Abstract
Aims
There is considerable unexplained interindividual variability in the methadone dose-effect relationship. The efflux pump P-glycoprotein (P-gp) regulates brain access and intestinal absorption of many drugs. Evidence suggests that methadone is a P-gp substrate in vitro, and P-gp affects methadone analgesia in animals. However the role of P-gp in human methadone disposition and pharmacodynamics is unknown. This investigation tested the hypothesis that the intestinal absorption and pharmacodynamics of oral and intravenous methadone are greater after inhibition of intestinal and brain P-gp, using the P-gp inhibitor quinidine as an in vivo probe.
Methods
Two randomized, double-blind, placebo-controlled, balanced crossover studies were conducted in healthy subjects. Pupil diameters and/or plasma concentrations of methadone and the primary metabolite EDDP were measured after 10 mg intravenous or oral methadone HCl, dosed 1 h after oral quinidine (600 mg) or placebo.
Results
Quinidine did not alter the effects of intravenous methadone. Miosis tmax (0.3 ± 0.3 vs 0.3 ± 0.2 h (−0.17, 0.22)), peak (5.3 ± 0.8 vs 5.1 ± 1.0 mm (0.39, 0.84)) and AUC vs time (25.0 ± 5.7 vs 26.8 ± 7.1 mm h (−6.1, 2.5)) were unchanged (placebo vs quinidine (95% confidence interval on the difference)). Quinidine increased (P < 0.05) plasma methadone concentrations during the absorptive phase, decreased tmax (2.4 ± 0.7 vs 1.6 ± 0.9 h (0.33, 1.2)), and increased peak miosis (3.2 ± 1.5 vs 4.3 ± 1.6 mm (−1.96, −0.19)) after oral methadone. The Cmax (55.6 ± 10.3 vs 59.4 ± 14.1 ng ml−1 (−8.5, 0.65)) and AUC of methadone (298 ± 46 vs 316 ± 74 ng ml−1 h (−54, 18)) were unchanged, as were the EDDP : methadone AUC ratios. Quinidine had no effect on the rate constant for transfer of methadone between plasma and effect compartment (ke0) (2.6 ± 2.6 vs 2.5 ± 1.4 h−1 (−3.5, 4.2)).
Conclusions
Quinidine increased the plasma concentrations of oral methadone in the absorptive phase and the miosis caused by methadone, suggesting that intestinal P-gp affects oral methadone absorption and hence its clinical effects. Quinidine had no effect on methadone pharmacodynamics after intravenous administration, suggesting that if quinidine is an effective inhibitor of brain P-gp, then P-gp does not appear to be a determinant of the access of methadone to the brain.
Keywords: blood–brain barrier, intestinal absorption, methadone, miosis, P-glycoprotein, pharmacodynamics, quinidine
Introduction
Methadone maintenance is the cornerstone of opiate addiction therapy, with documented efficacy and cost-effectiveness in preventing opiate abstinence syndrome and in reducing mortality, use of illicit opiates and codependent drugs, the incidence of infectious disease and crime, as well as increasing social productivity [1–3]. Methadone maintenance is a vital public health strategy for HIV/AIDS risk reduction [3, 4]. Methadone is also highly useful in the treatment of acute pain and chronic cancer pain, either as a primary modality or as an alternative in an opioid rotation protocol [5, 6]. Methadone has several beneficial characteristics, including high oral bioavailability, minimally active metabolites, efficacy in treating pain which is unresponsive to other opioids, and a long half-life which permits infrequent dosing. These characteristics have led to the development of medical maintenance programs and office-based pharmacotherapy with methadone for opioid addiction, including increased use of take home medication [3].
Confounding the goal of reproducibly maintaining therapeutic methadone blood (and CNS) concentrations, and leading to opiate withdrawal, unwanted side-effects, addiction treatment failures, and inadequate analgesia, is the considerable and (currently) unpredictable inter- and intra-individual variability in methadone disposition, including both pharmacokinetic and pharmacodynamic variability [7, 8]. This results in clinically significant variability in the dose-effect relationship for oral and intravenous methadone. Methadone is metabolized by human intestinal and hepatic microsomes, with 20–30% of a dose undergoing first-pass extraction [8, 9]. The primary route of metabolism and inactivation is N-demethylation to EDDP, catalyzed predominantly by CYP3A [8, 9]. There is considerable interindividual variability in methadone bioavailability (36–100%), intestinal and hepatic metabolism, first-pass extraction, systemic clearance (0.023–2.1 l min−1), and ke0 (the first order rate constant for transfer between plasma and the effect compartment) [7, 8]. There is also considerable variability in the pharmacodynamics of methadone, specifically the EC50 for analgesia, the subjective self-assessment of mood and well-being, and the effect of withdrawal [7, 8]. Together these pharmacokinetic and pharmacodynamic differences result in clinically significant variability in the dose-effect relationship, the mechanism(s) of which are not well understood.
The drug efflux pump P-glycoprotein (P-gp) is located on the luminal surface of brain capillary endothelial cells, where it is an integral component of the blood–brain barrier. Its function is to actively transport substrate drugs out of the brain thereby limiting brain access, and decreasing pharmacological effects [10–12]. P-gp is also expressed on the apical surface of intestinal epithelial cells, where it actively transports drugs back into the gut lumen and limits their oral bioavailability [10, 13], and also in renal proximal tubules [14]. Gender differences in P-gp activity have been suggested to explain differences in the disposition of some CYP3A substrates. This is supported by results from liver biopsies, but phenotyping did not find a difference [15].
There is some evidence to indicate that methadone is a substrate for P-gp. Using an in vitro gut sac model, Bouër et al. showed that methadone transport was increased in the presence of the P-gp inhibitors verapamil and quinidine [16]. Methadone also inhibited P-gp activity, particularly P-gp-mediated rhodamine123 transport across Caco-2 cell monolayers [17, 18]. In P-gp knockput mice, methadone analgesia was significantly increased [19]. Thus, methadone is a substrate for P-gp in vitro, and P-gp in animals affects methadone access to the brain and analgesia.
The antidiarrhoeal opioid loperamide decreases gut motility but is normally without central nervous system effects, because it is efficiently excluded from the brain by P-gp [20]. Loperamide alone had no effect on respiration, but markedly depressed breathing in subjects pretreated with the P-gp inhibitor quinidine [21]. This finding was originally attributed to a pharmacodynamic effect of quinidine on loperamide, since quinidine was reported to have no effect on plasma loperamide concentrations.
