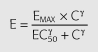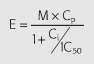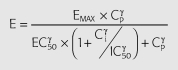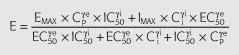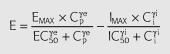Abstract
Aims
Recent reports have called into question the safety of ephedra supplements especially with regards to their cardiovascular effects. The purpose of this analysis was to characterize, via pharmacokinetic/pharmacodynamic modelling, the cardiovascular effects of ephedrine, the main active ingredient of ephedra, in apparently healthy, overweight volunteers.
Methods
In a randomized, double-blind, crossover, placebo-controlled study, eight subjects received either placebo, 0.25, 0.5 or 1.0 mg kg−1 ephedrine sulphate by mouth with a 7-day washout between treatments. Plasma ephedrine concentrations, heart rate and blood pressure were determined for 8 h postdose.
Results
The pharmacokinetics of ephedrine were best described by a one-compartment model with first-order absorption and elimination. The percentage change in heart rate was described by a linear model with a resulting slope of 0.14%·l µg−1 (CV = 59%). The percentage change in systolic blood pressure demonstrated clockwise hysteresis, and a sigmoidal tolerance model was used to describe the data. The mean maximum predicted effect (Emax) was 53.7% (CV = 41%) with an EC50 of 107 µg·l−1 (CV = 65%) and an inhibitory maximum (Imax) of 39.8% (CV = 60%). Tolerance developed with a mean half-life of 15 min (range 6–140 min).
Conclusions
This is the first study to apply a comprehensive pharmacokinetic/pharmacodynamic model to the cardiovascular effects of orally administered ephedrine. Although systolic blood pressure increases quickly after administration, the increase is nearly abolished by compensatory mechanisms.
Keywords: adverse events, ephedra, safety, tolerance, weight-loss
Introduction
Ephedra is a natural source of the alkaloid ephedrine. Recently, the use of ephedra-containing products has become popular as a weight-loss aid used, in the United States, by the general population [1], collegiate athletes [2], and health club members [3]. Manufacturer-recommended doses of ephedrine range from 10 to 30 mg two to three times a day. However, recent reports have called into question the safety of ephedra supplements especially with regard to their cardiovascular effects, which include hypertension, myocardial infarction, seizures and stroke, all of which are sometimes fatal [4–7]. A recent study examined the database maintained by the American Association on Poison Control Centers and found that ephedra-related adverse events accounted for more than one-half of all reported dietary supplement related adverse reactions even though ephedra-product sales account for < 1% of the marketplace [8]; this finding suggests the need for better methods to predict possible adverse reactions related to ephedrine use. The adverse events associated with ephedrine are consistent with the pharmacology of ephedrine. Ephedrine is a sympathomimetic amine, the effects of which are mediated through the α- and β-adrenergic receptors. Activation of these systems can be elicited by either direct interactions with receptors or by stimulating release of endogenous catecholamines [9].
Ephedrine from botanical sources demonstrates similar pharmacokinetics to the synthetic agent [10] and therefore should elicit similar pharmacodynamic effects. Previous studies have investigated the effects of ephedrine, both herbal and synthetic, on cardiovascular function [11–17]. However, no reports have communicated the effects of various doses of ephedrine on cardiovascular function. The ability to predict the extent of cardiovascular effects induced by ingestion of ephedrine, through pharmacokinetic/pharmacodynamic modelling, would aid in determining a possible therapeutic index for this supplement. Therefore, the purpose of this analysis was to characterize the cardiovascular effects (i.e. heart rate and systolic blood pressure) of ephedrine in relation to circulating concentrations of ephedrine after oral administration at three dose levels of synthetic ephedrine.
Methods
The data reported herein are an auxiliary analysis from a parent study conducted by our group (see ref [18]). The methods are summarized briefly here.
Subjects
Eight otherwise apparently healthy, overweight, nonsmoking volunteers (age range 21–44 years, median 30.5 years) entered the study. All subjects underwent a full medical history and physical examination (Table 1); none was taking any medication during the study. Subjects were excluded if they had taken prescription medications in the previous 4 weeks or an over-the-counter medication in the previous 2 weeks. There was no screening for prior use of ephedrine, and caffeine consumption was prohibited the day prior to testing. The University of North Carolina's Institutional Review Board approved all protocols. After determining the subject's suitability for enrolment, but prior to enrolment, written informed consent was obtained in response to a written and verbal explanation of the study.
