Abstract
Aims
Since 2002, there have been five major outcome trials of statins reporting findings from more than 47 000 subjects. As individual trial results differed, we performed a meta-analysis to ascertain the effectiveness and safety of statins overall and in subgroups. The aim of the study was to estimate the effect of statins on major coronary events and strokes, all-cause mortality and noncardiovascular mortality, and in different subgroups.
Methods
PubMed was searched for trials published in English. Randomized placebo-controlled statin trials with an average follow up of at least 3 years and at least 100 major coronary events were included. For each trial, the statin used, number and type of subjects, proportion of women, mean age and follow up, baseline and change in lipid profile, cardiovascular and non-cardiovascular outcomes were recorded.
Results
Ten trials involving 79 494 subjects were included in the meta-analysis. Due to heterogeneity, ALLHAT-LLT was excluded from some analyses. Statin therapy reduced major coronary events by 27% (95%CI 23, 30%), stroke by 18% (95%CI 10, 25%) and all-cause mortality by 15% (95%CI 8, 21%). There was a 4% (95%CI −10, 3%) nonsignificant reduction in noncardiovascular mortality. The reduction in major coronary events is independent of gender and presence of hypertension or diabetes. The risk reduction was greater in smokers (P < 0.05). Coronary events were reduced by 23% (95%CI 18, 29%) in pravastatin trials and 29% (95%CI 25, 33%) in five trials using other statins. Pravastatin reduced strokes by 12% (95%CI 1, 21%) whilst other statins reduced strokes by 24% (95%CI 16, 32%) (P = 0.04).
Conclusions
Statins reduce coronary events, strokes and all-cause mortality without increasing noncoronary mortality. The benefits accrue in men and women, hypertensives and normotensives, diabetics and nondiabetics, and particularly in smokers. Pravastatin appears to have less impact on strokes.
Keywords: cardiovascular disease prevention, clinical trials, coronary heart disease, HMG-CoA reductase inhibitor, lipids, meta-analysis, statin
Introduction
In the last decade, several large scale randomized controlled trials on statins have proven impressively the effect of 3-hydroxy-2-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) on cardiovascular events, including myocardial infarction and stroke [1–6]. At the same time, statins are now widely used in secondary prevention. Since 2002, several more large scale outcome trials have been completed [7–11]. The MRC/BHF Heart Protection Study (HPS) showed that the benefits of statins extended to a wide range of patients at risk from cardiovascular events, including those with peripheral vascular disease, cerebrovascular disease, diabetes (type 1 and type 2) and hypertension (men over 65 years old) [7]. Notably, hypercholesterolaemia was not an inclusion criterion; the nonfasting total cholesterol only needed to be at least 3.5 mmol l−1 (135 mg dl−1). Therefore, it seems that the majority of middle age to elderly men and women benefit from statins. On the other hand, a reduction in strokes was not observed in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) [9], whilst there was no significant reduction in coronary events or all-cause mortality in the subjects randomized to pravastatin treatment in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial-Lipid Lowering Trial (ALLHAT-LLT) [10]. The problem with the latter study was that the control group received usual care, and lipid lowering therapy was widely used by the time the study was in progress, thus reducing the contrast between treatment and control. The Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA) study also examined the effect of statin treatment in hypertensive patients [11]. Patients with a total cholesterol of less than 6.5 mmol l−1 were randomized to statin or placebo. This part of the study was terminated early after a median follow-up of just 3.3 years showing a benefit that was statistically significant but not very large in absolute terms. ASCOT-LLA is remarkable for the short duration of the study and the modest dosage of statin used.
Issues of risk-benefit and cost-benefit arise with the wider usage of statins. In terms of coronary heart disease (CHD) and overall mortality, patients with the characteristics of Scandinavian Simvastatin Survival Study (4S) are likely to benefit most from statins, as evidenced by the number needed to treat/year (NNT/year) [12]. The NNT/year for CHD events is high for subjects with the characteristics of the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) [12]. Statin therapy is not without danger; cerivastatin was withdrawn from the market after being launched worldwide because of reports of death due to rhabdomyolysis and liver dysfunction. Secondly, the divergent NNTs for different patient populations lead to large variation in cost-effectiveness. Owing to the current costs of statin therapy, their indiscriminate use poses a challenge to any healthcare system. Consequently, it is clinically important and economically sound to look at different patient populations to identify subgroups that benefit most from such treatment. By summating the available information from large-scale clinical trials, a meta-analysis can provide a more robust estimate of the effect of statins on different endpoints and in different subgroups.
Methods
Searching
A computerized literature search was performed using the Pub-Med database covering the period January 1990-April 2003. The following search terms were used: statin, HMG-CoA reductase inhibitor, atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, simvastatin, coronary disease, coronary heart disease and myocardial infarction. In addition, the references cited in articles on statins were examined.