These observations raise the possibility that P-gp may influence brain access and pharmacodynamics of methadone in humans. P-gp may also explain important clinical differences between opioids with respect to their onset times for analgesia. P-gp may also be a determinant of the oral absorption of methadone, its bioavailability, and clinical effects. Therefore, the purpose of this investigation was to assess the role of intestinal and brain P-gp in determining plasma concentrations and clinical effects of oral methadone, and of the clinical effects of intravenous methadone in humans. The P-gp inhibitor quinidine, used previously to evaluate the role of P-gp in the disposition of loperamide in the brain [21], was used as the in vivo probe.
Methods
Subjects and clinical protocol
Two randomized, double-blind, placebo-controlled, balanced crossover studies were conducted in healthy subjects. The protocols were approved by the University of Washington Institutional Review Board and each subject provided written informed consent. Subjects were in good health, within 25% of ideal body weight, had no history of hepatic or renal disease, were taking no prescription medications (except oral contraceptives), and were taking no non-prescription preparations known to alter CYP3A activity. Both smokers and non-smokers were enrolled. Subjects were instructed to consume no grapefruit-containing foods or juices for 5 days before and on each study day, and to consume no alcohol or caffeine for 1 day before and on each study day. Subjects were also asked to eat or drink nothing after midnight before each study day.
On each occasion, a catheter was placed in an arm vein for blood sampling or drug administration. Subjects were supine and were monitored with a pulse oximeter for 2 h after methadone administration, and received supplemental oxygen for an oxygen saturation less than 94%. After baseline measurements of methadone concentration and pupil diameter, subjects received either a 600 mg oral dose of immediate release quinidine sulphate or an oral placebo (lactose). Subjects and all investigators were blinded to the identity of this pretreatment. A 12-lead electrocardiogram was obtained 0.5, 1, 1.5, 2, 3 and 8 h after quinidine or placebo. Subjects were fed a standard breakfast 2 h after methadone administration and had free access to food and water thereafter. The washout period between placebo and quinidine phases was approximately 1 week.
In the first protocol subjects (n = 12, six males and six females aged 24 ± 5 (20–32) years (mean, SD, range) and weighing 69 ± 15 (54–106) kg) received methadone (10 mg HCl, 8.95 mg methadone base) as a 5 min IV infusion via a syringe pump 1 h after receiving quinidine or placebo. Dark-adapted pupil diameters were measured each minute during the infusion and 1, 3, 5, 7, 10, 15, 30, 45, 60, 75, 90, 105, 120, 150, 180, 240, 300, 360, and 480 min after the end of the infusion, using a Pupilscan Model 12 A infrared pupillometer (Keeler USA) as described previously [22]. The pupil diameter measurement obtained prior to the methadone infusion was taken as the baseline value, and used to calculate pupil diameter change at each time point.
In the second protocol oral methadone was administered. Ten subjects were studied (five males and five females aged 25 ± 5 (19–33) years and weighing 71 ± 14 (51–103) kg. Thirty min after quinidine or placebo, subjects received ondansetron (4 mg IV) for the prophylaxis of nausea. Thirty min later they were given 10 mg oral methadone HCl with 50 ml water. Venous blood samples were obtained before and 5, 10, 15, 30, 45, 60, 90, 120, 180, 240, 300, 360, and 480 min after methadone dosing. Plasma was separated and stored at −20 °C for later analysis. Dark-adapted pupil diameter was measured coincident with blood sampling (and also at 2.5 and 3.5 h).
Subjective self-assessment of feelings or mood states was measured by Visual Analogue Scales (VAS). The effects assessed (and scored from 0 to 100) included the level of alertness/sedation (almost asleep to wide awake), energy level (no energy to full of energy), confusion (confused to clear headed), clumsiness (extremely clumsy to well coordinated), anxiety (calm/relaxed to extremely nervous), and nausea (no nausea to worst nausea). Subjects were tested at baseline (prior to quinidine or placebo) and following each pupil diameter measurement.
The dose of methadone has been used safely by us in a number of previous studies [61], and is lower than that used by other investigators [62]. The dose of quinidine is within the range used clinically.
Analytical methods
Chemicals and reagents
Plasma concentrations of methadone and the primary metabolite 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolinium perchlorate (EDDP) were determined by HPLC-mass spectrometry (LCMS) using an Agilent (Palo Alto, CA) 1100 LC-MSD. Racemic methadone HCl was obtained from Sigma Corp. (St Louis, MO) and EDDP was obtained from the National Institutes of Drug Abuse. Methanol and acetonitrile (HPLC-grade) were from Fisher Scientific (Pittsburgh, PA). Oasis MCX solid-phase extraction (SPE) cartridges were from Waters Corp (Milford, MA). All stock drug solutions, buffers, and HPLC mobile phase were prepared using Milli-Q (Millipore, Bedford, MA) water.
Plasma (0.5 ml), 0.01 ml phosphoric acid, 400 µl water, and 2.5 ng internal standard (7-dimethylamino-5,5-diphenyl-4-octanone) were mixed in glass culture tubes by vortexing for 2 min, then loaded (0.5 ml min−1) onto MCX SPE cartridges (1ml, 30 mg) which had been conditioned with 1 ml methanol, then 1 ml 0.1 n HCl. Cartridges were washed with 1 ml 0.1 n HCl followed by 1 ml methanol, thoroughly dried under vacuum for 2 min, and analytes eluted by gravity in 1 ml of 5% ammonium hydroxide in methanol. Samples were evaporated to dryness at 45 °C under nitrogen, reconstituted with 50 µl of methanol (with 0.05%TFA) : water (with 0.05% TFA) (45 : 55 v : v), transferred to glass autosampler vials, and 15 µl was injected onto the HPLC. Separation was achieved with a Zorbax Eclipse XDB-C18 column (2.1 × 50 mm, 5 µm) with an Eclipse XDB-C8 guard column (2.1 × 12.5 mm, 5 µm) (Agilent). The binary HPLC mobile phase consisted of (a) 0.05% trifluoroacetic acid in water and (b) 0.05% trifluoroacetic acid in methanol. The initial solvent system (48% b) was maintained for 2 min then increased to 75% over the next 4.5 min, further increased to 90% over the next 0.5 min, and maintained at 90% for 1 min before returning to 48%, followed by 3 min for column equilibration, at a flow rate of 0.25 ml min−1. Mass spectrometer settings were: nitrogen drying gas (325 °C, 6 l min−1), nebulizer pressure 20 psi, capillary voltage 4000 V, fragmentor 70 V and SIM resolution set to high. Retention times were 3.2, 6.3, and 7.2 min for EDDP, methadone and the internal standard, respectively, which were monitored at m/z 278.1, 310.1 and 324.1, respectively. Calibration curves were obtained by analyzing drug-free plasma fortified with methadone (0.5, 1, 2, 5, 15, 50, 100, 200 ng ml−1) and EDDP (0.05, 0.1, 0.2, 0.5, 1, 2, 5, 10 ng ml−1). Quality control samples (2 and 100 ng ml−1 methadone; 1 and 5 ng ml−1 EDDP) were prepared from separate dilutions of stock solutions than those used for the calibration curves. The limit of quantification was the lowest point on the calibration curve. Samples from each subject were all analyzed on the same day. Standard curves were linear (mean r2 > 0.993 for both methadone and EDDP). Interday coefficients of variation were 12% and 10% at 2 and 100 ng ml−1 methadone, and 16% and 20% at 1 and 5 ng ml−1 EDDP, respectively.
