Abstract
Background and methods
Loop diuretic therapy is an essential part of chronic systolic heart failure (CH)F management, yet response to treatment can be variable. We analysed diuretic responsiveness in 39 stable patients with CHF in the community over 2 years. We measured serum ACE as a marker of adherence to ACE inhibitor therapy and urinary furosemide as a marker of diuretic adherence and action. Patients’ clinical outcome was stable and not hospitalized (Group 0); alive but hospitalized (Group 1); or dead during follow up (Group 2).
Results
Prescribed furosemide dose was variable (range 20–370 mg generally once daily) and progressive dose increments were common. Failed furosemide adherence (defined as <10% of a dose excreted in 24 h urine where normal average excretion =50% of an oral dose) during static prescribed dosing was infrequent relative to all days of therapy; yet was equally common across all outcome groups. Furosemide non-adherence appeared to be independent of non-adherence with ACE inhibitor (as marked by serum ACE activity >20 U l−1) treatment. Furosemide responsiveness (mm of sodium excreted per mg furosemide in urine) showed no relationship to prescribed dose and paradoxically tended to rise in patients with higher basal aldosterone concentrations. Furosemide responsiveness fell by outcome class despite increased dose. Within-patient responsiveness remained relatively constant although highly variable between individuals.
Conclusions
Furosemide responsiveness varied greatly between individuals but was constant within an individual. Non-adherence with furosemide was less common among those who died and appeared to occur at different time points from non-adherence with ACE inhibitor treatment, which was slightly more common in all outcome groups. Patients who died were prescribed higher furosemide doses and had greater furosemide excretion yet had similar sodium excretion. The main factor in response to chronic furosemide therapy was intrarenal diuretic resistance. Gross non-adherence was less important.
Keywords: aldosterone, clinical outcome, diuretic resistance, furosemide responsiveness, non-adherence
Introduction
Chronic systolic heart failure (CHF) is a major healthcare cost across the world [1]. Diuretic therapy is a routine part the management of the majority of patients with CHF [2]. Diuretics have variable effects on the neurohormonal responses of heart failure [3], and have the potential either to reduce readmission rates [4] or to generate iatrogenic morbidity through renal dysfunction [5, 6]. Minor changes can induce large fluctuations in haemodynamic status [7]. Despite this, many patients manipulate diuretic therapy in response to symptoms and/or body weight [8], although the impact of this has not been defined in controlled circumstances [9]. The decompensation of CHF resulting in hospital admission is a complex process [10] but may in part involve changes in diuretics. Episodes of diuretic resistance [11] might explain some episodes of hospitalization [12]. Equally, non-adherence with diuretic treatment could also play a part in this process. The natriuretic response to furosemide can be defined by relating the urinary concentration of drug to the sodium excretion in the same sample of urine. There are no data examining how furosemide responsiveness varies chronically over time in CHF patients. It is not clear whether increased doses used in patients with CHF or decompensation are associated with a proportionately higher response to furosemide, although diuretic resistance is a marker of poor outcome [13].
In this study we report longitudinal patterns of within- and between-patient furosemide responses in CHF over a 2-year follow-up period. We examine the incidence of non-adherence to furosemide, poor bioavailability of furosemide and variability in furosemide responsiveness over time for a link between these factors and hospital admissions. We also wished to see if non-adherence with furosemide occurred at the same time as the patients were non-adherent with their ACE inhibitor, and finally if diuretic resistance was simply related to the counter-regulatory effect of aldosterone.
Patients and methods
The study population consisted of 39 patients [29 men/10 women, mean age 68 (SD 8) years] with proven systolic left ventricular dysfunction recruited prospectively from general medical outpatient clinics or a specialized heart failure clinic. The majority had an index myocardial infarction and all had stable CHF for at least 18 months (clinically stable for 3 months preceding the study) with New York Heart Association (NYHA) class II to IV symptomatology. Heart failure was due to coronary artery disease (89%) or idiopathic dilatated cardiomyopathy (11%). Evidence of impaired left ventricular systolic function was documented in all by ejection fraction (EF) of <40% (either by radionuclide ventriculography and/or quantitative echocardiography). No patient had had an acute coronary event within the last 6 months. All patients received therapy with ACE inhibitor throughout and seven received additional digoxin. β-Blockade was not routine at the time of the study, although 14 subjects did receive this as part of their treatment. No changes were made in these aspects of therapy (Table 1). The local research and ethics committee (Tayside Regional Research and Ethics Committee) approved the study prior to recruitment. All patients gave written and informed consent to participate in the study before enrolling.
Table 1.
