Abstract
Aims
Nifedipine is a short-acting calcium antagonist formulated into several different oral preparations, each of which may have different effects on haemodynamics and autonomic nervous function. We compared the effects of nifedipine controlled-release (CR) and nifedipine retard on 24-h blood pressure, heart rate, rate–pressure product, and power spectral measures of heart rate variability in patients with essential hypertension.
Methods
After 4 weeks of a drug-free period, 25 patients were randomized to receive either once-daily treatment with nifedipine CR (20–40 mg daily; 12 patients) or twice-daily treatment with nifedipine retard (20–40 mg daily; 13 patients) for 12 weeks. The ambulatory blood pressure, heart rate, and ECG R–R intervals were measured during a 24-h period using a portable recorder (TM-2425) at the end of the drug-free and the treatment periods. A power-spectral analysis of R–R intervals was performed to obtain the low-frequency (LF) and high-frequency (HF) components.
Results
Nifedipine CR and nifedipine retard reduced 24-h blood pressure significantly by 15.9 ± 3.2 (SE)/8.7 ± 1.4 mmHg and by 10.9 ± 2.8/9.4 ± 1.7 mmHg, respectively, after the 12-week treatment. Nifedipine CR did not change the 24-h heart rate sig-nificantly, while nifedipine retard increased it significantly by 3.9 ± 2.1 beats min−1. Nifedipine CR produced a significant reduction in rate–pressure product throughout a 24-h period, while nifedipine retard did not change the rate–pressure product significantly. In addition, nifedipine retard significantly decreased the 24-h and daytime average values of the LF and HF components, while nifedipine CR affected the nighttime LF component alone and did not change the HF component throughout a 24-h period.
Conclusions
These results demonstrate that both nifedipine CR and nifedipine retard are effective as antihypertensive agents, but nifedipine CR has less influence on the autonomic nervous system and heart rate than nifedipine retard.
Keywords: autonomic nervous function, blood pressure, heart rate, nifedipine controlled-release, nifedipine retard
Introduction
Much debate has recently taken place regarding the relation between the short-acting calcium antagonists of dihydropyridines and the risk of myocardial infarction [1, 2]. Short-acting calcium antagonists cause an increase in sympathetic nerve activation and reflex tachycardia [3]. Increased sympathetic tone may represent one of the ‘pressure-independent’ coronary risk factors in hypertensive patients [4, 5]. Moreover, the clinical utility of the short-acting calcium antagonists is limited by the incidence of unfavourable side-effects such as headache, flushing, dizziness and palpitations. It is thought that such side-effects are caused by the acute vasodilatation and reflex activation of the sympathetic nervous system [6]. A certain degree of success has been achieved in reducing the incidence of such adverse effects by the use of slow-release formulations such as nifedipine retard. However, side-effects still occur with sufficient frequency to cause unfavourable effects in a significant number of patients compared with long-acting dihydropyridines such as amlodipine [7].
Nifedipine controlled-release (CR), a long-acting once-daily formulation of nifedipine, has a polymer matrix delivery system composed of a slow-release coat and a fast-release core and has recently become available in Japan [8]. The dissolution rates for the two layers are different, with the outer layer dissolving at a slower rate than the internal layer. This allows the plasma nifedipine concentration to remain relatively stable over 24 h. Therefore, nifedipine CR is expected to cause less activation of the sympathetic nervous system than nifedipine retard.
In the present study, we compared the effects of once-daily nifedipine CR and twice-daily nifedipine retard on 24-h blood pressure (BP), heart rate, and autonomic nerve activity in hypertensive patients. Autonomic nerve activity was evaluated non-invasively by a power spectral analysis of heart rate variability.
Methods
Patients
Twenty-seven outpatients with essential hypertension (age, mean 53.0 ± 1.5 years, range 39–69 years; 15 males and 12 females) participated in this study.
Written informed consent was obtained from all patients after a detailed explanation of the study protocol. The study protocol was approved by the Ethics Committee of Dokkyo University School of Medicine. The patients had systolic BP >150 mmHg, diastolic BP >90 mmHg, or both on at least three occasions at the outpatient clinic. Secondary causes of hypertension were ruled out through a comprehensive checkup including medical history, physical findings, urinalysis, blood chemistry, and endocrinological and radiological examinations when needed. All patients showed normal renal function as judged by the endogenous creatinine clearance. According to the World Health Organization criteria for organ damage, all patients were classified as having stage I (n = 4) or II (n = 23) hypertension. Seventeen patients were taking antihypertensive medication which consisted mainly of calcium antagonists or angiotensin-converting enzyme inhibitors, while the other 10 patients were newly diagnosed as having essential hypertension and had not received prior treatment.