Data analysis
The areas under the curve (AUC) during the measurement interval (0–8 h) for plasma methadone and EDDP concentration and miosis vs time, maximum observed plasma concentration or pupil change (Cmax, maximum effect), and time to maximum plasma concentration or effect (tmax) were determined by noncompartmental analysis using WinNonlin (Pharsight, Palo Alto, CA). Concentration-effect data were analyzed by nonparametrically collapsing the hysteresis loops to determine the value of ke0, the first order rate constant for transfer between plasma and the effect compartment [23]. This was accomplished using the program ke0obj (written by Steve Shafer at Stanford University, available at http://anesthesia.stanford.edu/pkpd/, last accessed August 23, 2003). Methadone and EDDP plasma concentrations, pupil diameter changes, and VAS scores were evaluated by two-way repeated measures analysis of variance followed by the Student-Newman-Keuls test for multiple comparisons using SigmaStat (SPSS, Chicago IL). AUCs, tmax, maximum effect, tmax and ke0 were compared by paired t-tests. All results are reported as mean ± SD. Statistical significance was assigned at P < 0.05.
Results
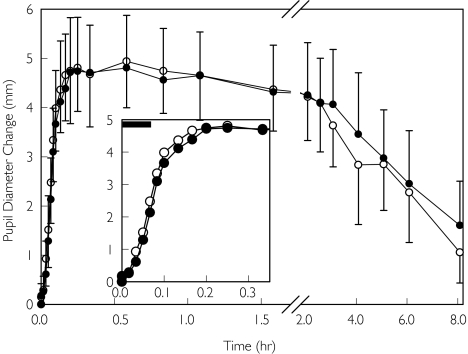
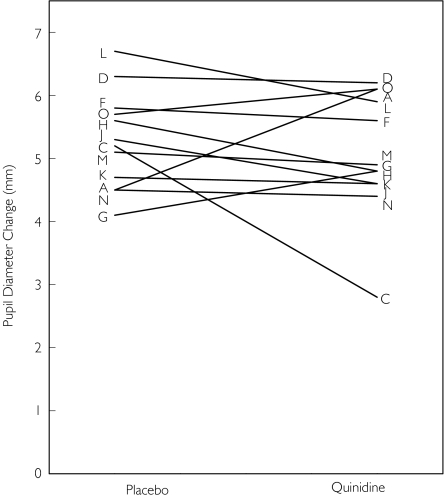
Intravenous methadone was administered as a 5 min infusion to reduce peak concentrations and thus side-effects such as respiratory depression and sedation. The onset of drug effect was relatively rapid, with maximal miosis occurring approximately 10–15 min after the end of the infusion (Figure 1 and Table 1). The influence of quinidine pretreatment on miosis after intravenous methadone is shown in Figure 1. There were no significant differences between pretreatment with quinidine and placebo in pupil diameters at any point in time. Similarly, there was no effect of quinidine on the time to or magnitude of maximum miosis (Table 1). Furthermore, quinidine pretreatment did not influence the apparent elimination rate for miosis or the AUC for miosis. Individual responses to intravenous methadone are shown in Figure 2. There were relatively small interindividual differences in miosis, with coefficients of variation of 15–20% and 25%, respectively, for peak miosis and AUC, even without weight-adjusted dosing. VAS scores for the subjective effects of methadone were unchanged by quinidine pretreatment (results not shown).
Figure 1.
Influence of quinidine on the miotic effects of IV methadone. Subjects received 10 mg IV methadone HCl as a 5 min infusion (shown as a solid bar in the insert), 1 h after quinidine (•) or placebo (○). Results are the change from baseline in dark-adapted pupil diameter, presented as the mean ± SD. The insert shows the first 20 min in greater detail. There were no significant differences between treatment groups
Table 1.
The effects of intravenous methadone
| Placebo | Quinidine | 95% confidence interval | |
|---|---|---|---|
| tmax (h) | 0.3 ± 0.3 | 0.3 ± 0.2 | (−0.17, 0.22) |
| Peak miosis (mm) | 5.3 ± 0.8 | 5.1 ± 1.0 | (−0.39, 0.84) |
| AUC(0,8 h) (mm h) | 25.0 ± 5.7 | 26.8 ± 7.1 | (−6.1, 2.5) |
| kel (h−1) | 0.24 ± 0.14 | 0.17 ± 0.08 | (−0.019, 0.15) |
There were no significant differences between groups.
Figure 2.
Influence of quinidine on miosis after IV methadone. Shown are peak miotic effects for each subject. Each set of data points is an individual subject, identified by a letter
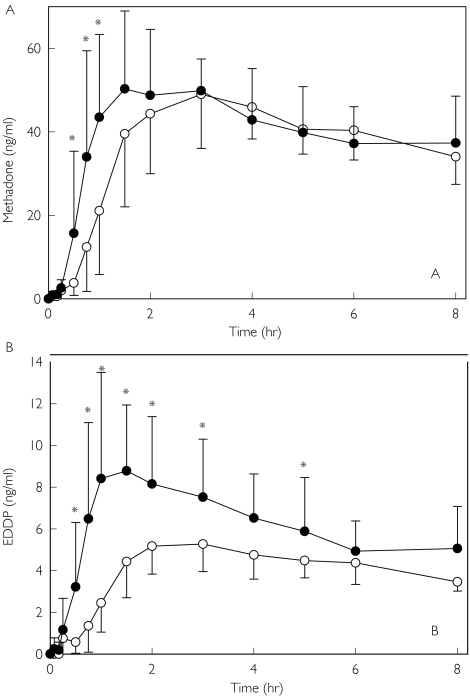
Plasma methadone and EDDP concentrations following oral methadone administration after placebo or quinidine pretreatment are shown in Figure 3. Quinidine significantly increased methadone concentrations during the absorptive phase (0.5–1 h), and tmax was decreased by one third. However methadone Cmax and AUC were unchanged (Table 2). Since the focus of this investigation was the early phase of the pharmacokinetics of methadone, plasma concentrations were not sampled sufficiently long enough to characterize methadone elimination. Quinidine also significantly increased plasma EDDP concentrations (Figure 3b) and the EDDP : methadone AUC(0,8 h) ratio.