Selection
Only clinical trials of statins conducted in man and reported in English were selected. One hundred and eighty-four trials were identified in the search, of which 137 were randomized controlled trials. These were reviewed by two researchers independently. Differences were resolved by consensus. The criteria for inclusion in the meta-analysis are: (1) randomized treatment allocation; (2) a placebo arm; (3) no other specified difference in management; (4) double-blinding at least in the assessment of endpoints; (5) follow up of at least 3 years; (6) a hard endpoint that is a cardiovascular event as the primary or secondary endpoint; (7) at least 100 major coronary events. Nine trials fitted the criteria and were included in the meta-analysis [1–5, 7–9, 11]. The ALLHAT-LLT was treated as a special case [10]. Although the control arm was standard care rather than placebo, it involved large numbers of patients and was an important trial. Our approach was to perform the meta-analysis with or without ALLHAT-LLT data and highlight instances where inclusion of data from this trial significantly increases heterogeneity.
Data abstraction
The main variables abstracted from the trials were: number of subjects, prior history of cardiovascular disease, statin drug name, dosage and duration, the range, mean and SD of age, sex, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride concentrations and corresponding percentage decrease in concentrations.
Study characteristics
The primary endpoints in these 10 studies differed. Nevertheless, they all analyzed all-cause and cardiovascular mortality and what were termed major CHD events. In the West of Scotland Coronary Prevention Study (WOSCOPS), Cholesterol and Recurrent Events Trial (CARE), Long-term Intervention with Pravastatin in Ischemic Disease Study (LIPID), HPS, Lescol Intervention Prevention Study (LIPS), PROSPER, ALLHAT-LLT and ASCOT-LLA, major CHD events meant coronary death or nonfatal myocardial infarction. In AFCAPS/TexCAPS, the term CHD event also included unstable angina, and in 4S it also included resuscitated cardiac arrest. In most of these trials, major CHD events were analyzed in subgroups according to gender, age, smoking, diabetes and hypertension status. In HPS, subgroup analysis was directed at major vascular events that included major CHD events, strokes and coronary and noncoronary revascularization procedures, thus invalidating comparisons with other statin trials.
Quantitative data synthesis
Relative risk ratios were calculated from the number of events in different treatment groups in the clinical trials using a meta-analysis software programme (Comprehensive Meta Analysis,TM Biostat Inc., Englewood, New Jersey, US). They differ slightly from the hazard ratios in the trial reports. Relative risk ratios were combined using a random effects model (DerSimonian & Laird) [13]. A number of predefined subgroup analyses were performed. These included stratification by gender, smoking, diabetes, hypertension and drug (pravastatin vs other statins). For sensitivity analysis, excluded studies [10, 14–19] were included to explore their impact on the results. In particular, estimates of relative risk ratios were calculated with and without data from ALLHAT-LLT [10].
Results
Trial flow
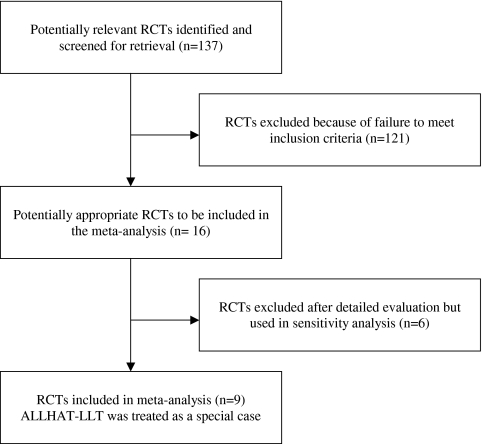
Figure 1 shows the stages of the meta-analysis.
Figure 1.
Trial flow
Study characteristics
Table 1 lists the trials used in the meta-analysis together with the respective trial drug, number and summary characteristics of patients, duration of follow-up, baseline and final lipid concentrations and corresponding percentage changes.
Table 1.