Study protocol
After completing the 4-week drug-free period, patients were randomly assigned to the nifedipine CR or nifedipine retard group. Patients assigned to the nifedipine CR group received 20 mg orally once daily for 4 weeks. Patients assigned to the nifedipine retard group received 10 mg orally twice daily for 4 weeks. Doses were increased to nifedipine CR 40 mg once daily or nifedipine retard 20 mg twice daily if BP was not adequately controlled after the initial 4-week treatment period. Each treatment period lasted for 12 weeks. Nifedipine CR was administered orally once daily between 07.00 h and 09.00 h after breakfast, and nifedipine retard was administered orally twice daily between 07.00 h and 09.00 h and between 18.00 h and 20.00 h after food. Patients visited our outpatient clinic every 4 weeks during the study period. At each visit, systolic and diastolic BPs were measured twice using a mercury sphygmomanometer with the patient in the sitting position. At the end of the drug-free and treatment periods, monitoring of the 24-h ambulatory BP and electrocardiogram was carried out.
Twenty-four hour ambulatory blood pressure measurement
The 24-h ambulatory BP was monitored every 30 min using the cuff-oscillometric device, TM-2425 (A&D Co., Tokyo, Japan). This device satisfied the criteria of the Association for the Advancement of Medical Instrumentation (AAMI) and British Hypertension Society (BHS) [9]. The usefulness of this recorder in clinical hypertension research was previously reported [6, 9–13]. The patients were asked to carry the device for 26 h, and the first 2 h of recordings made at or near the hospital were omitted from the later analysis. The ambulatory monitoring was performed during an average working day. The daytime and nighttime BPs were defined as the average values in the awake period between 07.00 h and 21.59 h and in the sleeping period between 22.00 h and 06.59 h, respectively. The same device was used in each individual for the entire protocol to avoid different BP readings caused by different recorders. To minimize the effect of the patients’ physical activities on BP, the ambulatory BP monitoring was performed on the same day of the week. In the present study, the product of heart rate and systolic BP (rate–pressure product; heart rate · systolic BP · 10−2) was also calculated from the ambulatory data. The rate–pressure product was reported to be the index which best correlates with myocardial oxygen consumption [14].
Power spectral analysis of R–R intervals
The ambulatory BP recorder used in this study, TM-2425, can also monitor the R–R interval of the electrocardiogram. The procedures of the power spectral analysis of R–R intervals in this device were previously reported in detail [13].
Briefly, electrocardiogram tracings were obtained with a pericardial lead (V5). All R–R intervals were recorded at a resolution of 7.8 ms throughout a 24-h period. Ectopic beats or artefacts were excluded automatically. After these procedures, spectral R–R interval variability was computed with the autoregressive model from every 5-min block over a 24-h period. The Hz range of 0.05–0.15 was computed as the low-frequency (LF) component, which is an index of both sympathetic and parasympathetic nerve activities [15]. The Hz range of 0.15–0.40 was computed as the high-frequency (HF) component, which reflects exclusively parasympathetic nerve activity [16]. The ratio of the LF to the HF component was also calculated.
Statistical analysis
Values are summarized where appropriate as means ± SE. The Fisher's exact test or the paired or unpaired Student's t-test was employed where appropriate with a statistically accepted significance at P < 0.05. For the comparisons of power spectral densities, the naturally logarithmic values, i.e. ln (the LF component), ln (the HF component), or ln (the ratio of the LF to the HF component), were used to normalize the skewness of the data [17].
Results
Of 27 patients, 13 were assigned to the nifedipine CR group and the other 14 to the nifedipine retard group. Two patients withdrew from the study due to adverse effects. One patient experienced dizziness and chest discomfort during the treatment period with nifedipine CR, and the other experienced facial flushing during the treatment period with nifedipine retard. The baseline characteristics of the 25 patients who completed the study are shown in Table 1. There were no significant differences in age, sex distribution, height, body weight, or body mass index between the nifedipine CR and nifedipine retard groups.
Table 1.