Figure 3.
Influence of quinidine on the disposition of (A) methadone and (B) EDDP. Subjects received 10 mg oral methadone HCl 1 h after quinidine (•) or placebo (○). Results are plasma concentrations (mean ± SD). Asterisks denote significant differences between treatment groups (P < 0.05)
Table 2.
The disposition and effects of oral methadone
| Placebo | Quinidine | 95% confidence interval | |
|---|---|---|---|
| Pharmacokinetics | |||
| Methadone tmax (h) | 2.4 ± 0.7 | 1.6 ± 0.9* | (0.33, 1.2) |
| Methadone Cmax (ng ml−1) | 55.6 ± 10.3 | 59.4 ± 14.1 | (−8.5, 0.65) |
| Methadone AUC(0,8 h) (ng ml−1 h) | 298 ± 46 | 316 ± 74 | (−54, 18) |
| EDDP Cmax (ng ml−1) | 5.9 ± 1.2 | 10.6 ± 3.7* | (−7.1, −2.3) |
| AUC(0,8 h) EDDP : AUC(0,8 h) methadone | 0.11 ± 0.02 | 0.16 ± 0.06* | (−0.087, −0.011) |
| Pharmacodynamics | |||
| tmax (h) | 3.0 ± 1.1 | 2.8 ± 1.3 | (−0.59, 1.09) |
| Peak miosis (mm) | 3.2 ± 1.5 | 4.3 ± 1.6* | (−1.96, −0.19) |
| AUC(0,8 h) (mm h) | 14.8 ± 8.9 | 19.9 ± 10.1 | (−11.2, 1.2) |
| ke0 (h−1) (median) | 2.6 ± 2.6 (1.5) | 2.5 ± 1.4 (2.2) | (−3.5, 4.2) |
Significantly different from placebo (P < 0.05).
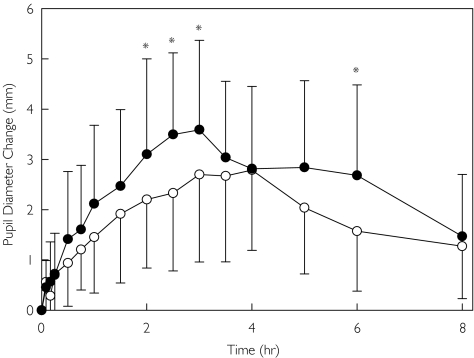
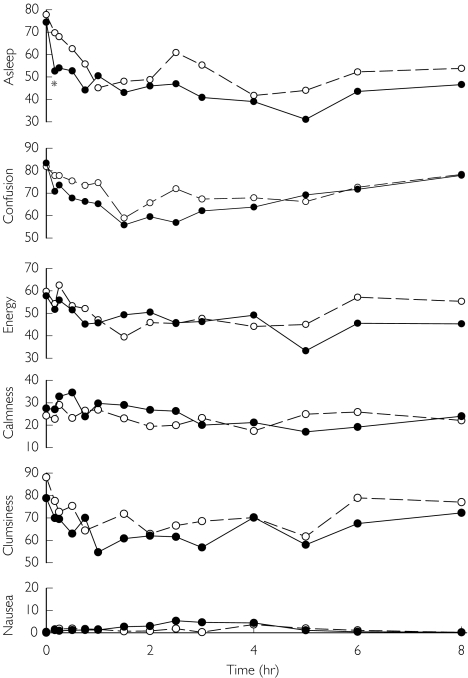
Quinidine pretreatment also increased the effects of oral methadone, assessed by dark-adapted pupil diameter change vs baseline (Figure 4). Maximum miosis was significantly increased by quinidine, although the AUC for miosis did not reach statistical significance due to interindividual variability and a small subject population (Table 2). Quinidine pretreatment did not significantly alter any subjective measure of the effect of oral methadone, based on subject self-assessment (Figure 5).
Figure 4.
Influence of quinidine on oral methadone effects. Results are the change from baseline in dark-adapted pupil diameter. Results as the mean ± SD (placebo (○), quinidine (•)). Asterisks denote significant differences between treatment groups (P < 0.05)
Figure 5.
Influence of quinidine on the subjective effects of oral methadone. Attributes assessed (scored from 0 to 100) were sedation (almost asleep to wide awake), energy level (no energy to full energy), confusion (confused to clear headed), clumsiness (extremely clumsy to well coordinated), anxiety (calm to extremely nervous), and nausea (no nausea to worst nausea). Results are the mean (placebo (○), quinidine (•)). SDs are omitted for clarity. Asterisks denote significant differences between treatment groups (P < 0.05)
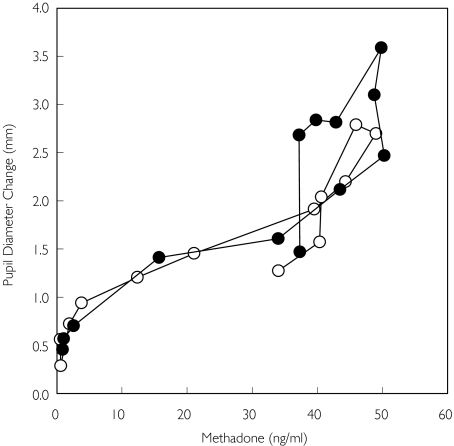
Concentration-effect data for oral methadone are provided in Figure 6. A hysteresis plot of the mean concentration-effect relationship showed negligible counter-clockwise hysteresis, consistent with the minimal delay in onset of miosis after intravenous methadone (Figure 1). The hysteresis loop after oral methadone was shifted slightly to the right and upward by quinidine pretreatment, commensurate with higher plasma methadone concentrations. Nonetheless, there was no collapse of the ascending and descending limbs, which would have reflected accelerated brain entry of methadone. There was no effect of quinidine on ke0 for miosis (Table 2).
Figure 6.
Influence of quinidine on the pharmacodynamics of oral methadone, shown as a counter-clockwise hysteresis plot. Each data point is the mean of 12 subjects, with SD omitted for clarity (placebo (○), quinidine (•))
The mean increase in uncorrected QT interval was 0.04 ± 0.03 s, and the maximum increase in QT interval in any subject at any time point was 0.12 s. These were well below the predefined safety criteria of a 50% increase above baseline. One subject had an oxygen saturation less than 94%, after IV methadone, and briefly received supplemental oxygen. Treatment of nausea and/or vomiting with ondansetron was required in 11 and 9 subjects, respectively, in the intravenous and oral methadone groups. All safety related events were considered minor.