Baseline characteristics of the study population arranged by eventual outcome group on follow up
| Group 0 No admissions Alive at follow up | Group 1 Admitted Alive at follow up | Group 2 Died during follow up | |
|---|---|---|---|
| N | 15 | 11 | 13 |
| Male (n) | 9 | 9 | 11 |
| Age | 70 ± 9 | 67 ± 7 | 68 ± 5 |
| Ischaemic aetiology | 13 | 11 | 11 |
| Non-ischaemic aetiology | 2 | 2 | |
| LVEF (mean ± SD) | 29 ± 5 | 32 ± 4 | 27 ± 7 |
| Therapy (n = treated minimum 1 month at some point) | |||
| Furosemide (n) | 15 | 11 | 13 |
| Thiazide (n) | 3 | 1 | 2 |
| Spironolactone (n) | 2 | 0 | 1 |
| Amiloride (n) | 2 | 2 | 3 |
| ACEI (n) | 15 | 11 | 13 |
| Beta blocker (n) | 8 | 4 | 2 |
| Digoxin (n) | 2 | 2 | 3 |
| Nitrate (n) | 7 | 8 | 4 |
| Calcium channel blocker (n) | 3 | 1 | 0 |
| Aspirin (n) | 12 | 9 | 9 |
Patient review
Patients were seen at intervals of 4–6 weeks in their own home (21–23 visits per patient over 2 years), having taken all their routine cardiac medications as prescribed by their general medical practitioner. Changes in drug therapy from the previous visit were noted and those therapies actually taken carefully documented. After recording body weight, the patient was rested supine for at least 20 min. Blood pressure was documented supine and blood samples were taken for electrolytes, hormone assay and diuretic analysis. A spot sample of urine was collected and a 24-h urinary collection started thereafter. Both urine collections (spot sample and 24-h collection) and blood were assayed for electrolytes and furosemide by standard methodology (see below). Patients completed the Minnesota Living with Heart Failure (LHF) questionnaire [14] at each visit calculating the total, emotional and physical scores longitudinally.
Analyses
Data grouping
Where relevant, results were grouped in accord with the prospective clinical outcome of the patients concerned. Patients were either stable in the community for the duration of follow-up (Group 0), or were alive at the end of the study but had been admitted to hospital for decompensated CHF at some point during follow-up (Group 1) or had died during the course of the programme (Group 2). A within-patient mean was calculated for each variable for each patient and then summarized per outcome. Variance is given for the outcome group mean unless otherwise stated. Patients who died in general did not die in close temporal relationship to a study visit. It was not possible therefore to make inference from the subsequent analysis of the relationship to an individual death. Equally with hospital admission data, again study sampling was not done in relation to the process of hospitalization.
Blood and urinary furosemide
Urine furosemide concentrations (in both spot and 24-h samples) were determined using high-performance liquid chromatography (HPLC) with fluorescence detection as described previously [15, 16]. Spot samples were used to determine furosemide responsiveness and 24-h collections to define excretion. After the addition of the internal standard (metolazone 1500 ng), 0.25 ml of urine was mixed with 2 ml of acetonitrile, and centrifuged at 2800 r.p.m. (∼3G) for 10 min. The supernatant was transferred to a clean test tube, evaporated to dryness using a Savant Roto-vap (Thermo Savant, Waltham, MA, USA), and reconstituted with 150 µl of mobile phase (35 : 65 acetonitrile: 50 mm sodium acetate, pH 3.6), of which a portion was injected into an HPLC. Furosemide and the internal standard were separated using a 5-µm Beckman (Beckman Coulter Fullerton, CA, USA) Ultrasphere™ (25 cm × 4.6 mm inner diameter) C-18 equipped with a 2-cm guard column. The mobile phase was delivered at a rate of 1 ml min−1, and the eluate was monitored at an emission wavelength of 378 nm after excitation at a wavelength of 234 nm using a Hewlett Packard (Agilent Technologies Palo Alto, CA, USA) 1146A fluorescence detector. Interassay coefficient of variation was <4% and 6% at furosemide concentrations of 0.3 µg ml−1 and 16 µg ml−1.
Serum and urinary electrolytes
Spot samples and aliquots of 24-h urine were assayed on the day of collection for sodium, potassium urea and creatinine using standard autoanalyser technology in our routine hospital clinical chemistry service operating with quality control standards (± 2.5% interassay coefficient of variation).
Hormones
Serum ACE activity
Angiotensin converting enzyme was determined using direct radioimmunoassay [17] operating within 2% intra- and 4% interassay coefficient of variation and with a lower limit of detection of 0.5 Enzymatic units (EU) l−1.
Plasma aldosterone
Plasma aldosterone was measured using a solid-phase (coated tube) radioimmunoassay technique (DPC Coat-a-Count assay; DPC plc, Llanberis, Caernarfon, Gwynedd, UK).
Statistical analysis
Statistical analysis was conducted using Minitab (version 12). All data are presented as mean ± 1 SD. Mean values at different time points were compared with a general linear model and repeated measures anova for normally distributed values and the Kruskal–Wallis test for those variables that were not normally distributed (aldosterone). Values of P < 0.05 were considered to be statistically significant.
Results
General
Of the total cohort of 39 subjects entering the programme, 15 patients completed the period of follow-up alive with no CHF hospitalization (Group 0); 11 were alive at the end but had been hospitalized for CHF decompensation (Group 1); and 13 patients died during follow-up (Group 2).