Baseline characteristics of patients
| Nifedipine CR | Nifedipine tetard | P | |
|---|---|---|---|
| Age (years) | 55.4 ± 2.6 | 51.3 ± 2.0 | NS |
| Males/females | 6 : 6 | 8 : 5 | NS |
| Height (cm) | 160.1 ± 2.1 | 162.8 ± 2.3 | NS |
| Body weight (kg) | 65.0 ± 3.1 | 67.7 ± 3.6 | NS |
| Body mass index (kg m−2) | 25.3 ± 0.9 | 25.4 ± 0.9 | NS |
In the nifedipine CR group, office BP during the drug-free period was 159.0 ± 2.8/98.2 ± 1.3 mmHg, with a significant reduction at the end of the treatment period of 131.0 ± 3.4/81.8 ± 2.7 mmHg {−17.4%[95% confidence interval (CI) −22.3, −12.4; P < 0.01]/−16.4% (95% CI −22.4, −10.5; P < 0.01)}. In the nifedipine retard group, office BP during the drug-free period was 155.4 ± 2.8/100.0 ± 2.6 mmHg, with a significant reduction at the end of the treatment period of 136.5 ± 2.5/88.5 ± 2.3 mmHg [−12.0% (95% CI −15.0, −9.0; P < 0.01)/−11.3% (95% CI −14.2, −8.5; P < 0.01)]. No significant differences in BP were found between the two groups at the end of the drug-free period or at the end of the treatment period. The mean doses of nifedipine CR and nifedipine retard were 30.0 ± 3.0 mg day−1 and 30.8 ± 2.9 mg day−1, respectively, at the end of the treatment period (P = NS).
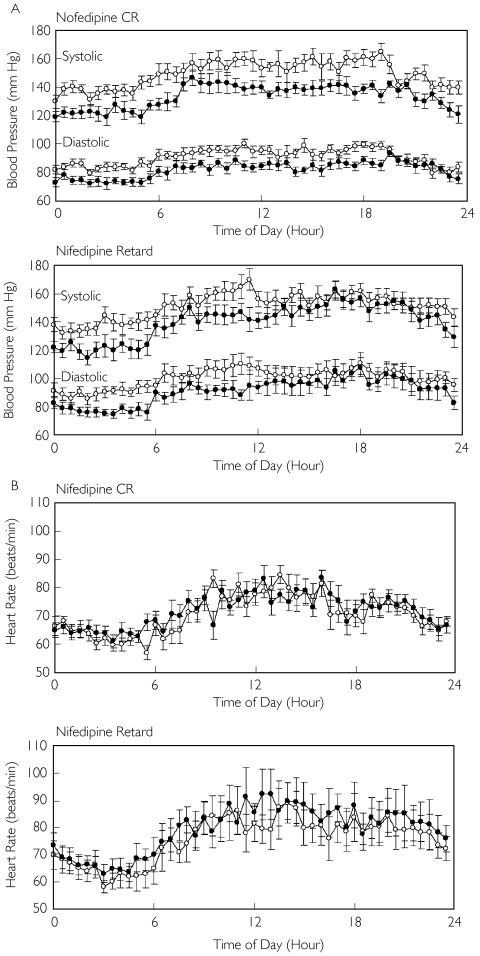
Figure 1 depicts the 24-h trendgram of BP and heart rate of the drug-free and treatment periods in the two groups. Table 2 lists the average values of BP, heart rate and the rate–pressure product for the entire 24-h period, the daytime and the nighttime, in the two groups. Both nifedipine CR and nifedipine retard significantly lowered the systolic and diastolic BP during the daytime and the nighttime. The decreases in the 24-h BPs were −15.9 ± 3.2 (95% CI −22.0, −9.7)/−8.7 ± 1.4 (95% CI −11.4, −6.1) mmHg during the treatment period with nifedipine CR and −11.1 ± 2.8 (95%CI −16.7, −5.5)/−9.4 ± 1.7 (95% CI −12.8, −6.0) mmHg during the treatment period with nifedipine retard. No significant differences in BP were found between the two groups at the end of the drug-free period or at the end of the treatment period. With regard to HR, nifedipine CR did not change the heart rate during the daytime or during the nighttime. In contrast, nifedipine retard significantly increased the 24-h average heart rate [+ 3.9 ± 2.1 beats min−1 (95% CI −0.3, 8.1; P < 0.05)] and the daytime heart rate [+ 4.3 ± 1.9 beats min−1 (95% CI 0.5, 8.1; P < 0.05)], although such a change was not significant during the nighttime. Nifedipine CR significantly decreased the 24-h average and the daytime rate–pressure product (P < 0.01 for each), while nifedipine retard did not change the rate–pressure product significantly during the daytime or during the nighttime.