Discussion
The major finding of this investigation was that quinidine significantly increased concentrations of orally administered methadone in the absorptive phase, with a concomitant increase in miosis. This supports the hypothesis that a pathway inhibited by quinidine mediates, at least in part, methadone intestinal transport and absorption.
Quinidine inhibits both CYP3A and P-gp function, and hence the results may be due to inhibition of methadone first-pass metabolism and/or transepithelial transport. Quinidine might have altered the renal excretion of methadone, since that of digoxin is significantly decreased by quinidine, probably through inhibition of P-gp [24]. However, this is unlikely since renal clearance accounts for only a small fraction of the overall elimination of methadone [8] which was unaffected by quinidine. The effects of quinidine on methadone absorption might also be explained by increased gastric emptying [25].
Methadone undergoes CYP3A-catalyzed N-demethylation to EDDP [8, 9]. Quinidine is a CYP3A substrate and inhibitor; thus quinidine inhibition of first-pass methadone metabolism might explain the increased plasma concentrations after oral methadone. However, quinidine did not decrease the EDDP : methadone concentration or AUC ratios making it unlikely that inhibition of CYP3A metabolism by quinidine accounts for the increased methadone concentrations. Although quinidine increased plasma EDDP concentrations more than those of methadone, an explanation is not apparent. Quinidine can stimulate the metabolism of certain CYP3A substrates [26]; however, it did not stimulate human liver microsomal methadone metabolism [27]. Quinidine inhibition of P-gp-mediated methadone efflux from the liver might have lead to greater metabolism; however, this is unproven. Lack of inhibition of methadone first-pass metabolism by quinidine is consistent with the inhibitory selectivity of quinidine for CYP2D6 in vivo[28], and the lack of effect on first-pass loperamide metabolism by quinidine in humans [21]. Although some studies have suggested a role for CYP2D6 in methadone metabolism in vivo[8], the lack of inhibition of methadone first-pass metabolism or systemic clearance by quinidine does not support this hypothesis.
The present findings support the view that methadone is a substrate for human intestinal P-gp (or some other transporter that is inhibited by quinidine) and that this transport activity is a determinant of the oral absorption of methadone. This is consistent with the substantially lower IC50 for quinidine inhibition of P-gp (2 µm) compared with that for CYP3A (51 µm) [29]. An apparent role for P-gp in methadone absorption is also consistent with previously observed effects of rifampicin and troleandomycin on methadone disposition [30]. The CYP3A and P-gp inducer rifampicin markedly reduced the plasma concentrations and bioavailability of oral methadone, and caused a four-fold increase in apparent oral clearance [30]. In contrast, the highly effective and selective CYP3A inhibitor troleandymycin [31–33], which significantly increased the oral bioavailability of the CYP3A substrates midazolam, alfentanil and erythromycin [34, 35], had no effect on methadone bioavailability [30]. This suggests an effect of rifampicin on P-gp-mediated intestinal methadone transport. Together, the effects of rifampicin and quinidine on methadone disposition support the hypothesis that methadone is a substrate for human intestinal P-gp, and that P-gp activity is an important determinant of plasma concentrations after oral methadone and hence its clinical effects.
The involvement of P-gp in methadone absorption is supported by previous in vitro and animal data. Callaghan & Riordan first showed that opioids such as morphine and methadone are P-gp substrates in vitro[17]. Methadone has been shown, using an in vitro gut sac model, to undergo P-gp-mediated transport that was increased by the P-gp inhibitors verapamil and quinidine [16]. Methadone also inhibited P-gp-mediated rhodamine123 transport in Caco-2 cells [18].
One clinical implication of the present findings is that P–gp mediated drug interactions may contribute to interindividual variability in methadone dose–response relationships. The effects of quinidine and rifampicin on oral methadone disposition are the first examples, and are similar to effects on the absorption of oral morphine [36, 37]. Diet, food and herbal preparations such as grapefruit juice, orange juice and St John's Wort affect P-gp activity [38], and thus may influence oral methadone disposition. There may be methadone interactions even more profound than the moderate effect of quinidine, for example with inhibition or induction of P-gp by HIV/AIDS drugs, most notably HIV protease inhibitors [39–41]. Ritonavir, nelfinavir, amprenavir, efavirenz and nevirapine all cause methadone withdrawal, associated with decreased plasma concentrations [8]. Whether these effects are due to P-gp induction, CYP3A induction, or both, remains to be determined. Pharmacogenetic variability in P-gp [14] may also potentially contribute to interindividual differences in methadone disposition. Further studies are needed to determine the influence of P-gp pharmacogenetics and drug interactions on oral methadone disposition.
A further finding of this investigation was that quinidine had no influence on miosis after intravenous methadone or on the plasma concentration-effect relationship after oral methadone. This was unexpected. Indeed, the intravenous methadone protocol was performed first (and without blood sampling because an unambiguous quinidine potentiation of methadone miosis was anticipated). The effects of quinidine on loperamide, reported to be caused by P-gp inhibition and increased brain access of loperamide [21], on which the present protocol was based, were therefore re-evaluated. We considered that perhaps there was a greater effect of quinidine on oral loperamide bioavailability (adding to an apparent effect on loperamide pharmacodynamics) than initially appreciated. Consequently, the second protocol evaluated the effects of quinidine on oral methadone disposition and the concentration-effect relationship. The latter was unchanged, hence the effects of quinidine on miosis after oral methadone were due entirely to increased methadone plasma concentrations in the absorption phase rather than on brain penetration and blood brain barrier P-gp.
The lack of an apparent role for P-gp in the access of of methadone to the brain is consistent with the rapid onset of the effects of methadone, whereby peak miosis occurred within 10–15 min after the infusion, and with previous studies showing rapid onset of methadone analgesia and sedation [42, 43]. This suggests a relatively small delay in the transit of methadone from plasma to the effect compartment. Rapid onset of the effects of methadone resembles that of fentanyl [44, 45], and differs from that of morphine. For example, using identical 5 min infusions, maximal miosis (tmax) for methadone, fentanyl and morphine occurred 0.3 ± 0.3, 0.13 ± 0.04 and 1.0 ± 0.9 h after the start of the infusion, respectively [46]. This is consistent with the effect compartment equilibration half-life (t1/2ke0) of 4 min for methadone analgesia in chronic pain patients [42] and 8–9 min for methadone analgesia and sedation in cancer patients [43], compared with 5 min for fentanyl [47] and 210 min for morphine [48].