Patterns of diuretic use
All 39 patients were treated with oral furosemide during follow-up (two were transferred from bumetanide to furosemide after hospital admission). Three patients were, for short periods, prescribed bumetanide alone (results not included in analysis) and seven patients received amiloride in addition to furosemide at some point, in the form of combination treatment (co-amilofruse =furosemide 40 mg +amiloride 5 mg). This combination does not affect total natriuresis during chronic dosing and therefore does not affect the interpretation of furosemide responsiveness [18]. Three patients received additional spironolactone and one had short-term bendrofluazide in addition to loop diuretic (these results are not included in analysis).
The variations in diuretic dose (Table 1) were striking (range 20–370 mg daily). The highest doses were found in the patients who died during follow-up (Group 2). Predictably the prescribed daily diuretic dose was most constant in the patients who were not admitted (Group 0). Eleven of 15 in this group had no change in diuretic treatment (neither increase or decrease) during the follow-up period. Four in this group had dose increments and one had the addition of a potassium-sparing agent. In Group 1 (admitted for CHF decompensation), only four of the 11 patients maintained a constant dose and none had a dose reduction. Seven patients had dose increments, three of which had the addition of amiloride. Eight of the 13 patients who died during follow-up (Group 2) had dose increments, including two who had a further diuretic agent added.
The majority of patients in the programme (30/39) were prescribed once-daily diuretic treatment. Patients in Group 2 (died during follow-up) often received single daily doses exceeding 160 mg day−1 exceeding maximum dose responsiveness in terms of diuresis and natriuresis. Prescribed daily dose was not associated with prior or concurrent episodes of symptomatic decompensation or hospital admissions. Dosage increments had no relationship to prior or concomitant symptomatic state as indicated by longitudinal Minnesota LHF questionnaire scores. The numbers of patients in Group 2 (died during follow-up) fell with duration of follow-up. Symptomatic score tended to remain static throughout follow-up, regardless of hospitalization state or current body weight (Figure 1). Dose increments had little impact on net natriuresis, kaliuresis or on furosemide responsiveness (Table 2).
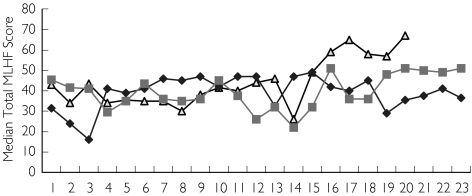
Figure 1.
Longitudinal total Minnesota Living with Heart Failure scores in patients who remained stable (Group 0). Group 2 (▵), Group 0 (♦), Group 1 ( )
)
Table 2.
Comparison of mean electrolyte and hormone measurements during the study period in terms of outcome Group. Group 0 = not hospitalised, Group 1 = hospitalised but no deaths, Group 2 = Died during follow up. (* = p < 0.05 vs Group 0)
| Group 0 (n =15) | Group 1 (n = 10) | Group 2 (n = 13) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome Group Variable | Mean value ± 1SD | Range of mean values | Range of Intra-subject SD | Mean value ± 1SD | Range of mean values | Range of Intra-subject SD | Mean value ± 1SD | Range of mean values | Range of Intra-subject SD |
| Serum Sodium (mM) | 139.4 ± 1.6 | 136–141 | 1.9–3.5 | 138.7 ± 3.9 | 129–147 | 1.2–5.2 | 138.7 ± 3.4 | 134–144 | 1.3–6.3 |
| Serum Potassium (mM) | 4.8 ± 0.3 | 4–5.2 | 0.3–0.7 | 5.0 ± 0.4 | 4.4–5.7 | 0.0–0.8 | 4.9 ± 0.6 | 3.2–5.5 | 0.1–0.7 |
| Urea (mM) | 7.1 ± 2.3 | 3.6–10.7 | 0.4–1.8 | 7.6 ± 2.3 | 5.1–12.2 | 0.8–2.9 | 9.5 ± 1.4* | 4.3–17.2 | 0.2–2.6 |
| Creatinine (µM) | 129.9 ± 41.7 | 67–211 | 6–42.3 | 142.2 ± 37 | 104–220 | 10.5–44.2 | 162.8 ± 43* | 95–246.8 | 5.1–33 |
| Urinary sodium (mM/24hr) | 88.2 ± 35 | 8.6–219.2 | 114 ± 59 | 14–281 | 102.3 ± 49 | 24–316 | |||
| Urinary Potassium (mM/24hr) | 29.2 ± 9 | 7.7–66.4 | 30.6 ± 10 | 10.4–64.6 | 28.3 ± 11 | 6.3–72.4 | |||
| ACE activity (IEU/L) | 10.8 ± 9.8 | 4.6–45.7 | 1.6–13 | 8.5 ± 3.8 | 5–19.8 | 1.7–13.9 | 14.5 ± 14.8 | 3.6–45 | 0–29.3 |
| BNP (pg/ml) | 48.2 ± 28.5 | 19.3–99.7 | 7.3–48.1 | 39.8 ± 28 | 14.6–103 | 5.1–58.9 | 100.2 ± 44.3* | 15.8–161 | 8–131.3 |
| Aldosterone (pg/ml) | 161.8 ± 130.8 | 22.7–497.4 | 0–156.4 | 111.7 ± 62.7 | 30.4–219.2 | 0–151.6 | 230.2 ± 276 | 50.9–1141.7 | 0–143.1 |
Drug analyses
Twenty-four-hour urinary furosemide excretion
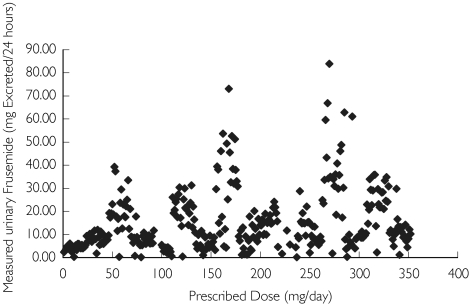
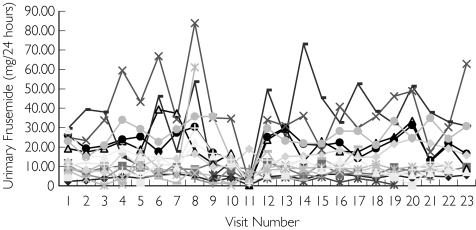
Des-pite large interindividual variability there was an approximately linear relationship (r = 0.57) between prescribed dose and measured urinary furosemide excretion (Figure 2). Comparison of measurements within subjects revealed important information. While between-subject concentrations tended to vary widely (and regardless of prescribed dose), within-subject measurements much showed less (∼ 20%) variability (Figure 3), suggesting that within-subject kinetics of urinary excretion of furosemide was relatively constant.