Table 1.
Description of subjects
| Subject | Age (years) | Race | Gender | Body weight (kg) | BMI (kg m−2) | Body fat (%) | CLCr (mL min−1) |
|---|---|---|---|---|---|---|---|
| 1 | 25 | African-American | Male | 77.0 | 24.6 | 11.0 | 105 |
| 2 | 32 | African-American | Male | 84.4 | 25.2 | 12.9 | 97.1 |
| 3 | 44 | Caucasian | Female | 65.6 | 27.0 | 28.2 | 69.0 |
| 4 | 29 | Caucasian | Male | 84.2 | 28.8 | 11.0 | 94.8 |
| 5 | 23 | Caucasian | Male | 84.7 | 24.5 | 14.7 | 119 |
| 6 | 32 | African-American | Female | 74.1 | 24.2 | 19.4 | 83.5 |
| 7 | 42 | Caucasian | Female | 60.7 | 25.6 | 24.5 | 75.4 |
| 8 | 21 | Caucasian | Male | 85.3 | 24.1 | 12.9 | 151 |
BMI, body mass index; CLCr, creatinine clearance calculated from Cockcroft-Gault using ideal body weight or adjusted body weight for those subjects with actual weight >120% ideal body weight [36]; body fat determined by 4-site skinfold assessment.
Study design
The study was designed as a 5-way, randomized, double-blind, placebo-controlled trial with three doses of ephedrine sulphate USP (0.25, 0.5 and 1 mg kg−1) dissolved in 240 ml cranberry juice given orally followed by an open-labelled sibutramine (10 mg) treatment with a 7-day washout between treatments. The sibutramine results have been reported elsewhere (see ref [18]). Because of the unavailability of appropriate placebos and longer half-life (and greater potential for carry-over effects), treatment with sibutramine was the final, open-labelled arm. Subjects were admitted to the General Clinical Research Center (GCRC) at the University of North Carolina Hospitals the night prior to the experiment. After an overnight fast, subjects received the respective treatment, and pharmacokinetic/pharmacodynamic measurements (heart rate and blood pressure) were obtained for 8 h postdose. Standard, room-temperature meals were allowed 5 h after dosing.
Pharmacokinetic measurements and analyses
Blood samples (5 mL) for determining plasma drug concentrations were drawn predose and at 0.25, 0.75, 1.25, 1.75, 2.25, 3, 4, 5, 6 and 8 h postdose during the ephedrine and placebo treatments. All times were relative to the start of dosing. Bioanalysis of ephedrine in human plasma was performed by Oneida Research Services (Whitesboro, NY) using a proprietary, validated LC/MS/MS method. The accuracy of the method (expressed as percent of nominal) was > 95% over the concentration range of 0.1–10 µg l−1. The precision of the method (expressed as percent relative standard deviation) was < 10% over the concentration range. The ephedrine concentration-time data from individual subjects for all three doses were fit simultaneously with a one-compartment model assuming first-order absorption with a lag time and first-order elimination (WinNonlin 3.2, Pharsight Corp., Mountain View, CA) (Equations 1 and 2):
| (1) |
| (2) |
where Xa is the mass of ephedrine at the absorption site (per kg body weight); Cp is the plasma ephedrine concentration, ka is the absorption rate constant; V/F is the apparent volume of distribution (per kg body weight); and CL/F is the apparent oral clearance (per kg body weight). Parameter estimates from the pharmacokinetic model were then fixed to obtain pharmacodynamic parameter estimates.
Pharmacodynamic measurements and analysis
Blood pressure (systolic and diastolic) and heart rate were recorded in duplicate (Dinamap Monitor, Critikon, Tampa, FL) in the semirecumbent position at the same points as pharmacokinetic blood sampling. All pharmacodynamic data were derived as percentage change from the time-respective, placebo-controlled condition in order to account for diurnal variations (Figure 1). Various models were evaluated to analyze the relationship between pharmacologic effect (i.e. either percentage change in heart rate or systolic blood pressure) and concentration. Selection of the appropriate model was based on Akaike's Information Criteria (AIC), standard error of the parameter estimates and visual inspection of the fit of the pooled data. Percentage change in heart rate was described with a linear model after condensing all three ephedrine doses into a single data set for individual subjects.
| (3) |
where Se is the slope of the relationship of the percentage change in heart rate vs plasma ephedrine concentration (Cp).