The lack of effect of quinidine on methadone pharmacodynamics in humans differs from the reported role of P-gp in animals. In mdr1a/(−/−) P-gp knockout mice, methadone analgesia was increased three-fold compared with wild-type mice [19]. There may be pertinent species differences in methadone transport by P-gp. Indeed, there are known differences between mice and humans in P-gp transport of several compounds [20, 49]. Unfortunately, there is relatively little information regarding methadone transport in humans or animals. However lack of quinidine effects on methadone pharmacodynamics in humans was similar to the lack of effect on morphine and fentanyl pharmacodynamics [46], and in contrast to evidence for carrier-mediated transport of these opioids in animals [50–54]. Further investigations are needed to determine the relevance of animal models for human methadone brain transport.
The lack of effect of quinidine on methadone pharmacodynamics also differs from the effects of quinidine on loperamide pharmacodynamics in humans [21]. Loperamide is an unambiguous P-gp substrate in vitro and in animals in vivo[20, 55]. Unfortunately there are no direct comparisons of loperamide and methadone transport. If methadone is a comparatively weaker P-gp substrate than loperamide, then this might affect their susceptibility to P-gp inhibition and explain differences between quinidine effects on methadone and loperamide pharmacodynamics in humans.
Differences in the effects of quinidine on methadone pharmacodynamics compared with those on intestinal absorption may also be related to quinidine concentrations in the brain and gut. Quinidine is a comparatively weak P-gp inhibitor, and plasma quinidine concentrations may have been inadequate to inhibit brain P-gp activity [56]. The IC50 for quinidine inhibition of P-gp was 2–34 µmin vitro[29, 57, 58], whereas oral quinidine (600 mg) gives peak plasma concentrations of only about 6 µm[59]. Thus systemic quinidine concentrations may not have been sufficiently high to inhibit brain P-gp activity and P-gp mediated methadone transport. Since, intestinal drug concentrations are substantially higher than in plasma after oral dosing, even a comparatively weak inhibitor of P-gp like quinidine might achieve sufficient concentrations to enhance oral bioavailability [56]. Lower systemic vs intestinal quinidine concentrations, particularly if methadone has a low affinity for P-gp compared with loperamide, might also account for the effect of quinidine on plasma concentrations but not methadone pharmacodynamics after oral methadone. This does not explain however, why quinidine enhanced loperamide [21] but not methadone brain access. More generally, it remains unknown whether quinidine is the optimal in vivo probe for determining P-gp participation in brain drug penetration, as discussed previously [60].
One potential limitation of this investigation merits mention. Methadone used clinically is a racemate, although the (R)-enantiomer is 10–50 times more potent than its antipode [8]. This investigation evaluated the clinical effects, pharmacokinetics and pharmacodymanics of the racemate, rather than individual enantiomers. Since quinidine did not affect the concentration-effect relationship of the racemate, it is unlikely that those of the more active (R)-enantiomer were affected. It remains unknown, however, whether there are stereoselective differences in the effects of quinidine on oral methadone absorption.
In summary, this investigation showed that quinidine, used as an in vivo inhibitor probe for intestinal and brain P-gp, increased the plasma concentrations of oral methadone in the absorption phase and the extent of miosis in healthy subjects, but had no influence on methadone pharmacodynamics in humans. This suggests a role for P-gp in the intestinal disposition of oral methadone and a potential for P–gp mediated drug interactions and pharmacogenetic variability, but the exact role for P-gp in brain methadone access requires further investigation.
Acknowledgments
This study was supported by an award from the National Institutes of Drug Abuse to the University of Washington Alcohol and Drug Abuse Institute, and by NIH grants R01-DA14211, K24-DA00417 and M01-RR00037 to the University of Washington General Clinical Research Center.
References
- 1.NIH Consensus Conference. Effective medical treatment of opiate addiction. JAMA. 1998;280:1936–43. [PubMed] [Google Scholar]
- 2.Barnett PG, Hui SS. The cost-effectiveness of methadone maintenance. Mt Sinai J Med. 2000;67:365–74. [PubMed] [Google Scholar]
- 3.Kreek MJ, Vocci FJ. History and current status of opioid maintenance treatments: blending conference session. J Subst Abuse Treat. 2002;23:93–105. doi: 10.1016/s0740-5472(02)00259-3. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen JL, Copeland AL. Drug abuse treatment as an HIV prevention strategy: a review. Drug Alcohol Depend. 2000;59:17–31. doi: 10.1016/s0376-8716(99)00104-0. [DOI] [PubMed] [Google Scholar]
- 5.Mercadante S, Casuccio A, Fulfaro F, et al. Switching from morphine to methadone to improve analgesia and tolerability in cancer patients: a prospective study. J Clin Oncol. 2001;19:2898–2904. doi: 10.1200/JCO.2001.19.11.2898. [DOI] [PubMed] [Google Scholar]
- 6.Ripamonti C, Bianchi M. The use of methadone for cancer pain. Hematol Oncol Clin North Am. 2002;16:543–55. doi: 10.1016/s0889-8588(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 7.Garrido MJ, Troconiz IF. Methadone: a review of its pharmacokinetic/pharmacodynamic properties. J Pharmacol Toxicol. 1999;42:61–6. doi: 10.1016/s1056-8719(00)00043-5. [DOI] [PubMed] [Google Scholar]
- 8.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–93. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- 9.Oda Y, Kharasch ED. Metabolism of methadone and levo-alpha-acetylmethadol (LAAM) by human intestinal cytochrome P450 3A4 (CYP3A4). potential contribution of intestinal metabolism to presystemic clearance and bioactivation. J Pharmacol Exp Ther. 2001;298:1021–32. [PubMed] [Google Scholar]