Figure 2.
Relationship between prescribed dose and excreted furosemide (mg 24 h−1) for Group 0 patients (all data points included)
Figure 3.
Longitudinal urinary furosemide excretion within subjects in Group 0
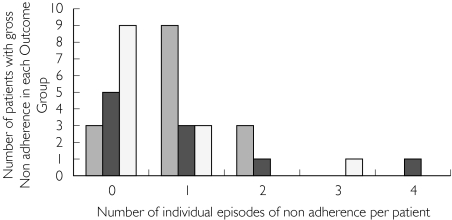
At certain time points in individual patients, it was clear that 24-h urinary furosemide excretion was at the lower limit of assay detection (< 1 mg furosemide excreted in urine over 24 h or less than 10% of the dose with constant or increased prescribed dose) despite continued treatment. These values are not realistically compatible with prescribed drug intake and were therefore considered to reflect non-adherence. The number of individual episodes of non-adherence (as defined) with furosemide prescription is illustrated in Figure 4. Over the period of study they were infrequent but demonstrable in these terms.
Figure 4.
Frequency of gross non-adherence with furosemide over 2 years’ (23 measurements) follow-up in each outcome group. Group 0 (n = 15) ( ), Group 1 (n = 10) (
), Group 1 (n = 10) ( ), Group 2 (n = 13) (□)
), Group 2 (n = 13) (□)
Thus 11 of 15 patients in Group 0 showed failure of adherence at some point during their 21–23 visits for follow-up. Of these 11, nine patients had one episode in isolation, two patients had two episodes of failed adherence. Six of 11 patients in Group 1 (hospitalized for heart failure during follow-up) showed episodes of adherence failure. Four patients showed one episode in isolation. One patient had four episodes and one had eight episodes of adherence failure. Five of 13 patients in Group 2 (died in follow-up) showed adherence failure at some point. Three patients showed one episode each. One patient had two and another patient had three episodes of compliance failure (Figure 4 and Table 3). There was no statistical difference in the number of episodes of gross furosemide non-adherence between the three outcome groups. We conclude that this was unlikely to be a determinant of outcome (hospitalization or death) in CHF patients.
Table 3.
Summary Individual Furosemide mean dose and response data summarised in terms of outcome Group. Group 0 = not hospitalised, Group 1 = hospitalised but no deaths, Group 2 = Died during follow up. (* = p < 0.05 vs Group 0 and baseline)
| Variables baseline group mean | Follow up group mean | ||||||
|---|---|---|---|---|---|---|---|
| Outcome group | Prescribed Dose (mg/24hr) | Prescribed Dose (mg/24hr) | Median | Max | Min | Total N = | Group Sd |
| Group 0 | 57.3 | 75.7 | 40.0 | 240.0 | 20.0 | 301 | 53.1 |
| Group 1 | 48.6 | 55.1 | 40.0 | 200.0 | 20.0 | 162 | 37.6 |
| Group 2 | 93.5 | 141.5* | 80.0 | 500.0 | 40.0 | 95 | 110.4 |
| Urinary Furosemide Concentration (mcg/ml) | |||||||
| Group 0 | 6.8 | 8.4 | 5.9 | 40.2 | 0.1 | 329 | 7.4 |
| Group 1 | 3.9 | 4.1 | 3.1 | 53.2 | 0.0 | 218 | 3.7 |
| Group 2 | 7.2 | 11.4 | 8.9 | 81.3 | 0.1 | 117 | 11.1 |
| Urinary Furosemide Excretion (mcg/min) | |||||||
| Group 0 | 8.5 | 10.5 | 7.0 | 58.3 | 0.1 | 338 | 81.8 |
| Group 1 | 4.6 | 5.9 | 4.7 | 44.0 | 0.2 | 177 | 4.8 |
| Group 2 | 11.2 | 13.2 | 10.4 | 57.4 | 0.1 | 116 | 10.3 |
| Furosemide Responsiveness (mM/mg) | |||||||
| Group 0 | 21.3 | 21.4 | 7.5 | 1005.2 | 0.0 | 310 | 81.4 |
| Group 1 | 26.1 | 27.7 | 12.5 | 384.1 | 0.0 | 158 | 46.9 |
| Group 2 | 18.4 | 16.7 | 7.0 | 568.3 | 0.0 | 115 | 56.1 |
Serum ACE suppression and diuretic compliance
We have previously shown that serum ACE activity can be used in CHF to identify non-adherence with long-acting chronic angiotensin converting enzyme inhibitor (ACEI) therapy [19]. Therefore the timing of individual episodes of furosemide non-adherence as identified by urinary assay was compared with the concomitant serum sample assayed for serum ACE activity in order to see if non-adherence to both was coincidental. The results are summarized in Table 4. We found little correlation between the patterns of non-adherence with the two groups of drugs.
