009-9236(99)70071-8. [DOI] [PubMed] [Google Scholar]
- 81.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, Cooper DM, Fahy JV, Fish JE, Ford JG, Kraft M, Kunselman S, Lazarus SC, Lemanske RF, Jr, Martin RJ, McLean DE, Peters SP, Silverman EK, Sorkness CA, Szefler SJ, Weiss ST, Yandava CN National Heart, Lung and Blood Institute's Asthma Clinical Research Network. Effect of polymorphism of the beta (2) -adrenergic receptor on response to regular use of albuterol in asthma. Int Arch Allergy Immunol. 2001;124:183–6. doi: 10.1159/000053705. [DOI] [PubMed] [Google Scholar]
- 82.Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;34:349–56. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- 83.Iiri T, Farfel Z, Bourne HR. G-protein diseases furnish a model for the turn-on switch. Nature. 1998;394:35–8. doi: 10.1038/27831. [DOI] [PubMed] [Google Scholar]