- 10.Fromm MF. P-glycoprotein. A defense mechanism limiting oral bioavailability and CNS accumulation of drugs. Int J Clin Pharmacol Ther. 2000;38:69–74. doi: 10.5414/cpp38069. [DOI] [PubMed] [Google Scholar]
- 11.Lee G, Dallas S, Hong M, Bendayan R. Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol Rev. 2001;53:569–96. [PubMed] [Google Scholar]
- 12.De Boer AG, Van Der Sandt IC, Gaillard PJ. The role of drug transporters at the blood–brain barrier. Annu Rev Pharmacol Toxicol. 2003;43:629–56. doi: 10.1146/annurev.pharmtox.43.100901.140204. [DOI] [PubMed] [Google Scholar]
- 13.Fricker G, Miller DS. Relevance of multidrug resistance proteins for intestinal drug absorption in vitro and in vivo. Pharmacol Toxicol. 2002;90:5–13. doi: 10.1034/j.1600-0773.2002.900103.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin JH, Yamazaki M. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet. 2003;42:59–98. doi: 10.2165/00003088-200342010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42:107–21. doi: 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- 16.Bouër R, Barthe L, Philibert C, Tournaire C, Woodley J, Houin G. The roles of P-glycoprotein and intracellular metabolism in the intestinal absorption of methadone: In vitro studies using the rat everted intestinal sac. Fundam Clin Pharmacol. 1999;13:494–500. doi: 10.1111/j.1472-8206.1999.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 17.Callaghan R, Riordan JR. Synthetic and natural opiates interact with P-glycoprotein in multidrug- resistant cells. J Biol Chem. 1993;268:16059–64. [PubMed] [Google Scholar]
- 18.Störmer E, Perloff MD, von Moltke LL, Greenblatt DJ. Methadone inhibits rhodamine123 transport in Caco-2 cells. Drug Metab Dispos. 2001;29:954–6. [PubMed] [Google Scholar]
- 19.Thompson SJ, Koszdin KK, Bernards CM. Opiate-induced analgesia as increased and prolonged in mice lacking P- glycoprotein. Anesthesiology. 2000;92:1392–9. doi: 10.1097/00000542-200005000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood–brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–24. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadeque AJ, Wandel C, He H, Shah S, Wood AJ. Increased drug delivery to the brain by P-glycoprotein inhibition. Clin Pharmacol Ther. 2000;68:231–7. doi: 10.1067/mcp.2000.109156. [DOI] [PubMed] [Google Scholar]
- 22.Phimmasone S, Kharasch ED. A pilot evaluation of alfentanil-induced miosis as a noninvasive probe for hepatic cytochrome P450 3A4 (CYP3A4) activity in humans. Clin Pharmacol Ther. 2001;70:505–17. doi: 10.1067/mcp.2001.119994. [DOI] [PubMed] [Google Scholar]
- 23.Fuseau E, Sheiner LB. Simultaneous modeling of pharmacokinetics and pharmacodynamics with a nonparametric pharmacodynamic model. Clin Pharmacol Ther. 1984;35:733–41. doi: 10.1038/clpt.1984.104. [DOI] [PubMed] [Google Scholar]
- 24.Hedman A, Angelin B, Arvidsson A, Dahlqvist R, Nilsson B. Interactions in the renal and biliary elimination of digoxin. stereoselective difference between quinine and quinidine. Clin Pharmacol Ther. 1990;47:20–6. doi: 10.1038/clpt.1990.3. [DOI] [PubMed] [Google Scholar]
- 25.Moghadamnia AA, Rostami-Hodjegan A, Abdul-Manap R, Wright CE, Morice AH, Tucker GT. Physiologically based modelling of inhibition of metabolism and assessment of the relative potency of drug and metabolite: dextromethorphan vs dextrorphan using quinidine inhibition. Br J Clin Pharmacol. 2003;56:57–67. doi: 10.1046/j.1365-2125.2003.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngui JS, Tang W, Stearns RA, et al. Cytochrome P450 3A4–mediated interaction of diclofenac and quinidine. Drug Metab Dispos. 2000;28:1043–50. [PubMed] [Google Scholar]
- 27.Foster DJ, Somogyi AA, Bochner F. Methadone N-demethylation in human liver microsomes. Lack of stereoselectivity and involvement of CYP3A4. Br J Clin Pharmacol. 1999;47:403–12. doi: 10.1046/j.1365-2125.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Branch RA, Adedoyin A, Frye RF, Wilson JW, Romkes M. In vivo modulation of CYP enzymes by quinidine and rifampin. Clin Pharmacol Ther. 2000;68:401–11. doi: 10.1067/mcp.2000.110561. [DOI] [PubMed] [Google Scholar]
- 29.Wandel C, Kim RB, Kajiji S, Guengerich P, Wilkinson GR, Wood AJ. P-glycoprotein and cytochrome P-450 3A inhibition: dissociation of inhibitory potencies. Cancer Res. 1999;59:3944–8. [PubMed] [Google Scholar]
- 30.Whittington D, Sheffels P, Hoffer C, Kharasch E. Role of CYP3A in the metabolism, disposition and clinical effects of methadone in humans. Clin Pharmacol Ther. 2003;73:P17. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Kolars JC, Schmiedlin-Ren P, Schuetz JD, Fang C, Watkins PB. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Invest. 1992;90:1871–8. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greiner B, Eichelbaum M, Fritz P, et al. The role of intestinal P–glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104:147–53. doi: 10.1172/JCI6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanwimolruk S, Paine MF, Pusek SN, Watkins PB. Is quinine a suitable probe to assess the hepatic drug-metabolizing enzyme CYP3A4? Br J Clin Pharmacol. 2002;54:643–51. doi: 10.1046/j.1365-2125.2002.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paine MF, Wagner DA, Hoffmaster KA, Watkins PB. Cytochrome P450 3A4 and P-glycoprotein mediate the interaction between an oral erythromycin breath test and rifampin. Clin Pharmacol Ther. 2002;72:524–35. doi: 10.1067/mcp.2002.128387. [DOI] [PubMed] [Google Scholar]
- 35.Kharasch E, Walker A, Hoffer C, Sheffels P. Oral alfentanil: a potential noninvasive probe for first-pass cytochrome P4503A activity. Anesthesiology. 2004;in press doi: 10.1067/mcp.2003.30. [DOI] [PubMed] [Google Scholar]
- 36.Fromm MF, Eckhardt K, Li S, et al. Loss of analgesic effect of morphine due to coadministration of rifampin. Pain. 1997;72:261–7. doi: 10.1016/s0304-3959(97)00044-4. [DOI] [PubMed] [Google Scholar]