Details of the trials used in the meta-analysis
| 4S [1] | WOSCOPS [2] | CARE [3] | AFCAPS/ TexCAPS [4] | LIPID [5] | HPS [7] | LIPS [8] | PROSPER [9] | ALLHAT-LLT [10]* | ASCOT-LLA [11] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 1994 | 1995 | 1996 | 1998 | 1998 | 2002 | 2002 | 2002 | 2002 | 2003 |
| Trial drug | Simvastatin | Pravastatin | Pravastatin | Lovastatin | Pravastatin | Simvastatin | Fluvastatin | Pravastatin | Pravastatin | Atorvastatin |
| Number of subjects | 4444 | 6595 | 4159 | 6605 | 9014 | 20 536 | 1677 | 5804 | 10 355 | 10 305 |
| Type of patients | CHD | Primary prevention | Post MI | Primary prevention | CHD | Primary prevention + CVD | CHD | Primary prevention + CVD | HT | HT |
| Women (%) | 19 | 0 | 14 | 15 | 17 | 25 | 16 | 52 | 49 | 19 |
| Mean age at baseline (years) | 59 | 55 | 59 | 58 | 62 | 60 | 75 | 66 | 63 | |
| Mean follow up (years) | 5.4 | 4.9 | 5.0** | 5.2 | 6.1 | 5.0 | 3.9** | 3.2 | 4.8 | 3.3** |
| Baseline total cholesterol (mmol l−1) | 6.8 | 7.0 | 5.4 | 5.7 | 5.6 | 5.9 | 5.2 | 5.7 | 5.8 | 5.5 |
| Baseline LDL-C (mmol l−1) | 4.9 | 5.0 | 3.6 | 3.9 | 3.9 | 3.4 | 3.4 | 3.8 | 3.8 | 3.4 |
| Baseline HDL-C (mmol l−1) | 1.2 | 1.1 | 1.0 | 1.0 | 0.9 | 1.1 | 1.0 | 1.3 | 1.2 | 1.3 |
| Baseline triglycerides (mmol l−1) | 1.5 | 1.8 | 1.8 | 1.8 | 1.6 | 2.1 | 1.7 | 1.5 | 1.7 | 1.7 |
| % change in total cholesterol | −26 | −20 | −20 | −19 | −18 | −20 | −23 | −10 | −19 | |
| % change in LDL-C | −36 | −26 | −28 | −27 | −25 | −29 | −27 | −32 | −17 | −29 |
| % change in HDL-C | + 7 | + 5 | + 5 | + 5 | + 5 | + 3 | 0 | + 5 | + 1 | 0 |
| % change in triglycerides | −17 | −12 | −14 | −13 | −11 | −14 | 0 | −12 | 0 | −14 |
Control group received usual care rather than placebo;
median CHD, coronary heart disease; MI, myocardial infarction; CVD, cardiovascular disease; HT, hypertension.
Primary vs secondary prevention
Table 2 shows the relative risk reduction, absolute risk reduction and number needed to treat/year for major coronary events, CHD and all-cause mortality in primary and secondary prevention trials. With the exception of ALLHAT-LLT, the relative risk reduction is similar, but the NNT/year varies considerably. The latter tends to be lower in secondary prevention than in primary prevention. Thus, the NNT/year for major coronary events ranges from 63 in 4S to 310 in ASCOT-LLA and 780 in ALLHAT-LLT. The latter two trials involved hypertensive patients, very few of whom had pre-existing CHD.
Table 2.
Relative risk reduction (RRR) and number needed to treat (NNT)/year in the trials
| All-cause mortality | CHD mortality | Major coronary events | ||||
|---|---|---|---|---|---|---|
| Trial | %RRR | NNT/year | %RRR | NNT/year | NNT/year | NNT/year |
| 4S [1] | 29 (14, 41) | 163 (352, 106) | 411 (25, 54) | 54 (270, 108) | 31 (21, 39) | 63 (89, 49) |
| WOSCOPS [2] | 22 (−2, 40) | 551 (−14134, 270) | 33 (0, 55) | 802 (165149, 402) | 30 (15, 43) | 217 (463, 141) |
| CARE [3] | 8 (−127, 254) | 639 (−502, 195) | 19 (−6, 39) | 449 (−1927, 202) | 22 (7, 36) | 167 (496, 100) |
| AFCAPS-Tex-CAPS [4] | −4 (−43, 25) | −1128 (−620, 787) | 27 (−62, 69) | 4281 (−2785, 1210) | 37 (20, 50) | 256 (514, 170) |
| LIPID [5] | 22 (12, 30) | 202 (374, 138) | 23 (10, 34) | 317 (737, 202) | 22 (13, 31) | 172 (294, 122) |
| HPS [7] | 12 (5, 18) | 286 (638,185) | 17 (7, 26) | 427 (1016, 271) | 26 (19, 32) | 163 (226, 128) |
| LIPS [8] | 24 (−6, 45) | 164 (−984, 76) | 47 (−6, 73) | 291 (−4095, 140) | 31 (−3, 54]) | 265 (−5458, 129) |
| PROSPER [9] | 2 (−15, 17) | 1626 (−228, 178) | 22 (−2, 41) | 342 (−5669, 166) | 21 (4, 35) | 181 (838, 101) |
| ALLHAT-LLT [10] | 1 (−10, 12) | 3808 (−530, 414) | 1 (−24, 21) | 20257 (−919, 843) | 9 (−4, 21) | 780 (−2139, 330) |
| ASCOT-LLA [11] | 13 (−6, 29) | 603 (−1562, 253) | 35 (17, 50) | 310 (730,197) | ||
For NNT/year, negative values indicate harm and whether positive or negative those exceeding 1000 imply negligible impact.