Figure 1.
Twenty-four-hour trendgram of blood pressure (A) and heart rate (B) at the end of the drug-free period (○) and the treatment period (•) in patients treated with once-daily nifedipine controlled-release (CR) and in patients treated with twice-daily nifedipine retard
Table 2.
Blood pressure and heart rate during the drug-free and treatment periods
| Drug-free period | Nifedipine CR Treatment period | Difference (95% CI) | Drug-free period | Nifedipine retard Treatment period | Difference (95% CI) | |
|---|---|---|---|---|---|---|
| 24-h | ||||||
| Systolic BP, mmHg | 149.3 ± 3.0 | 133.4 ± 2.6*** | −22.0, −9.7 | 150.8 ± 3.7 | 139.7 ± 4.0*** | −16.7, −5.5 |
| Diastolic BP, mmHg | 90.6 ± 1.5 | 81.8 ± 1.6*** | −11.4, −6.1 | 95.6 ± 3.1 | 86.2 ± 2.4*** | −12.8, −6.0 |
| Heart rate, beats min−1 | 70.6 ± 1.3 | 71.4 ± 1.6 | −2.5, 4.2 | 70.5 ± 1.8 | 74.4 ± 2.4* | −0.3, 8.1 |
| RPP, mmHg·beats min−1·10−2 | 106.0 ± 2.8 | 95.9 ± 3.0*** | −15.4, −4.8 | 106.8 ± 3.5 | 104.6 ± 4.2 | −8.2, 3.8 |
| Daytime (07.00–21.59 h) | ||||||
| Systolic BP, mmHg | 155.1 ± 3.6 | 139.0 ± 2.6*** | −22.6, −9.7 | 156.7 ± 3.9 | 147.9 ± 4.2*** | −14.3, −3.3 |
| Diastolic BP, mmHg | 94.3 ± 1.7 | 85.2 ± 1.7*** | −12.1, −6.1 | 99.3 ± 3.5 | 91.3 ± 2.9*** | −11.7, −4.3 |
| Heart rate, beats min−1 | 74.8 ± 1.7 | 75.1 ± 1.7 | −3.5, 4.2 | 74.5 ± 2.2 | 78.8 ± 2.6** | 0.5, 8.1 |
| RPP, mmHg·beats min−1·10−2 | 116.3 ± 3.6 | 104.5 ± 2.7** | −18.2, −5.5 | 116.7 ± 4.3 | 116.7 ± 5.3 | −6.2, 6.1 |
| Nighttime (22.00–06.59 h) | ||||||
| Systolic BP, mmHg | 139.6 ± 2.5 | 124.1 ± 3.4*** | −23.0, −7.8 | 140.9 ± 3.9 | 126.1 ± 4.8*** | −22.3, −7.2 |
| Diastolic BP, mmHg | 84.2 ± 1.5 | 76.1 ± 1.8*** | −11.2, −5.0 | 89.3 ± 2.7 | 77.6 ± 2.2*** | −15.4, −8.0 |
| Heart rate, beats min−1 | 63.6 ± 1.3 | 65.2 ± 2.1 | −2.4, 5.7 | 63.9 ± 1.7 | 67.1 ± 2.2 | −2.2, 8.6 |
| RPP, mmHg·beats min−1·10−2 | 88.6 ± 1.9 | 81.5 ± 4.2* | −14.5, 0.3 | 90.2 ± 2.9 | 84.4 ± 3.3 | −12.6, 1.0 |
BP, Blood pressure; RPP, rate–pressure product.
P < 0.1;
P < 0.05;
P < 0.01 compared with drug-free period.