Table 4.
Data showing those patients (who had concomitant data collection) suggesting Non- Adherence with an ACE inhibitor and non-adherence with Furosemide and how often they occurred together
| ACE Inhibitor Non-adherence ACE activity > 20EU/L | Diuretic Non-adherence Furosemide <10% average | Concomitant non adherence with both the ACEI and diuretic Rate (%) | |||
|---|---|---|---|---|---|
| Outcome group Criterion of Non Adherence → Elevated | Number of episodes | Number of Patients | Number of episodes | Number of Patients | |
| Group 0 n = 14 | 11 | 8 | 15 | 11 | 0/26 (0) |
| Group 1 n = 10 | 25 | 7 | 9 | 5 | 1/34 (2.9) |
| Group 2 n = 11 | 15 | 3 | 6 | 4 | 1/21 (4.7) |
In Group 0 from 351 sampling points collected from 14 patients (one Group 0 patient was treated with captopril and thus had no interpretable ACE suppression data), there were only 15 episodes of furosemide non-adherence that occurred in 11 of the 15 patients. Four patients had no evidence of failure of adherence to furosemide during the follow-up visits. None of these episodes of furosemide non-adherence coincided with failure of adherence to ACE inhibitor treatment in the same patients. There were 11 samples from eight patients where serum ACE was markedly elevated and not compatible with adherence to their ACE inhibitor prescription.
In Group 1 (patients admitted during follow-up) from 235 sampling points in 11 patients, there were nine episodes of furosemide non-adherence in five of the 11 patients. In this group there were 25 samples from seven patients where serum ACE was elevated and not compatible with adherence to ACE inhibitor prescription. In only one of the episodes was there concordant failure of adherence of both furosemide and ACE inhibitor.
In Group 2 (died during follow-up) from 132 sampling points in 11 patients (two Group 2 patients treated with captopril do not have interpretable ACE suppression data due to the poor binding affinity of this drug), there were six episodes of furosemide non-adherence in four of the 11 patients. There were 15 samples from three of the patients where serum ACE was elevated and not compatible with adherence to the ACE inhibitor prescription. In only one episode was there concordant failure of furosemide and ACE inhibitor adherence.
Diuretic responsiveness
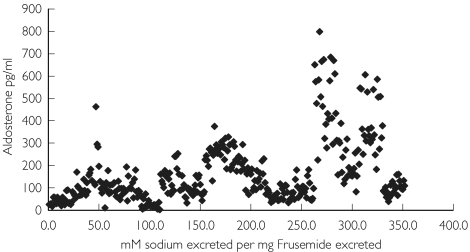
Within-patient furosemide responsiveness, assessed as sodium excretion relative to amount of urinary furosemide in the spot sample of urine (mm of sodium excreted per mg of furosemide excreted in the same urine), was relatively constant. Within-patient values for furosemide responsiveness were more consistent than the pattern of total urinary drug excretion (Table 2). There was no simple relationship between responsiveness and prescribed diuretic dose. Responsiveness if anything tended to be greater in patients where concomitant aldosterone concentrations were higher. This was most evident in patients alive and not admitted during follow-up (Group 0), but was evident in all outcome groups, and appeared to have a tri-modal distribution (Figure 5). The outcome grouped mean (± SD) responsiveness (outliers included) showed a clear but nonstatistically significant trend for responsiveness to be reduced in those patients who died during follow-up (Table 3).
Figure 5.
Furosemide responsiveness vs. basal aldosterone in patients in Group 0
Discussion
The clinical pharmacology of furosemide is well defined and the most important determinant of response is the amount of drug reaching the renal tubule [20]. Drug effect can be quantified by the ratio of sodium excretion relative to urinary diuretic concentration [21]. Nearly all patients with significant left ventricular (LV) systolic dysfunction will require loop diuretic treatment as part of standard therapy. Attempts at diuretic withdrawal are successful only in a small minority in this patient group [22] and this is often complicated by short-term antinatriuresis. Despite this role, there are no data on the longitudinal impact of chronic loop diuretic therapy despite the repeated assumption that many CHF re-admissions are due to disease-related fluid retention or to problems with non-adherence and/or resistance to diuretic therapy [13, 23].