Effect on coronary events
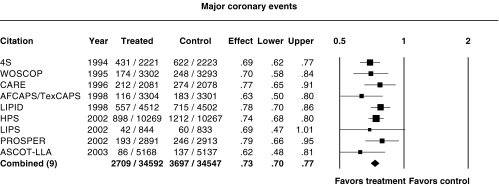
Figure 2 shows the relative risk ratios for major coronary events (CHD death and nonfatal myocardial infarction) in these trials. To ensure that ASCOT-LLA was comparable with the other trials, silent myocardial infarctions were not counted though they formed the composite primary endpoint in that trial [11]. There was a highly significant reduction in this endpoint in all the trials except LIPS and ALLHAT-LLT, where the 95% confidence intervals of the relative risk ratio crossed 1 (Table 2). The point estimate of the relative risk ratio in LIPS was close to the combined estimate but because of the relatively small number of events, the confidence interval was wide. Excluding ALLHAT-LLT, the relative risk ratio of all trials combined was 0.73 (95%CI 0.70, 0.77) (Figure 1). Inclusion of ALLHAT-LLT leads to increased heterogeneity (P = 0.08) and results in a relative risk ratio of 0.75 (95% CI 0.70, 0.80), which was still highly significant (P < 0.0001).
Figure 2.
Relative risk ratios and 95% confidence intervals for major coronary events (CHD death and nonfatal myocardial infarction) in the major trials. P = 0.57 for heterogeneity
Effect on mortality
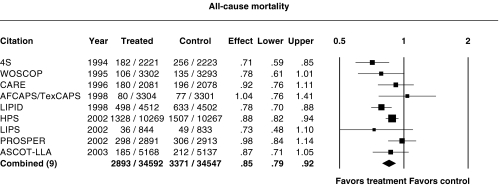
Figure 3 shows the relative risk ratios for all-cause mortality. Only 4S, LIPID and HPS showed a statistically significant reduction in all-cause mortality. In AFCAPS/TexCAPS, PROSPER and ALLHAT-LLT, the point estimate was very close to 1. The relative risk ratio for the combination of all the trials except ALLHAT-LLT was 0.85 (95%CI 0.79, 0.92). Inclusion of ALLHAT-LLT led to significant heterogeneity (P = 0.02) and a relative risk ratio of 0.87 (95%CI 0.80, 0.94), although the point estimate was not significantly altered and the overall reduction in all-cause mortality was still highly significant (P = 0.0003).
Figure 3.
Relative risk ratios for all-cause mortality. P = 0.10 for heterogeneity
Noncardiovascular mortality
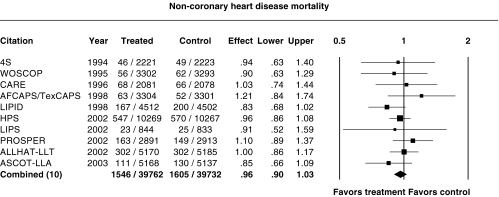
Figure 4 shows the relative risk ratio for non-CHD mortality. Non-CHD mortality ranged from a 17% decrease in LIPID (P = 0.08) to a 21% increase in AFCAPS/TexCAPS (P = 0.3). There was no significant change in noncardiovascular mortality in any of the trial. Inclusion of ALLHAT-LLT did not result in significant heterogeneity (P = 0.68). Overall, there was a nonsignificant reduction in non-CHD mortality, the relative risk ratio being 0.96 (95%CI 0.90, 1.03).
Figure 4.
Relative risk ratios for non-CHD mortality. P = 0.68 for heterogeneity
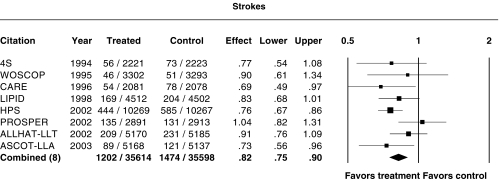
Effect on strokes
Figure 5 shows the relative risk ratio for strokes. For the sake of uniformity, transient ischaemic attacks were not included in the analysis. In CARE, HPS and ASCOT-LLA, there was a significant reduction in strokes. Inclusion of ALLHAT-LLT did not result in significant heterogeneity (P = 0.66). When all the trial data on strokes were combined, there was a significant reduction in strokes, with a relative risk ratio of 0.82 (95%CI 0.75, 0.90).
Figure 5.