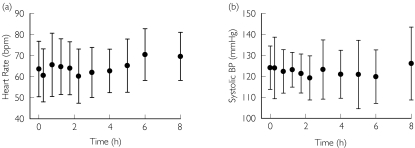
Figure 1.
Intra-day (diurnal) variability in semirecumbent (a) heart rate and (b) systolic blood pressure during the placebo condition (mean ± SD, n = 8)
Percentage change in systolic blood pressure was modelled with an integrated PK/PD system incorporating tolerance based on the scheme described in Figure 2 according to the following mathematical model:
| (4) |
| (5) |
where Cp is the predicted plasma concentration from Equation 3, Ci is the hypothetical ‘inhibitor’ concentration; k1i is the rate constant of tolerance associated with the production of the hypothetical inhibitor which was assumed to be equal to ki0, the rate of loss of the inhibitor; Emax is the maximum effect; EC50 is the plasma ephedrine concentration corresponding to one-half the maximum effect; γe is the slope factor for the effect; Imax is the maximum inhibitory effect; IC50 is the hypothetical ‘inhibitor’ concentration corresponding to one-half the maximum inhibitory effect; γI is the slope factor for the inhibitory effect.
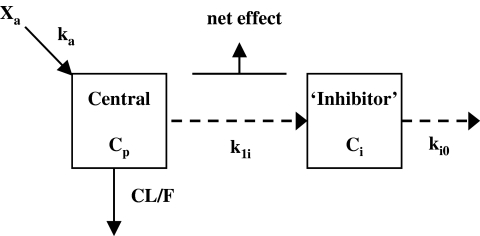
Figure 2.
Schematic of the tolerance model used to describe the effects of ephedrine on systolic blood pressure. ka, the absorption rate constant; CL/F, the apparent oral clearance; Cp, plasma concentration; k1i, rate constant for tolerance development; ki0, rate constant for the disappearance of tolerance; Ci, hypothetical ‘inhibitor’ concentration
Applicability of the tolerance model
To test the applicability of the model, plasma ephedrine concentrations and the respective systolic blood pressure data were obtained from two independent sources [11, 15]. Two conditions were used to evaluate the developed model (Table 2). First, for each study the pooled data were first modelled to obtain pooled pharmacokinetic parameter estimates using Equations 2 and 3. In the first scenario (‘blood pressure prediction’), pharmacokinetic parameter estimates for each study were used to simulate the pharmacodynamic outcomes using pharmacodynamic parameter estimates from this current data set and Equations 4 and 5. Subsequently in the second scenario (‘model re-evaluation’), data sets were modelled to convergence to define new pooled, study-specific pharmacodynamic parameter estimates for each given study using the already obtained pooled study-specific pharmacokinetic parameter estimates.
Table 2.
Methods to evaluate applicability of tolerance model
| Scenario 1 ‘Blood pressure prediction’ | Scenario 2 ‘Model re-evaluation | |
|---|---|---|
| Pharmacokinetics (PK) | Study-specific | Study-specific |
| Pharmacodynamics (PD) | Predicted based on current study PD parameter estimates (Table 5) | Data modelled to convergence (i.e. obtain new set of parameter estimates) (Table 6) |
| Goal | To predict blood pressure response given blood concentrations | To re-evaluate PD parameters based on different experimental designs. |
Results
Pharmacokinetics
The pharmacokinetic data were analysed with a one-compartment model assuming first-order input and output (Figure 3, Table 3). The one compartment model with a lag-time is consistent with previous publications [10, 17, 19]. Linear pharmacokinetics were assumed and therefore all three doses of ephedrine were fitted simultaneously to yield common parameter estimates. Oral clearance (CL/F) of ephedrine correlated significantly with creatinine clearance (r2 = 0.66, P < 0.05), consistent with the hypothesis that renal clearance is the predominant route of ephedrine elimination [15, 20].