In this study we observed the community-based use of furosemide in otherwise stable CHF. Individual reasons for hospital referral and admission in the CHF population can be very variable and many admissions are not directly due to cardiac decompensation [24] but may represent intercurrent illness social instability or practical problems surrounding drug therapy. In the same way, death can come suddenly without warning in the community or be related to differing factors in the CHF population hospitalized prior to their final illness [25]. In this project we attempted primarily to gain insight into whether the factors of diuretic resistance, poor diuretic absorption and/or diuretic non-adherence were linked to outcome. Importantly, patients in the study were not aware of the pattern of drug monitoring and were managed routinely by their community physician. This remains the main management paradigm for the vast majority of CHF patients and is therefore by far the most appropriate setting to complete such a study.
Within the follow-up period there was a progressive rise in prescribed daily dose of diuretic. Quality of life and symptom indices (MLHF scores) were static between outcome groups and unaffected by this change (Figure 1). Diuretic responsiveness within an individual patient remained constant despite variation in prescribed dose and regardless of outcome. Patients who died during follow-up (Group 2) were in general treated with higher doses and these doses were reaching their site of action as shown by the higher urine furosemide levels. However, this failed to produce a proportionately greater natriuresis or kaliuresis. This suggests that diuretic resistance (impaired sodium excretion per unit drug reaching the tubule) was present. Poor furosemide absorption might still have had a role, as double the prescribed furosemide dose in Group 2 led to only a 35% increase in mean urinary furosemide. It has been known for many years that furosemide is erratically absorbed in CHF [26]. Thus, the effectiveness of upward titration of dose in these patients is blunted.
Higher prescribed doses were associated with poorer renal function in those who died during follow-up. One would ordinarily presume that this renal deterioration occurs because of diuretic-induced volume depletion, but in this study a higher dose did not produce a proportionally higher delivery of either furosemide or sodium into the urine. While it seems unlikely that volume depletion alone was the cause of the decreased renal function, it may equally also be a surrogate for the severity of the patient's overall disease [6, 13].
Non-adherence to prescribed therapy is an important and practical fact of clinical response, yet is little studied. We have examined this issue before in CHF using different techniques in relation to ACE inhibitor therapy [19]. There are no perfect answers in this area as to how to define the problem. We feel our assumption that minimal 24-h urine furosemide excretion on the same dose with continued treatment equates to gross non-adherence, is a reasonable deduction and is valid. We are not able to exclude partial non-adherence without detailed pharmacokinetic modelling or by utilizing techniques such as computerized caps that register when a medication container is opened. The latter are not fool proof and easily open to manipulation. Our surrogate was gross non-adherence (nominally <10% of average 24-h urinary furosemide excretion), and this is a degree of non-adherence that would be expected to have clinical significance. Excretion rates of furosemide at this level will have no meaningful diuretic effect and are associated if anything with antinatriuresis. Overall, we found the frequency of such events during repeated measurements to be reassuringly rare. Clearly, by point sampling we may have missed important episodes. Diuretic non-adherence could be expected to have important effects on clinical state through missed doses, rebound antinatriuretic effects [27] and also important effects on the diuretic interaction with ACE inhibition [28].
The patients in this study were not being instructed to vary either their dose of diuretic (or ACE inhibitor) informally [8], which is an increasingly common yet formally untested pattern [29]. The fact that the isolated episodes of non-adherence with furosemide and non-adherence with an ACE inhibitor did not appear to correspond is intriguing. One simple explanation is that patients stop taking whichever supply has run out in their bathroom cabinet. Alternatively, it may be that they dislike the diuretic effect of furosemide and deliberately omit only their diuretic when they are likely to be away from home. Our visits were all done in the patients’ homes to avoid this being a complicating factor. Non-adherence with diuretic treatment from previous work does not appear to relate to social class [30].
While much is made of the lack of controlled trial evidence of efficacy for diuretic therapy reducing morbidity and mortality in CHF, there are some encouraging data that suggest diuretic strategies may be more important than previously anticipated [4]. The use of spironolactone in severe heart failure is complex [31], but some workers have suggested that at least some of the benefits are mediated by improved diuresis [32]. In our small study, non-adherence did not seem to relate to outcome. Thus, its importance might be overestimated. However, the data here show that while episodes of gross furosemide non-adherence are quite rare, this non-adherence occurs at a different time from non-adherence with an ACE inhibitor and could have a potentially vital impact on both the efficacy of hormone suppression and also on the net diuresis [32, 33]. Our data raise the notion that patients could have periods of time when they will be taking furosemide without the ACE inhibitor, and vice versa. The variability in natriuresis created by this could easily lead to fluid retention and hospitalization. This possibility should be examined in a larger prospective group and with samples secured rapidly on admission and before pulsed diuresis.