Relative risk ratios for strokes (excluding transient ischaemic attacks). P = 0.28 for heterogeneity
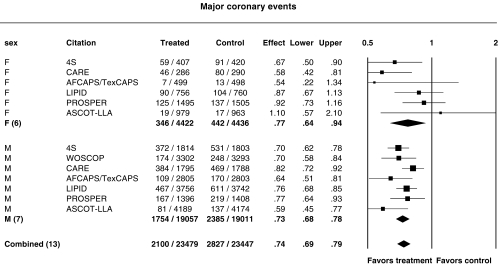
Influence of gender
Figure 6 shows the relative risk ratios for major coronary events in men and women. The incidence of major coronary events in men and women has been reported for 4S, CARE, LIPID, AFCAPS/TexCAPS, LIPID, PROSPER and ASCOT-LLA. All the subjects in WOSCOPS were men. The combined estimate of the relative risk ratio for major coronary events was 0.73 (95% CI 0.68, 0.78) in men and 0.77 (95%CI 0.64, 0.94) in women. Whilst the confidence intervals for women were large because of fewer female participants, there was no evidence of a difference between the sexes (P = 0.37). ALLHAT-LLT was not included because of heterogeneity.
Figure 6.
Relative risk ratios for major coronary events in females (F) and males (M). P = 0.14 and P = 0.20 for heterogeneity in female and male subgroups, respectively
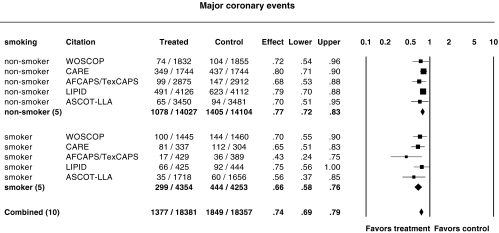
Influence of smoking status
Figure 7 shows the relative risk ratios for major coronary events in smokers and nonsmokers. The incidence of major coronary events in men and women has been reported for WOSCOPS, CARE, AFCAPS/TexCAPS, LIPID and ASCOT-LLA. Although outnumbered by nonsmokers in these trials, the smokers appeared to benefit more (P = 0.048); the relative risk ratio for major coronary events was 0.66 (95% CI 0.58, 0.76) in smokers and 0.77 (95%CI 0.72, 0.83) in nonsmokers.
Figure 7.
Relative risk ratios for major coronary events in nonsmokers and smokers. P = 0.74 and P = 0.42 for heterogeneity in subgroups of nonsmokers and smokers, respectively
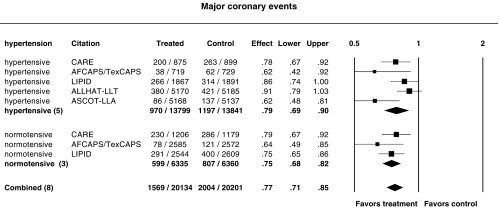
Influence of hypertension status
Figure 8 shows the relative risk ratios for major coronary events in hypertensive and normotensive subjects. ALLHAT-LLT and ASCOT-LLA were conducted in hypertensive patients only. The relative risk ratio for major coronary events was 0.79 (95% CI 0.69, 0.90) in hypertensive persons and 0.75 (95%CI 0.68, 0.82) in normotensive persons, there being no statistically significantly difference (P = 0.15). Exclusion of the ALLHAT-LLT data resulted in a relative risk ratio of 0.75 (95%CI 0.64, 0.88) in hypertensive persons, same as in normotensive persons (P = 0.6).
Figure 8.
Relative risk ratios for major coronary events in hypertensive and normotensive subjects. P = 0.14 and P = 0.45 for heterogeneity in hypertensive and normotensive subgroups, respectively
Influence of diabetes
The available outcome data on diabetics in the statin trials are limited. The incidence of major coronary events in diabetics and non-diabetics was reported in CARE, AFCAPS/TexCAPS, LIPID and ASCOT-LLA. The relative risk ratio for major coronary events was 0.81 (95% CI 0.68, 0.95) in diabetics and 0.72 (95%CI 0.63, 0.82) in non-diabetics. In HPS, diabetes was an inclusion criterion and in this subgroup there was a significant reduction in major vascular events, the primary endpoint [7].
Pravastatin vs other statins
Among the 10 trials, five used pravastatin. The relative risk ratio for major coronary events for pravastatin was 0.79 (95% CI 0.73, 0.86) and 0.71 (95% CI 0.67, 0.75) for other statins (P = 0.01). Respective relative risk ratios for all-cause mortality were 0.84 (95% CI 0.75, 0.94) and 0.89 (95% CI 0.79, 1.01) (P = 0.34). After exclusion of ALLHAT-LLT data, relative risk ratios for pravastatin were 0.77 (95% CI 0.71, 0.82) (P = 0.12) for major coronary events and 0.86 (0.76, 0.98) for all-cause mortality. There was no significant increase in non-CHD mortality in trials involving pravastatin or other statins. The relative risk ratio for strokes was 0.76 (95% CI 0.68, 0.84) for other statins and 0.88 (95% CI 0.79, 0.99) for pravastatin. The reduction in strokes in the pravastatin trials was half that of other statins (P = 0.039). The relative risk ratio for new diagnosis of cancer was 0.99 (95% CI 0.92, 1.07) for other statins and 1.05 (95% CI 0.96, 1.16) for pravastatin. The difference between pravastatin and other statins was not significant (P = 0.32). Apart from PROSPER, there was no significant increase in cancer in the other trials.