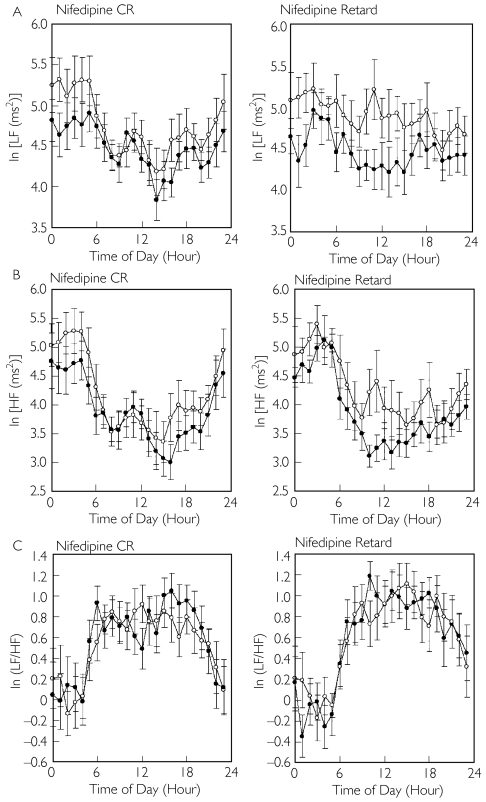
Figure 2 depicts the 24-h trendgram of the LF component, the HF component and the ratio of the LF to the HF component of the drug-free and treatment periods in the two groups, and Table 3 lists the average values for the 24-h period, the daytime and the nighttime, in the two groups. Nifedipine retard significantly decreased the 24-h average and the daytime HF component (each for P < 0.05), while nifedipine CR did not change the HF component significantly during the daytime or the nighttime. Neither nifedipine CR nor nifedipine retard changed the ratio of the LF to the HF component significantly during the daytime or the nighttime.
Figure 2.
Twenty-four-hour trendgram of the low-frequency (LF) component (A), the high-frequency (HF) component (B), and the ratio of the LF to the HF component (LF/HF) (C) at the end of the drug-free period (○) and the treatment period (•) in patients treated with once-daily nifedipine controlled-release (CR) and in patients treated with twice-daily nifedipine retard
Table 3.
Parameters of heart rate variability during the drug-free and treatment periods
| Drug-free period | Nifedipine CR Treatment period | Difference (95% CI) | Drug-free period | Nifedipine retard Treatment period | Difference (95% CI) | |
|---|---|---|---|---|---|---|
| 24-h | ||||||
| LF, ln (ms2) | 4.74 ± 0.19 | 4.48 ± 0.16 | −0.54, 0.02 | 4.90 ± 0.21 | 4.47 ± 0.17** | −0.67, −0.19 |
| HF, ln (ms2) | 4.21 ± 0.27 | 3.91 ± 0.26 | −0.65, 0.06 | 4.30 ± 0.28 | 3.88 ± 0.16* | −0.77, −0.06 |
| ln (LF/HF) | 0.54 ± 0.14 | 0.57 ± 0.15 | −0.10, 0.16 | 0.61 ± 0.16 | 0.59 ± 0.14 | −0.26, 0.24 |
| Daytime (07.00–21.59 h) | ||||||
| LF, ln (ms2) | 4.50 ± 0.17 | 4.32 ± 0.13 | −0.46, 0.10 | 4.83 ± 0.23 | 4.39 ± 0.18* | −0.69, −0.20 |
| HF, ln (ms2) | 3.75 ± 0.27 | 3.55 ± 0.21 | −0.57, 0.17 | 3.96 ± 0.33 | 3.50 ± 0.20* | −0.88, −0.05 |
| ln (LF/HF) | 0.75 ± 0.14 | 0.77 ± 0.12 | −0.12, 0.16 | 0.87 ± 0.20 | 0.89 ± 0.16 | −0.25, 0.30 |
| Nighttime (22:00–06.59 h) | ||||||
| LF, ln (ms2) | 5.14 ± 0.26 | 4.74 ± 0.22* | −0.76, −0.05 | 5.02 ± 0.20 | 4.61 ± 0.18* | −0.73, −0.09 |
| HF, ln (ms2) | 4.96 ± 0.31 | 4.51 ± 0.35 | −0.88, −0.02 | 4.86 ± 0.24 | 4.53 ± 0.16 | −0.66, 0.00 |
| ln (LF/HF) | 0.19 ± 0.19 | 0.23 ± 0.21 | −0.16, 0.25 | 0.16 ± 0.16 | 0.09 ± 0.16 | −0.34, 0.19 |
LF, Low-frequency component; HF, high-frequency component.
P < 0.05;
P < 0.01, compared with drug-free period.
Discussion
The current study demonstrated that once-daily nifedipine CR produced a reduction in the 24-h BP similar to twice-daily nifedipine retard after chronic treatment for 12 weeks in patients with mild-to-moderate essential hypertension. However, the effects of the two formulations of nifedipine on haemodynamic and power spectral measures of heart rate variability differed in some respects; nifedipine retard significantly decreased the 24-h and daytime average values of the LF and HF components and significantly increased the 24-h and daytime heart rate, while nifedipine CR affected the nighttime LF component alone and did not significantly change the heart rate or the HF component throughout a 24-h period. In addition, nifedipine CR decreased the 24-h and daytime average values of the rate–pressure product, while nifedipine retard did not significantly change the rate–pressure product throughout a 24-h period. These findings suggest that nifedipine retard caused a decrease in parasympathetic nerve activity, whereas nifedipine CR did not produce such effects on autonomic nervous function. Reflex tachycardia was observed in the patients treated with nifedipine retard but not in those treated with nifedipine CR. It is also suggested that nifedipine CR but not nifedipine retard reduced myocardial oxygen consumption significantly in these patients.