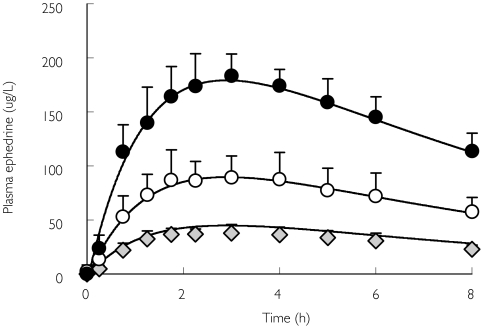
Figure 3.
Plasma ephedrine concentration (mean ± SD, n = 8)-time profile after 0.25 ( , 0.5 (○) and 1.0 (•) mg kg−1 oral dosing. Data were modelled simultaneously with a one-compartment model incorporating a lag time, assuming first order absorption and elimination (solid line)
, 0.5 (○) and 1.0 (•) mg kg−1 oral dosing. Data were modelled simultaneously with a one-compartment model incorporating a lag time, assuming first order absorption and elimination (solid line)
Table 3.
Summary of ephedrine pharmacokinetic parameters for all three doses of ephedrine sulphate (0.25, 0.50, 1.0 mg kg−1)
| Subject | ka (h−1) | CL/F(l h−1 kg−1) | V/F(l kg−1) | tlag (min) |
|---|---|---|---|---|
| 1 | 1.30 | 0.39 | 4.4 | 5.2 |
| 2 | 0.94 | 0.46 | 4.4 | 13.2 |
| 3 | 1.50 | 0.38 | 4.5 | 12.6 |
| 4 | 0.96 | 0.51 | 4.5 | 6.1 |
| 5 | 0.89 | 0.50 | 4.6 | 2.4 |
| 6 | 0.84 | 0.37 | 3.4 | 8.7 |
| 7 | 0.86 | 0.43 | 3.6 | 5.5 |
| 8 | 0.29 | 0.72 | 2.5 | 2.1 |
| Mean | 0.95 | 0.47 | 4.0 | 7.0 |
| SD | 0.36 | 0.11 | 0.8 | 4.2 |
| Pooled* | 0.93 | 0.45 | 4.2 | 7.1 |
ka, rate constant of absorption; CL/F, apparent oral clearance; V/F, apparent volume of distribution; tlag, lag time;
parameter derived from modelling data from all subjects (n = 8).
Pharmacodynamics
The relationship between heart rate and ephedrine concentrations did not show evidence of significant hysteresis on an individual basis (data not shown) or on a pooled basis (Figure 4). The data were best described by a linear model suggesting the effects were well below the potential Emax of the system and therefore were in the linear portion of the concentration-response relationship. Results of the pharmacodynamic analysis are summarized in Table 4.
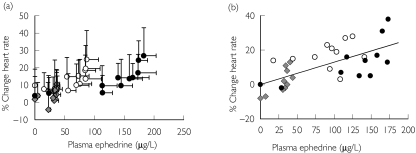
Figure 4.
Percentage change in heart rate vs ephedrine plasma concentration after oral dosing. (a) mean ± SD, n = 8 for all subjects and doses and (b) representative model fit for one subject (subject 1). Symbols represent actual data and line represents model prediction
Table 4.
Summary of heart rate model fits using a linear model collapsing all three doses. Peak heart rate is described by both the maximum heart rate from ephedrine treatment and the % change from placebo condition
| Subject | Se (%Δ·l µg−1) | Peak heart rate (beats min−1) (%Δ) |
|---|---|---|
| 1 | 0.13 | 72 (38) |
| 2 | 0.17 | 86 (37) |
| 3 | 0.15 | 101 (33) |
| 4 | 0.065 | 76 (23) |
| 5 | 0.056 | 76 (30) |
| 6 | 0.18 | 97 (49) |
| 7 | 0.078 | 112 (39) |
| 8 | 0.31 | 77 (57) |
| Mean | 0.14 | 87 (38) |
| SD | 0.08 | 15 (11) |
| Median | 0.14 | 82 (38) |
| Pooled* | 0.11 | – |
Se, rate of change of the % change in heart rate from placebo condition; peak heart rate; highest % change in heart rate in the effect-time profile;
parameters derived by modelling data from all subjects (n = 8).