Loop diuretics have long been accepted to have a powerful stimulant effect on renin and aldosterone [34] and elevations in aldosterone would be expected to limit the natriuretic response to furosemide. Because of this we were keen to examine the relationship between diuretic responsiveness and endogenous aldosterone. Using repeated measures from the whole patient sample, there appeared to be a positive rather than the expected negative relationship between aldosterone and furosemide responsiveness. This implies that the effect of furosemide is sufficient to overwhelm the sodium retaining effect of aldosterone. While it remains likely that aldosterone does in part restrain the natriuretic effect of a loop diuretic, our data suggest that the furosemide can overcome this despite the fact that raising the diuretic dose probably further elevates aldosterone.
In summary, this community-based project following stable patients under routine conditions shows the response to chronic furosemide varies greatly between individuals but is reasonably constant within an individual subject. Non-adherence with treatments among CHF patients seems to be a selective process and is overall rare. We found non-adherence was, if anything, less common among those who died. While patients who died were prescribed higher furosemide doses and had greater furosemide excretion, they had no greater level of sodium excretion. Diuretic resistance is likely to be a key factor. We did not find the level of aldosterone to be a major cause of diminished diuretic response. Our data did not suggest that occasional gross non-adherence was a factor in determining outcome in CHF.
Acknowledgments
The authors thank Jessamine Robson and Amanda Duncan for nursing support and the British Heart Foundation and Scottish Office for Financial support to complete these studies.
References
- 1.Schrier RW, Abdullah JG, Weinberger HH, Abraham WT. Therapy of heart failure. Kidney Int. 2000;57:1418–25. doi: 10.1046/j.1523-1755.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- 2.Brater DC. Diuretic therapy. New Engl J Med. 1998;339:387–95. doi: 10.1056/NEJM199808063390607. [DOI] [PubMed] [Google Scholar]
- 3.Van Zweiten PA. Neuroendocrine effects of diuretics in heart failure. Br Heart J. 1994;72(Suppl):51–3. doi: 10.1136/hrt.72.2_suppl.s51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosin J, Diez J. Effects of type of diuretic on mortality in patients with chronic congestive heart failure. Eur J Heart Failure. 2000;2(Suppl. 2):20–1. [Google Scholar]
- 5.Channer KS, McLean KA, Lawson-Matthew P, Richardson M. Combination diuretic therapy treatment in severe heart failure: a randomised controlled trial. Br Heart J. 1994;71:146–50. doi: 10.1136/hrt.71.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith GL, Vaccarino V, Kosiborod M, et al. Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail. 2003;9:13–25. doi: 10.1054/jcaf.2003.3. [DOI] [PubMed] [Google Scholar]
- 7.Braunschweig F, Linde C, Eriksson MJ, Hofman-Bang C, Ryden L. Continuous haemodynamic monitoring during withdrawal of diuretics in patients with congestive heart failure. Eur Heart J. 2002;23:59–69. doi: 10.1053/euhj.2001.2690. [DOI] [PubMed] [Google Scholar]
- 8.Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary home based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354:1077–83. doi: 10.1016/s0140-6736(99)03428-5. [DOI] [PubMed] [Google Scholar]
- 9.Broadley AJM, Marshall AJ. Self-administration of Metolazone reduces readmissions with decompensated congestive cardiac failure. Heart. 1999;82:397–8. doi: 10.1136/hrt.82.3.397a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felker GM, Adams KF, Jr, Konstam MA, O'connor CM, Gheorghiade M. The problem of decompensated heart failure: nomenclature, classification, and risk stratification. Am Heart J. 2003;145(2 Suppl):S18–S25. doi: 10.1067/mhj.2003.150. [DOI] [PubMed] [Google Scholar]
- 11.Brater DC. Resistance to loop diuretics. Why it happens and what to do about it. Drugs. 1985;30:427–43. doi: 10.2165/00003495-198530050-00003. [DOI] [PubMed] [Google Scholar]
- 12.Opasich C, Febo O, Riccardi PG, et al. Concomitant factors of decompensation in chronic heart failure. Am J Cardiol. 1996;78:354–7. doi: 10.1016/s0002-9149(96)00294-9. [DOI] [PubMed] [Google Scholar]
- 13.Neuberg GW, Miller AB, O'Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144:31–8. doi: 10.1067/mhj.2002.123144. [DOI] [PubMed] [Google Scholar]
- 14.Rector TS, Kubo SH, Cohn JN. Patient's self-assessment of their congestive heart failure Part 2: Content, reliability and validity of a new measure, the Minnesota Living with Heart Failure Questionnaire. Heart Failure. 1987;3:198–209. [Google Scholar]