Nifedipine is a dihydropyridine calcium antagonist with documented efficacy in the treatment of patients with hypertension and angina [18]. It has a short plasma half-life, requiring multiple daily administrations of the immediate-release product. Nifedipine CR, a long-acting once-daily formulation of nifedipine, has recently become available in Japan. It consists of a coat-core tablet and a hydrophillic matrix (an expandable hydrogel layer of polyethylene oxide and hydroxypropylmethylcellulose) that releases the drug slowly as it erodes. The diameters of the coat-core tablet and the core are 9 mm and 5 mm, respectively. The coat-core tablet is coated with common film-forming materials and pigments used to protect against photodegradation of nifedipine. Drug concentrations after administration of the nifedipine CR formulation peak within 4 h after administration, and are sustained at that level for at least 24 h after administration [8]. It has been shown that nifedipine CR has a smaller peak–trough fluctuation of plasma concentartions than does nifedipine retard [19]. In the light of these properties, it is expected that nifedipine CR has less influence on the autonomic nervous system and heart rate than does nifedipine retard in the treatment of hypertension. However, no published data are available as to the comparison of nifedipine CR with nifedipine retard in these respects.
There has been some controversy concerning the safety of treating cardiovascular patients with calcium antagonists [20–22]. Short-acting 1,4-dihydropyridines such as nifedipine capsule increase the risk of cardiac death and myocardial reinfarction in patients with coronary artery disease [1, 2]. The US Food and Drug Administration (FDA) issued a statement warning physicians about the potential hazard associated with the use of short-acting dihydropyridines [20]. In addition to a rapid fall in BP, increased sympathetic activity may participate in these adverse events. It was suggested that intermittent increases in sympathetic activity persist during even chronic treatment with those 1,4-dihydropyridines that have a poor trough–peak ratio [3]. Moreover, there is considerable evidence that increased sympathetic tone may both directly and indirectly cause the development or maintenance of left ventricular hypertrophy [23] and the development and progression of atherosclerosis [24]. Thus, high sympathetic tone may, at least in part, explain the increased cardiovascular events in patients treated with short-acting calcium antagonists. A certain degree of success has been achieved in reducing the incidence of such adverse effects by the use of slow-release formulations such as nifedipine retard. However, side-effects still occur with sufficient frequency to cause unfavourable effects in a significant number of patients compared with long-acting dihydropyridines such as amlodipine [7]. In the present study, reflex tachycardia was observed in patients treated with nifedipine retard but not in those treated with nifedipine CR.
In the present study, autonomic nervous function was evaluated using a power spectral analysis of heart rate variability. Power spectral analyses of heart rate variability have been widely accepted as a non-invasive method of assessing the autonomic nervous function of patients with various cardiovascular disorders [25]. It has been shown that the estimation of heart rate variability by ambulatory monitoring offers prognostic information beyond that provided by the evaluation of traditional cardiovascular risk factors. For example, Tsuji et al.[26, 27] found that reductions in measures of heart rate variability including the LF and HF components were significantly associated with the onset of a cardiac event in subjects who participated in the Framingham Heart Study. In the present study, nifedipine retard significantly decreased the 24-h and daytime average values of the LF and HF components, while nifedipine CR affected the nighttime LF component alone and did not change the HF component throughout a 24-h period. These results suggest that nifedipine CR has less influence on the autonomic nervous system than nifedipine retard.
In the current study, nifedipine retard significantly decreased the 24-h and daytime average values of the HF component, which represents parasympathetic nerve activity, while nifedipine CR did not change the HF component throughout a 24-h period. These findings are consistent with previous observations, including ours. We examined the autonomic effects of nifedipine retard on hypertensive patients in a 4-week comparative study with amlodipine [12]. A significant (P < 0.01) decrease in the HF component was observed with nifedipine retard. Although the mechanism of the decrease in parasympathetic nerve activity caused by nifedipine retard was not clarified by the present study, it is suggested that nifedipine affects parasympathetic nervous activity directly. For example, Izumi et al.[28] reported that nifedipine inhibited parasympathetic lower lip vasodilatation in anaesthetized cats.