Sensitivity analysis
Relative risk ratios with or without including ALLHAT-LLT have been presented above. There were six randomized controlled trials that were excluded because of the low number of coronary events [14–16, 19], short duration [17], or active control [18]. The point estimates of the relative risk ratios in these studies were not significantly different from those derived in the meta-analysis. Inclusion of data from these six studies did not materially alter the point estimates, confidence intervals or conclusions.
Discussion
Statins represent a major advance in the fight against cardiovascular diseases. They are able to lower total cholesterol and LDL cholesterol effectively and safely. The incidence of side-effects and toxicity is low and therefore within limits, high doses can be used to lower the cholesterol concentration drastically. As a result, there were large changes in cholesterol concentration in the clinical trials and significant reductions in cardiovascular events. Despite the differences in inclusion criteria, prior history of cardiovascular disease and baseline lipid concentrations, all the trials except ALLHAT-LLT and LIPS showed similar significant reductions in the relative risks of coronary events. It had been suspected that there are diminishing benefits to cholesterol lowering as the baseline cholesterol decreases [20], and so patients with higher concentrations of cholesterol stood to benefit more and should be treated with a higher degree of urgency and priority. However, the relative risk reduction is similar at different concentrations of baseline cholesterol, whilst the baseline absolute risk is determined by many factors besides cholesterol. Thus, the absolute risk reduction and the NNT/year are scarcely affected by baseline cholesterol concentrations. Patients with pre-existing coronary artery disease, who are at high absolute risk, benefit from treatment, almost regardless of their baseline cholesterol concentration. Conversely, there is little impact on incidence of cardiovascular events when statins are given to subjects who have a high cholesterol concentration but no evidence of cardiovascular disease or risk factors. Thus, if statin therapy has to be constrained due to limitation of resources, priority should be accorded to patients with CHD, diabetes or multiple risk factors. However, if resources are not an issue, use of these agents could be considered for all subjects who stand to benefit.
The reduction in coronary events is translated to a reduction in all-cause mortality. Though the latter endpoint did not reach statistical significance in most of the clinical trials, in the meta-analysis it was highly significant. Safety is very much a major concern following the withdrawal of cerivastatin. The clinical trials in this meta-analysis involved altogether 79 494 patients and reassuringly, there was no increase in non-CHD mortality. Indeed, there was a nonsignificant 5% reduction and the upper limit of the 95% confidence interval was a 3% increase. This puts to rest the concerns about lipid lowering therapy and noncoronary heart disease deaths [21]. Statin therapy in those with atherosclerotic vascular disease or those at risk of such disease, decreases mortality without increasing deaths from other causes.
In four out of five trials involving pravastatin, an increase in cancer was observed in the pravastatin arm. In PROSPER, there was a statistically significant increase in cancer [9]. Overall, the increase in cancer in the pravastatin trials, about 5%, was not statistically significant. However, the period of follow up in the trials was short relative to the development of malignancies. As statins are carcinogenic in certain animal models [22], data on the long-term effects of statins are needed.
In epidemiological studies, the association between strokes and hypercholesterolaemia is weak [23]. In CARE, there was a 31% reduction in stroke that was surprising at the time [3]. In 4S, there was a significant reduction in cerebrovascular events, including strokes and transient ischaemic attacks [1]. Unfortunately, a counting error slightly exaggerated the relative reduction in the initial report [24]. The HPS included patients with previous cerebrovascular disease and showed prospectively a definite reduction in strokes. In PROSPER, the reduction in stroke was not significant and there was no significant effect on cognitive function [9]. The apparent lack of effect on stroke and cognitive decline might be due to the short follow up in this study, although whether statins have any effect on dementia is controversial [25]. Our meta-analysis showed that all the trial results were consistent and that overall there was a 21% reduction in strokes. However, pravastatin has a weaker impact on stroke than the other statins; its effect is only half that of the others. How statins prevent strokes is uncertain, so it is possible that pravastatin might differ from the other statins with respect to effects other than cholesterol synthesis. The outcome of statin treatment may be related to the cholesterol reduction [10, 26], so the relatively poorer outcome with pravastatin could also be related to its lower efficacy in lowering cholesterol [27, 28]. Nevertheless, the degree of lipid lowering in the pravastatin trials in this meta-analysis, 27.3%, was not substantially lower than that in the trials using other statins, 29.3%, and could not fully explain the differences in the hard endpoints.