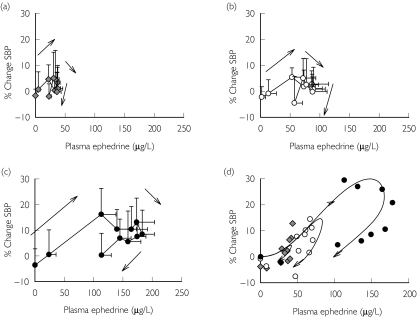
Percentage changes in systolic blood pressure were best described by a tolerance model as suggested by clockwise hysteresis in the plasma concentration-effect profile (Figure 5 and Table 5). Other models including those without a tolerance component, those with nonsigmoid tolerance models (i.e. linear effect model) and various other receptor–ligand interaction models (i.e. agonist-competitive antagonist, agonist-partial agonist) were examined but did not characterize satisfactorily the effect-time profiles for the pooled data (see Appendix). These models were also used with an ‘effect’ compartment but this addition did not aid in the characterization of the effect-time profiles (i.e. did not lower the AIC). Figure 5d is a representative model fit from one subject. Tolerance developed with a mean half-life of 15 min (range 6–140 min). However, subject 6 showed very little tolerance, and these data were best described by a sigmoid Emax model (Table 4). Tolerance offset ∼70% (CV = 47%) of the increase in systolic blood pressure and only subject 3 demonstrated some rebound effect (i.e. Imax > Emax). Estimates for Emax, and EC50 demonstrated similar variability (CV = 42% and 61%, respectively). In this study, no concentration-effect relationship was noted for diastolic blood pressure (data not shown).
Figure 5.
Percentage change in systolic blood pressure vs ephedrine plasma concentration after oral dosing of ephedrine sulphate. (a) 0.25 mg kg−1 (grey diamonds), (b) 0.5 mg kg−1 (open circles), (c) 1.0 mg kg−1 (closed circles) and (d) representative model prediction for one subject after each dose (subject 4). Data shown as mean ± SD, n = 8. Symbols represent actual data and line represents model fit
Table 5.
Summary of systolic blood pressure model fits using a tolerance model
| Subject | Emax (%Δ) | EC50 (µg l−1) | γe | Imax (%Δ) | IC50 (µg l−1) | γi | ki0 (h−1) |
|---|---|---|---|---|---|---|---|
| 1 | 68.2 | 121 | 3.6 | 44.2 | 93.0 | 5.3 | 3.6 |
| 2 | 54.8 | 250 | 1.5 | 18.4 | 46.5 | 6.0 | 0.6 |
| 3 | 36.4 | 73.3 | 2.2 | 43.5 | 71.3 | 1.0 | 1.2 |
| 4 | 52.9 | 115 | 2.2 | 31.3 | 79.6 | 2.3 | 0.3 |
| 5 | 29.9 | 123 | 0.6 | 9.6 | 41.0 | 8.1 | 6.4 |
| 6 | 38.6 | 134 | 0.9 | – | – | – | – |
| 7 | 99.9 | 91.5 | 1.5 | 82.9 | 81.8 | 1.4 | 4.2 |
| 8 | 49.0 | 46.8 | 2.3 | 48.9 | 43.0 | 1.9 | 3.1 |
| Mean | 53.7 | 107 | 1.9 | 39.8 | 65.2 | 3.7 | 2.8 |
| SD | 22.2 | 69.0 | 1.0 | 23.9 | 21.3 | 2.7 | 2.2 |
| Median | 50.9 | 103 | 1.9 | 43.5 | 71.3 | 2.3 | 3.1 |
| Mean without subject 6 | 55.9 | 103 | 2.0 | 39.8 | 65.2 | 3.7 | 2.8 |
| Pooled* | 64.2 | 174 | 1.9 | 26.5 | 90.6 | 2.8 | 2.0 |
Emax, maximum effect; EC50, plasma ephedrine concentration corresponding to one-half the maximum effect, γe, slope factor for the effect, Imax, maximum inhibitory effect; IC50, theoretical ‘inhibitor’ concentration corresponding to one-half the maximum inhibitory effect, γi, slope factor for the inhibitory effect; ki0, rate constant of tolerance development;
parameters derived by modelling data from all subjects (n = 8).