In the present study, neither nifedipine CR nor nifedipine retard changed the ratio of the LF to the HF component significantly during the daytime or the nighttime. This is in good agreement with the study by Sato et al.[29]. They found that the HF component was lower during the day after nifedipine retard therapy, while the ratio of the LF to the HF component was unchanged during the daytime or the nighttime. Although the ratio of the LF to the HF component is considered to represent sympathovagal balance, there are some problems with the interpretation of this index [30]. Therefore, further studies are needed to compare the effects of the two formulations of nifedipine on the sympathetic nervous system.
In addition, with regard to the present finding that neither nifedipine CR nor nifedipine retard changed the ratio of the LF to the HF component significantly, we must acknowledge the statistical power limitations of the sample size. In the present study, the number of patients to be enrolled was determined based on the size of earlier studies conducted with other antihypertensive agents [31], since there were no standardized methods to estimate appropriate sample size in order to obtain sufficient statistical power for the main endpoints measured.
Dihydropyridine calcium antagonists are effective alone and in combination with other agents in lowering BP [32]. They are especially effective in isolated systolic hypertension; in the Systolic Hypertension in Europe (SYST-EUR) trial [33], strokes were reduced by approximately 40%, and all-cause cardiovascular morbidity and mortality were reduced by approximately 30% in isolated systolic hypertension subjects who received the dihydropyridine calcium antagonist, nitrendipine, compared with those on placebo. The Hypertension Optimal Treatment (HOT) trial [34] demonstrated that aggressive BP lowering to values <140/90 mmHg with a felodipine-based regimen was safe and effective. In the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [35], the BP lowering with amlodipine was approximately 3–4 mmHg greater than that seen with lisinopril and 1–2 mmHg greater than chlorthalidone. It appears that these results apply well to nifedipine CR, because nifedipine CR is a long-acting once-daily formulation of nifedipine.
In summary, once-daily nifedipine CR and twice-daily nifedipine retard lowered the BP to a similar extent in the treatment of mild-to-moderate essential hypertension. Nifedipine retard caused a decrease in parasympathetic nerve activity with reflex tachycardia, while nifedipine CR did not produce such effects.
References
- 1.Psaty BM, Heckbert SR, Koepsell TD, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620–5. [PubMed] [Google Scholar]
- 2.Furberg CD, Psaty BM, Meyer JV. Nifedipine: dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326–31. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 3.Ruzicka M, Leenen FH. Relevance of 24 h blood pressure profile and sympathetic activity for outcome on short- versus long-acting 1,4-dihydropyridines. Am J Hypertens. 1996;9:86–94. doi: 10.1016/0895-7061(95)00350-9. [DOI] [PubMed] [Google Scholar]
- 4.Cohn JN. Sympathetic nervous system activity and the heart. Am J Hypertens. 1989;2:353S–356S. [PubMed] [Google Scholar]
- 5.Julius S. Sympathetic hyperactivity and coronary risk in hypertension. Hypertension. 1993;6:886–93. doi: 10.1161/01.hyp.21.6.886. [DOI] [PubMed] [Google Scholar]
- 6.Minami J, Kawano Y, Makino Y, Matsuoka H, Takishita S. Effects of cilnidipine, a novel dihydropyridine calcium antagonist, on autonomic function, ambulatory blood pressure and heart rate in patients with essential hypertension. Br J Clin Pharmacol. 2000;50:615–20. doi: 10.1046/j.1365-2125.2000.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremner AD, Fell PJ, Hosie J, et al. Early side-effects of antihypertensive therapy: comparison of amlodipine and nifedipine retard. J Hum Hypertens. 1993;7:79–81. [PubMed] [Google Scholar]
- 8.Shimoyama M, Ochi H, Takeda S, et al. Effect of controlled-release nifedipine on left ventricular hypertrophy in Japanese patients with hypertension: an open-label, uncontrolled study. Cuur Ther Res Clin Exp. 2001;62:773–82. [Google Scholar]
- 9.Tochikubo O, Ikeda A, Miyajima E, et al. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–24. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 10.Minami J, Yoshii M, Todoroki M, et al. Effects of alcohol restriction on ambulatory blood pressure, heart rate, and heart rate variability in Japanese men. Am J Hypertens. 2002;15:125–9. doi: 10.1016/s0895-7061(01)02265-8. [DOI] [PubMed] [Google Scholar]