- 37.Kharasch ED, Whittington D, Hoffer C, Altuntas TG, Sheffels P. Role of P-glycoprotein in the intestinal absorption of morphine, fentanyl and methadone. Clin Pharmacol Ther. 2003;73:P58. doi: 10.1016/j.clpt.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Deferme S, Augustijns P. The effect of food components on the absorption of P-gp substrates: a review. J Pharm Pharmacol. 2003;55:153–62. doi: 10.1211/002235702603. [DOI] [PubMed] [Google Scholar]
- 39.Perloff MD, von Moltke LL, Fahey JM, Daily JP, Greenblatt DJ. Induction of P-glycoprotein expression by HIV protease inhibitors in cell culture. AIDS. 2000;14:1287–9. doi: 10.1097/00002030-200006160-00034. [DOI] [PubMed] [Google Scholar]
- 40.Perloff MD, von Moltke LL, Greenblatt DJ. Fexofenadine transport in Caco-2 cells: inhibition with verapamil and ritonavir. J Clin Pharmacol. 2002;42:1269–74. doi: 10.1177/009127002762491370. [DOI] [PubMed] [Google Scholar]
- 41.de Maat MMR, Ekhart GC, Huitema ADR, Koks CHW, Mulder JW, Beijnen JH. Drug interactions between antiretroviral drugs and comedicated agents. Clin Pharmacokinet. 2003;42:223–82. doi: 10.2165/00003088-200342030-00002. [DOI] [PubMed] [Google Scholar]
- 42.Inturrisi CE, Colburn WA, Kaiko RF, Houde RW, Foley KM. Pharmacokinetics and pharmacodynamics of methadone in patients with chronic pain. Clin Pharmacol Ther. 1987;41:392–401. doi: 10.1038/clpt.1987.47. [DOI] [PubMed] [Google Scholar]
- 43.Inturrisi CE, Portenoy RK, Max MB, Colburn WA, Foley KM. Pharmacokinetic-pharmacodynamic relationships of methadone infusions in patients with cancer pain. Clin Pharmacol Ther. 1990;47:565–77. doi: 10.1038/clpt.1990.77. [DOI] [PubMed] [Google Scholar]
- 44.Scott JC, Ponganis KV, Stanski DR. EEG quantitation of narcotic effect: The comparative pharmacodynamics of fentanyl and alfentanil. Anesthesiology. 1985;62:234–41. doi: 10.1097/00000542-198503000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Chapman CR, Hill HF, Saeger L, Gavrin J. Profiles of opioid analgesia in humans after intravenous bolus administration: alfentanil, fentanyl and morphine compared on experimental pain. Pain. 1990;43:47–55. doi: 10.1016/0304-3959(90)90049-J. [DOI] [PubMed] [Google Scholar]
- 46.Kharasch ED, Whittington D, Hoffer C, Altuntas TG, Sheffels P. Role of P-glycoprotein in the clinical effects of morphine, fentanyl and methadone. Clin Pharmacol Ther. 2003;73:P17. doi: 10.1016/j.clpt.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Scott JC, Stanski DR. Decreased fentanyl and alfentanil dose requirements with age. A simultaneous pharmacokinetic and pharmacodynamic evaluation. J Pharmacol Exp Ther. 1987;240:159–66. [PubMed] [Google Scholar]
- 48.Dershwitz M, Walsh JL, Morishige RJ, et al. Pharmacokinetics and pharmacodynamics of inhaled versus intravenous morphine in healthy volunteers. Anesthesiology. 2000;93:619–28. doi: 10.1097/00000542-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Yamazaki M, Neway WE, Ohe T, et al. In vitro substrate identification studies for P-glycoprotein-mediated transport: species difference and predictability of in vivo results. J Pharmacol Exp Ther. 2001;296:723–35. [PubMed] [Google Scholar]
- 50.Henthorn TK, Liu Y, Mahapatro M, Ng KY. Active transport of fentanyl by the blood–brain barrier. J Pharmacol Exp Ther. 1999;289:1084–9. [PubMed] [Google Scholar]
- 51.Cirella VN, Pantuck CB, Lee YJ, Pantuck EJ. Effects of cyclosporine on anesthetic action. Anesth Analg. 1987;66:703–6. [PubMed] [Google Scholar]
- 52.Letrent SP, Polli JW, Humphreys JE, Pollack GM, Brouwer KR, Brouwer KL. P-glycoprotein-mediated transport of morphine in brain capillary endothelial cells. Biochem Pharmacol. 1999;58:951–7. doi: 10.1016/s0006-2952(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 53.Xie R, Hammarlund-Udenaes M, de Boer AG, de Lange EC. The role of P-glycoprotein in blood–brain barrier transport of morphine: transcortical microdialysis studies in mdr1a (−/−) and mdr1a (+/+) mice. Br J Pharmacol. 1999;128:563–8. doi: 10.1038/sj.bjp.0702804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zong J, Pollack GM. Morphine antinociception is enhanced in mdr1a gene-deficient mice. Pharm Res. 2000;17:749–53. doi: 10.1023/a:1007546719287. [DOI] [PubMed] [Google Scholar]
- 55.Polli JW, Wring SA, Humphreys JE, et al. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620–8. [PubMed] [Google Scholar]
- 56.Donahue JP, Dowdy D, Ratnam KK, et al. Effects of nelfinavir and its M8 metabolite on lymphocyte P-glycoprotein activity during antiretroviral therapy. Clin Pharmacol Ther. 2003;73:78–86. doi: 10.1067/mcp.2003.11. [DOI] [PubMed] [Google Scholar]
- 57.Weiss J, Dormann SM, Martin-Facklam M, Kerpen CJ, Ketabi-Kiyanvash N, Haefeli WE. Inhibition of P-glycoprotein by newer antidepressants. J Pharmacol Exp Ther. 2003;305:197–204. doi: 10.1124/jpet.102.046532. [DOI] [PubMed] [Google Scholar]
- 58.Ekins S, Kim RB, Leake BF, et al. Application of three-dimensional quantitative structure-activity relationships of P-glycoprotein inhibitors and substrates. Mol Pharmacol. 2002;61:974–81. doi: 10.1124/mol.61.5.974. [DOI] [PubMed] [Google Scholar]
- 59.Brosen K, Davidsen F, Gram LF. Quinidine kinetics after a single oral dose in relation to the sparteine oxidation polymorphism in man. Br J Clin Pharmacol. 1990;29:248–53. doi: 10.1111/j.1365-2125.1990.tb03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of P-glycoprotein in the intestinal absorption and clinical effects of morphine. Clin Pharmacol Ther. 2003;74:543–54. doi: 10.1016/j.clpt.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Dale O, Hoffer C, Sheffels P, Kharasch ED. Disposition of nasal, intravenous, and oral methadone in healthy volunteers. Clin Pharmacol Ther. 2002;72:536–45. doi: 10.1067/mcp.2002.128386. [DOI] [PubMed] [Google Scholar]
- 62.Boulton DW, Arnaud P, DeVane CL. Pharmacokinetics and pharmacodynamics of methadone enantiomers after a single oral dose of racemate. Clin Pharmacol Ther. 2001;70:48–57. doi: 10.1067/mcp.2001.116793. [DOI] [PubMed] [Google Scholar]