All the statin trials suffered from relatively low numbers of women participants. In WOSCOPS, only men were recruited. The totality of the data on women shows clearly that women benefit as much as men. This means that women at the same level of risk as men should receive statin treatment. Most cardiovascular events in women occur after the menopause, when their level of cardiovascular risk catches up with that of men. Thus, statin therapy is particularly pertinent in postmenopausal women, in view of the failure of hormonal replacement therapy to prevent cardiovascular events [29].
Cigarette smoking enhances the risk of cardiovascular events, so statins may be useful to counteract this. The relative risk reduction in smokers appears to be greater than in nonsmokers. Therefore, statins should not be withheld from those at risk of cardiovascular events until they stop smoking. It seems that statin therapy should be started, whilst every available means should be used to help the patient quit the habit.
It was shown in HPS that type 1 and type 2 diabetics benefited from statin therapy, regardless of the concentration of cholesterol and the presence of other risk factors. This is reasonable in view of their having similar event rates to patients who had a previous myocardial infarction [30]. The recent American guidelines on high blood cholesterol regard diabetes as CHD risk equivalent [31]. Our meta-analysis confirms that diabetics benefit from treatment as much as nondiabetics. On the other hand, there is no suggestion of greater relative risk reduction in diabetics. Hence, the criteria for starting statins in diabetics should be based on cardiovascular risk.
In HPS, hypertensive men aged 65 years or over benefited from lipid lowering therapy. This was confirmed in ASCOT-LLA although in ALLHAT, the benefit was modest and did not reach statistical significance. However, in the latter study, the control group received usual care that included lipid lowering therapy. Overall, it seems that hypertension alone justifies treatment, although undoubtedly many of these patients have other risk factors too.
HPS and ASCOT-LLA provide evidence on the effectiveness of lipid lowering in the new indications of diabetes and hypertension. One in 12 of the adult population has diabetes [32]. Half of elderly men over 70 years are hypertensive [33]. The number of individuals in the community who could benefit from statin therapy is therefore staggering. Consequently, healthcare providers are obliged to devise ways of prioritizing such therapy for those who can derive the most benefit. In recent years, this has depended on calculating the absolute risk of a cardiac event [31]. Our results also support this approach, as relative risk reductions vary little with respect to patient characteristics, gender, hypertensive and diabetic status. However, conventional methods of calculating absolute risk based on Framingham [34] or Münster [35] cohorts tend to emphasize risk in the elderly at the expense of the young, so decisions to start therapy based on absolute cardiovascular risk alone inevitably favour the elderly [36]. Most patients below 50 years of age do not have the 2% or 3% annual risk to mandate statin therapy, but death or disability due to myocardial infarction or stroke at this age causes a substantial loss in life years and quality of life. Thus, when making a decision on lipid lowering therapy, in addition to estimating cardiovascular risk, the anticipated cumulative benefits of treatment should also be considered.
The distinction between primary and secondary prevention is becoming blurred as most candidates for primary prevention have a certain level of cardiovascular risk, although by definition they have not had an event yet. It is increasingly realized that those who receive primary prevention may already be at an advanced stage of atherosclerosis. There are now an increasing number of biochemical markers such as homocysteine and C-reactive protein that may improve risk prediction. Primary prevention would therefore be an important area of focus in future.
In conclusion, through meta-analysis of the clinical trials on statins, we are able to show that statins reduce coronary events and all-cause mortality, with no increase in non-coronary mortality. With the exception of pravastatin, statins also have a strong effect on reducing the incidence of strokes. Benefits accrue in men and women, hypertensives and normotensives, diabetics and non-diabetics, and particularly in smokers. It may be more cost-effective to treat those at the highest cardiovascular risk, for example patients with CHD, diabetes and multiple risk factors.
Acknowledgments
B. M. Y. Cheung, C. R. Kumana and C.P. Lau are members of the Institute of Cardiovascular Science and Medicine, University of Hong Kong.
Competing interests: The authors have, at various times in the past 5 years, been reimbursed by MSD, Novartis, Pfizer and Sanofi for organizing, attending or speaking in scientific meetings.