- 11.Minami J, Ishimitsu T, Matsuoka H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension. 1999;33:586–90. doi: 10.1161/01.hyp.33.1.586. [DOI] [PubMed] [Google Scholar]
- 12.Minami J, Ishimitsu T, Kawano Y, Matsuoka H. Effects of amlodipine and nifedipine retard on autonomic nerve activity in hypertensive patients. Clin Exp Pharmacol Physiol. 1998;25:572–6. doi: 10.1111/j.1440-1681.1998.tb02254.x. [DOI] [PubMed] [Google Scholar]
- 13.Minami J, Kawano Y, Ishimitsu T, Takishita S. Blunted parasympathetic modulation in salt-sensitive patients with essential hypertension. evaluation by power-spectral analysis of heart-rate variability. J Hypertens. 1997;15:727–35. doi: 10.1097/00004872-199715070-00004. [DOI] [PubMed] [Google Scholar]
- 14.Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate–pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549–56. doi: 10.1161/01.cir.57.3.549. [DOI] [PubMed] [Google Scholar]
- 15.Pomeranz B, Macaulay RJB, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H–151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 16.Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–96. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 17.Akselrod S, Gordon D, Madwed JB, et al. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985;249:H867–H875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- 18.Murdoch D, Brogden RN. Sustained release nifedipine formulations. An appraisal of their current uses and prospective roles in the treatment of hypertension, ischaemic heart disease and peripheral vascular disorders. Drugs. 1991;41:737–79. doi: 10.2165/00003495-199141050-00006. [DOI] [PubMed] [Google Scholar]
- 19.Nakamichi N, Yanagida T, Hikima Y, et al. Phase I study of nifedipine sustained-release formulation (BAY a 1040-OD tablets): multiple administration study. Jpn Pharmacol Ther. 1995;23(Suppl):S257–S269. (in Japanese) [Google Scholar]
- 20.Gibson RS, Boden WE. Calcium channel antagonists: friend or foe in postinfarction patients? Am J Hypertens. 1996;9:172S–176S. doi: 10.1016/s0895-7061(96)00386-x. [DOI] [PubMed] [Google Scholar]
- 21.Messerli FH. What, if anything, is controversial about calcium antagonists? Am J Hypertens. 1996;9:177S–181S. doi: 10.1016/s0895-7061(96)00387-1. [DOI] [PubMed] [Google Scholar]
- 22.Kloner RA. The issue of the cardiovascular safety of dihydropyridines. Am J Hypertens. 1996;9:182S–186S. doi: 10.1016/s0895-7061(96)00388-3. [DOI] [PubMed] [Google Scholar]
- 23.Schobel HP, Langenfeld M, Gatzka C, et al. Treatment and post-treatment effects of alpha- versus beta-receptor blockers on left ventricular structure and function in essential hypertension. Am Heart J. 1996;132:1004–9. doi: 10.1016/s0002-8703(96)90013-7. [DOI] [PubMed] [Google Scholar]
- 24.Julius S. The evidence for a pathophysiologic significance of the sympathetic overactivity in hypertension. Clin Exp Hypertens. 1996;18:305–21. doi: 10.3109/10641969609088965. [DOI] [PubMed] [Google Scholar]
- 25.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 26.Tsuji H, Venditti FJ, Jr, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–83. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji H, Larson MG, Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 28.Izumi H, Nakamura I. Nifedipine-induced inhibition of parasympathetic-mediated vasodilation in the orofacial areas of the cat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R332–R339. doi: 10.1152/ajpregu.2000.279.1.R332. [DOI] [PubMed] [Google Scholar]
- 29.Sato H, Arai H, Fukuda E, et al. Effects of nifedipine retard on heart rate and autonomic balance in patients with ischemic heart disease. Int J Clin Pharmacol Res. 2001;21:65–71. [PubMed] [Google Scholar]
- 30.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96:3224–32. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 31.Hamada T, Watanabe M, Kaneda T, et al. Evaluation of changes in sympathetic nerve activity and heart rate in essential hypertensive patients induced by amlodipine and nifedipine. J Hypertens. 1998;16:111–18. doi: 10.1097/00004872-199816010-00016. [DOI] [PubMed] [Google Scholar]
- 32.Weir MR, Izzo JL. Calcium antagonists. In: Izzo JL, Black HR, editors. Hypertension Primer. 3. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 433–7. [Google Scholar]
- 33.Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–64. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 34.Hansson L, Zanchetti A, Carruthers SG, et al. HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 35.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]