Applicability of tolerance model
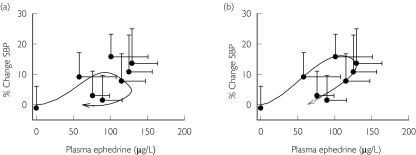
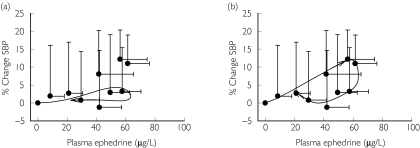
To test the generalizability and robustness of the model, two previously published data sets were analyzed first using the pooled pharmacodynamic parameter estimates from Table 5(Figure 6a and Figure 7a) with study-specific pooled pharmacokinetic parameter estimates. In Case 1, a 50 mg dose of synthetic ephedrine was administered and changes in systolic blood pressure were simulated using the pooled pharmacodynamic parameter estimates obtained from the current study (Figure 6a). However, the data were better described by allowing the model to converge to a different set of values (Figure 6b), with the major difference related to the tolerance half-life. In Case 2, data were obtained after administration of ephedra (Metabolift®, Twin Laboratories Inc.) that contained a combination of 17.3 mg ephedrine, 5.3 mg pseudoephedrine, and 175 mg caffeine. Again, % change in systolic blood pressure were simulated with pooled parameter estimates from this current study (Figure 7a); these estimates did not accurately predict the observed changes in systolic blood pressure with underestimation of peak effects. These data also were best described by allowing all pharmacodynamic parameters to be fitted simultaneously to obtain study-specific estimates. The estimate of tolerance half-life in case 1 and 2 was ∼46 min. Case 2 also required a lower value of Imax suggesting a lack of tolerance development that may be a function of the concomitant administration of caffeine or to a lesser extent pseudoephedrine (Table 6).
Figure 6.
Application of tolerance model for systolic blood pressure after administration of 50 mg ephedrine hydrochloride [11]. (a) Model fit from using pooled pharmacodynamic parameter estimates from this current study and study-specific pooled pharmacokinetic parameter estimates. (b) Model prediction using study-specific pharmacodynamic parameter estimates. Pharmacokinetic values: ka = 1.27 h−1, CL/F = 34.6 l h−1, V/F = 306 l, tlag = 9 min. Data shown as mean ± SD, n = 16. Symbols represent actual data and line represents model fit
Figure 7.
Application of tolerance model for systolic blood pressure after administration of ephedra (17.3 mg ephedrine, 5.3 mg pseudoephedrine, 175 mg caffeine) [15] (a) Model fit from using pooled pharmacodynamic parameter estimates from this current study and study-specific pooled pharmacokinetic parameter estimates. (b) Model fit using study-specific pharmacodynamic parameter estimates. Pharmacokinetic values: ka = 1.34 h−1, CL/F = 21.6 l h−1, V/F = 222 l, tlag = 25 min. Data shown as mean ± SD, n = 8. Symbols represent actual data and line represents model fit
Table 6.
Applicability of tolerance model to different data sets
| Emax(%Δ) | EC50 (µg l−1) | γe | Imax (%Δ) | IC50(µg l−1) | γi | ki0(h−1) | Imax/Emax | Dose | Synthetic or herbal | |
|---|---|---|---|---|---|---|---|---|---|---|
| Current study (n = 8) | 64.2 | 174 | 1.9 | 26.5 | 90.6 | 2.8 | 2.0 | 0.41 | 0.25, 0.5, 1.0 mg kg−1 | Synthetic |
| Berlin et al. (2001) (n = 16) | 54.3 | 143 | 1.7 | 20.4 | 74.4 | 1.9 | 0.8 | 0.38 | 50 mg | Synthetic |
| Haller et al. (2002) (n = 8) | 54.7 | 154 | 1.3 | 9.8 | 34.1 | 5.9 | 0.6 | 0.18 | 17.3 mga | Herbal |
Emax, maximum effect; EC50, plasma ephedrine concentration corresponding to one-half the maximum effect, γe, slope factor for the effect, Imax, maximum inhibitory effect; IC50, theoretical ‘inhibitor’ concentration corresponding to one-half the maximum inhibitory effect, γi, slope factor for the inhibitory effect; ki0, rate constant of tolerance development;
ephedrine also contained 175 mg caffeine.