References
- 1.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 2.Shepherd J, Cobbe M, Ford I, et al. for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 3.Sacks FM, Pfeffer MA, Moye LA, et al. for the Cholesterol and Recurrent Events Trial Investigators The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 4.The Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 5.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/ TexCAPS. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 6.La Rosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–6. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 7.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 8.Serruys PWJC, de Feyter Macaya C et al. for the Lescol Intervention Prevention Study (LIPS) Investigators. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention. A randomised controlled trial. JAMA. 2002;287:3215–22. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd J, Blauw GJ, Murphy MB, et al. on behalf of the PROSPER Study Group. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 10.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomised to pravastatin vs. usual care. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 11.Sever PS, Dahlof B, Poulter NR, et al. for the ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentration, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA) Lancet. 2003;361:1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 12.Kumana CR, Cheung BMY, Lauder IJ. Gauging the impact of statins using number needed to treat. JAMA. 1999;282:1899–901. doi: 10.1001/jama.282.20.1899. [DOI] [PubMed] [Google Scholar]
- 13.Fleiss JL. Measure of effect size for categorical data. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York, NY: Russell Sage Foundation; 1994. pp. 245–60. [Google Scholar]
- 14.Jukema JW, Bruschke AV, van Boven AJ, et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS) Circulation. 1995;91:2528–40. doi: 10.1161/01.cir.91.10.2528. [DOI] [PubMed] [Google Scholar]
- 15.The post coronary artery bypass graft trial investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med. 1997;336:153–62. doi: 10.1056/NEJM199701163360301. [DOI] [PubMed] [Google Scholar]
- 16.Serruys PW, Foley DP, Jackson G, et al. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J. 1999;20:58–69. doi: 10.1053/euhj.1998.1150. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GG, Olsson AG, Ezekowitz MD, et al. for the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of Atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL Study: a Randomized Controlled Trial. JAMA. 2001;285:1711–8. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 18.Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The Greek Atorvastatin and Coronary-Heart-Disease Evaluation (GREACE) Study. Curr Med Res Opin. 2002;18:220–8. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 19.Liem AH, van Boven AJ, Veeger NJ, et al. FLuvastatin On Risk Diminishment after Acute myocardial infarction Study Group. Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomised trial. Eur Heart J. 2002;23:1931–7. doi: 10.1053/euhj.2002.3291. [DOI] [PubMed] [Google Scholar]
- 20.Sacks FM, Tonkin AM, Shepherd J, et al. for the Prospective Pravastatin Pooling Project Investigator Group. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: the Prospective Pravastatin Pooling Project. Circulation. 2000;102:1893–900. doi: 10.1161/01.cir.102.16.1893. [DOI] [PubMed] [Google Scholar]
- 21.Wysowski DK, Gross TP. Deaths due to accidents and violence in two recent trials of cholesterol-lowering drugs. Arch Intern Med. 1990;150:2169–72. [PubMed] [Google Scholar]
- 22.Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA. 1996;275:55–60. [PubMed] [Google Scholar]
- 23.Prospective studies collaboration. Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Lancet. 1995;346:1647–53. [PubMed] [Google Scholar]
- 24.Pedersen TR, Kjekshus J, Pyorala K, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian Simvastatin Survival Study (4S) Am J Cardiol. 1998;81:333–5. doi: 10.1016/s0002-9149(97)00904-1. [DOI] [PubMed] [Google Scholar]
- 25.Cheung BMY, Kumana CR. Dementia and statins. Lancet. 2001;357:880. doi: 10.1016/s0140-6736(05)71806-7. [DOI] [PubMed] [Google Scholar]
- 26.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. Br Med J. 2003;326:1423–9. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hippisley-Cox J, Cater R, Pringle M, Coupland C. Cross sectional survey of effectiveness of lipid lowering drugs in reducing serum cholesterol concentration in patients in 17 general practices. Br Med J. 2003;325:689–92. doi: 10.1136/bmj.326.7391.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung BMY, Kumana CR. Effectiveness of lipid lowering drugs in general practice: Study had two major flaws (letter) Br Med J. 2003;327:51. doi: 10.1136/bmj.327.7405.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grady D, Herrington D, Bittner V, et al. HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 30.Haffner SM. Coronary heart disease in patients with diabetes. N Engl J Med. 2000;342:1040–2. doi: 10.1056/NEJM200004063421408. [DOI] [PubMed] [Google Scholar]
- 31.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults: Third National Health Nutrition Examination Survey, 1988–94. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 33.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988–91. Hypertension. 1995;25:305–13. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 34.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–8. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 35.Assmann G, Scholte H, von Eckardstein A. Hypertriglyceridemia and elevated levels of lipoprotein (a) are risk factors for major coronary events in middle-aged men. Am J Cardiol. 1996;77:1179–84. doi: 10.1016/s0002-9149(96)00159-2. [DOI] [PubMed] [Google Scholar]
- 36.Cheung BMY, Kumana CR. Should decisions on treatment be based on absolute benefit rather than absolute risk? NZ Med J. 2001;114:214–5. [PubMed] [Google Scholar]