- 15.Agarwal R, Gorski JC, Sundblad K, Brater DC. Urinary protein binding does not affect response to Furosemide in patients with nephritic syndrome. J Am Soc Nephrol. 2000;11:1100–5. doi: 10.1681/ASN.V1161100. [DOI] [PubMed] [Google Scholar]
- 16.Voelker JR, Cartwright-Brown D, Anderson S, et al. Comparison of loop diuretics in patients with chronic renal insufficiency. Kidney Int. 1987;32:572–8. doi: 10.1038/ki.1987.246. [DOI] [PubMed] [Google Scholar]
- 17.Fogarty Y, Fraser CG, Browning MCK. Intra and inter individual variability of serum angiotensin converting enzyme activity: clinical implications. Ann Clin Biochem. 1989;26:201–2. doi: 10.1177/000456328902600224. [DOI] [PubMed] [Google Scholar]
- 18.Hropot M, Sorgel F, Mutschler E. Pharmacodynamics and pharmacokinetics of Frusemide combinations with potassium retaining and thiazide like diuretics: clearance and micropuncture studies. Nauyn Schmiedebergs Arch Pharmacol. 1986;333:457–61. doi: 10.1007/BF00500025. [DOI] [PubMed] [Google Scholar]
- 19.Struthers AD, MacFadyen RJ, Fraser C, et al. Non adherence to angiotensin converting enzyme inhibitor therapy: a comparison of different ways of measuring it in patients with chronic heart failure. J Am Coll Cardiol. 1999;34:2072–7. doi: 10.1016/s0735-1097(99)00439-8. [DOI] [PubMed] [Google Scholar]
- 20.Brater DC, Chennavasin P, Day B, Burdette A, Anderson S. Bumetanide and Frusemide. Clin Pharmacol Ther. 1983;34:207–13. doi: 10.1038/clpt.1983.154. [DOI] [PubMed] [Google Scholar]
- 21.Benet LZ. Pharmacokinetics/pharmacodynamics of Frusemide in man: a review. J Pharmacokin Biopharm. 1979;7:1–27. doi: 10.1007/BF01059438. [DOI] [PubMed] [Google Scholar]
- 22.Grinstead WC, Francis MJ, Marks GF, Tawa CB, Zoghbi WA, Young JB. Discontinuation of chronic diuretic therapy in stable congestive heart failure secondary to coronary artery disease or to idiopathic dilated cardiomyopathy. Am J Cardiol. 1994;73:881–6. doi: 10.1016/0002-9149(94)90815-x. [DOI] [PubMed] [Google Scholar]
- 23.Bennett SJ, Huster GA, Baker SL, et al. Characterisation of the precipitants of hospitalization for heart failure. Am J Crit Care. 1998;7:168–74. [PubMed] [Google Scholar]
- 24.Khand AU, Gemmell I, Rankin AC, Cleland JG. Clinical events leading to progression of heart failure: insights from a national database. Eur Heart J. 2001;22:153–64. doi: 10.1053/euhj.2000.2175. [DOI] [PubMed] [Google Scholar]
- 25.Metcalfe C, Thompson SG, Cowie MR, Sharples LD. The use of hospital admission data as a measure of outcome in clinical studies of heart failure. Eur Heart J. 2003;24:105–12. doi: 10.1016/s0195-668x(02)00384-6. [DOI] [PubMed] [Google Scholar]
- 26.Vasko MR, Cartwright DB, Knochel JP, Nixon JV, Brater DC. Frusemide absorption altered in decompensated congestive heart failure. Ann Int Med. 1985;102:314–18. doi: 10.7326/0003-4819-102-3-314. [DOI] [PubMed] [Google Scholar]
- 27.Almeshari K, Ahlstrom NG, Capraro FE, Wilcox CS. A volume independent component to post diuretic sodium retention in humans. J Am Soc Nephrol. 1993;3:1878–83. doi: 10.1681/ASN.V3121878. [DOI] [PubMed] [Google Scholar]
- 28.Good JM, Brady AJ, Noormohammed FH, Oakley CM, Cleland JGF. Effect of intense angiotensin II suppression on the diuretic response to Frusemide during chronic ACE inhibition. Circulation. 1994;90:220–4. doi: 10.1161/01.cir.90.1.220. [DOI] [PubMed] [Google Scholar]
- 29.MacFadyen RJ, Struthers AD. Diuretic use and abuse in systolic cardiac failure: a recipe for renal impairment? Heart. 2000;83:468. doi: 10.1136/heart.83.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Struthers AD, Anderson G, Donnan P, MacDonald TM. Social deprivation increases cardiac hospitalisations in chronic heart failure independent of disease severity and diuretic non-adherence. Heart. 2000;83:12–16. doi: 10.1136/heart.83.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41:215–16. doi: 10.1016/s0735-1097(02)02694-3. [DOI] [PubMed] [Google Scholar]
- 32.Bauersachs J, Fraccarollo D, Ertl G, Gretz N, Wehling M, Christ M. Striking increase of natriuresis by low dose Spironolactone in congestive heart failure only in combination with ACE inhibition: mechanistic evidence to support RALES. Circulation. 2000;102:2325–8. doi: 10.1161/01.cir.102.19.2325. [DOI] [PubMed] [Google Scholar]
- 33.Beck FX, Ohno A, Muller E, Seppi T, Pfaller W. Inhibition of angiotensin converting enzyme modulates structural and functional adaptation to loop diuretic induced diuresis. Kidney Int. 1997;57:36–43. doi: 10.1038/ki.1997.5. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal J, Boucher R, Nowaczynski W, Genest J. Acute changes in plasma volume, renin activity, and free aldosterone levels in healthy subjects following frusemide administration. Can J Physiol Pharmacol. 1968;46:85–91. doi: 10.1139/y68-015. [DOI] [PubMed] [Google Scholar]