Discussion
With recent reports of adverse events associated with ephedra use [4–7], as well as highly publicized deaths of professional athletes who consumed ephedra, a more comprehensive understanding of the potential adverse events associated with ephedra use is needed. Although previous studies have demonstrated the cardiovascular effects of ephedrine (or ephedra) after oral administration, this is the first study to model these effects comprehensively. The pharmacokinetics of ephedrine were described by a one-compartment model with an oral clearance similar to that of other studies [11, 15]. White et al.[17] found a smaller absorption rate constant (ka) than in the present study after ingestion of E. sinica capsules, but others have reported slightly larger ka values with ephedrine hydrochloride in tablet and solution dosage forms [10, 19]. The difference with the latter study may be a function of dosage form (i.e. capsule vs tablet vs solution), ephedrine salt (i.e. sulphate vs hydrochloride), or co-ingestion of fruit juice.
In the present study, heart rate was proportional to plasma ephedrine concentrations in a time-independent manner. In contrast, a previous report indicated that ephedrine doses <1.0 mg kg−1 produced a counter-clockwise hysteresis for heart rate effects [15]. The linear nature of the concentration-effect relationship suggests the percentage change in heart rate was far below the maximal heart rate. The predicted maximum heart rate can be estimated as between 176 and 199 beats min−1 in this subject pool, using the age-predicted maximum heart rate estimate of 220 − age (or > 100% increase from the placebo condition). The maximum change in any subject for any dose was < 60%, and thus ephedrine may have a minimal effect on induction of heart rate. On average, a plasma concentration change of 10 µg l−1 would increase heart rate ∼1 beat min−1. Astrup et al.[21] also found a dose-dependent increase in heart rate with increasing ephedrine doses.
The increase in blood pressure due to ephedrine is a product of positive ionotropic effects, positive chronotropic effects and increased peripheral resistance as a result of vasoconstriction. The relative potency of ephedrine for β-receptors is β1 > β2 » β3 and suggests that at clinically relevant plasma concentrations, cardiovascular effects (i.e. effects governed by β1 and β2) would predominate over the lipid mobilization and thermogenic effects (i.e. β3 effects) of ephedrine [22, 23]. The cardiovascular effects of ephedrine can be reduced/eliminated with the concomitant use of a nonselective β-adrenoceptor blocker (e.g. nadolol) [24]. The effect-time relationship for systolic blood pressure exhibited clockwise hysteresis, suggesting acute tolerance; this behaviour was especially notable at 5–8 h postdose. Others have found similar effect-time relationships for the effects of ephedrine on systolic blood pressure for doses <1.0 mg kg−1 of ephedrine [11, 15]. Why systolic blood pressure demonstrates tolerance to ephedrine, but heart rate effects do not, is unknown. However this difference may be explained by differential desensitization between β1 and β2 receptors or between various tissues expressing these receptors [25]. As mentioned, clockwise hysteresis is indicative of pharmacodynamic tolerance; the mean half-life of tolerance development to systolic blood pressure effects was ∼15 min. Thus, complete tolerance should develop around 1.25 h (five half-lives) after ingestion. Other studies investigating pressor effects of compounds have found similar tolerance half-lives in humans: cocaine and heart rate (24 min) [26], caffeine and mean arterial pressure (57 min) [27], nicotine and systolic blood pressure (70 min) [28] and nitroglycerin and vessel contractility (2–3 h) [29]. Interestingly, cocaine is pharmacologically similar to ephedrine and elicited the most similar tolerance half-life. The elevation in systolic blood pressure induced by ephedrine was nearly abolished by the acute tolerance and suggests that over a single dosing interval, the overall change in systolic blood pressure would be small. This ‘rebound effect’ may explain the lack of change in systolic blood pressure in other studies [12, 13, 30]. However, simultaneous use of caffeine may offset some of the tolerance development as suggested by case 2 of the model validation.
Mechanisms responsible for the acute tolerance with regard to systolic blood pressure can include reduction in receptor number, counter-regulation, depletion of neurotransmitter pool or receptor desensitization. This latter factor has been implicated in in vitro work in cells transfected with β-receptors [31]. In this system, ephedrine (100 µm or 16.5 mg l−1) saturates the activation of the second messenger, adenylate cyclase, and thus desensitizes β-receptors to the effects of epinephrine without loss of surface receptors [31, 32]. Ephedrine reduced activity of epinephrine approximately 58% at 5 min and 65% after 30 min of incubation [31], a similar time course to this study. In addition, ephedrine was shown to have an EC50 of 565 nm (∼95 µg l−1) in activating adenylate cyclase in transfected cells, a similar EC50 to this study [31]. However, Shannon et al.[22] found higher EC50 values (9.1 µm and 3.6 µm) for β1 and β2 receptors, respectively, using a different cell line transfected with human receptors. This same study found that ephedrine (50 mg three times daily) decreased urinary excretion of norepinephrine, suggesting that tolerance may be a function of decreasing endogenous release of certain catecholamines [22]. Although these in vitro studies suggest a possible mechanism for ephedrine-induced tolerance to blood pressure effects, further work is needed to define the mechanism underlying tolerance development and the dissociation in the apparent tolerance to blood pressure but not to heart rate.
It has been suggested that increases in systolic blood pressure and increased cardiac contractility may lead to a cardiovascular event [33]. Therefore, the therapeutic implications of the basic understanding of the pharmacokinetic/pharmacodynamic relationships for the cardiovascular response to ephedrine ingestion can allow a better prediction of potential adverse events associated with its use. Although the absolute changes in systolic blood pressure are probably not clinically relevant in normotensive individuals, there are several implications of acute tolerance in the systolic blood pressure response. First, acute tolerance may increase risk for orthostatic hypotension as suggested by the findings of Berlin et al.[11]. These investigators noted a decrease in systolic blood pressure when standing compared with the supine position and this phenomenom lasted ∼4 h, the approximate time to loss of tolerance. It was noted that the decrease in standing blood pressure was not compensated by an increase in heart rate. In addition, exercise activates the sympathetic nervous system and thus increases systolic blood pressure, cardiac output and heart rate; tolerance induced by ephedrine may prevent the increase in systolic blood pressure. Recent research indicates no difference between placebo and ephedra (20 mg ephedrine) + caffeine (150 mg) treatment in terms of increases in systolic blood pressure during exercise initiated 60 min after ingestion [34]. There was no difference in systolic blood pressure during recovery (120 min postingestion) either, and these data suggest that vasodilatation associated with exercise can offset the pressor response to ephedrine.
One of the goals of pharmacokinetic/pharmacodynamic modelling is to help optimize drug therapy. Ephedra (ephedrine) has been associated with cardiovascular adverse events at typical recommended doses. The current study modelled the relationship between ephedrine exposure after a single dose and changes in heart rate and systolic blood pressure in nonobese, mildly overweight volunteers. Use of ephedrine-containing products is not limited to the overweight or the obese but is used by the general population. It is unknown if obese individuals (BMI > 30) respond to ephedrine differently from healthy, normal weight volunteers but the obese may have enhanced cardiovascular response to ephedrine due to an already high sympathetic stimulation in this population [35]. Government approved weight loss agents (e.g. fenfluramine, phenylpropanolamine) have been removed from the market because of safety issues and this suggests that ephedrine, with its similar mechanism of action, should be avoided in the obese with concomitant cardiovascular disease. In addition, the current study used data from a single dose of ephedrine; the pharmacodynamics of repeated doses of ephedrine and the adaptation to chronic dosing may be inherently different due to the development of tolerance or even sensitization [11]. Nonetheless, these data have the potential to be used to determine a therapeutic index providing an adequate marker of effectiveness (e.g. weight loss) can be obtained. For example, a low dose of ephedrine (17 mg) with caffeine (case 2), results in the same blood pressure increases as medium (30–40 mg) to high doses (70–80 mg) of ephedrine and such combinations, although effective in weight reduction, may increase the likelihood of an adverse event; these ephedrine combinations probably should be avoided. Future studies to determine the exposure-response relationship of mixtures containing ephedra (e.g. ephedrine + caffeine, ephedrine + caffeine + aspirin) for adverse events are necessary for confirmation. The better understanding of the relationship between ephedrine concentration and effect on heart rate and systolic blood pressure will help in defining the therapeutic role of ephedrine in weight management.
Acknowledgments
This work was supported by GlaxoSmithKline and the General Clinical Research Center program of the Division of Research Resources, National Institutes of Health (grant # RR00046). Dr Persky is supported by a Clinical Pharmacokinetics/Pharmacodynamics Fellowship sponsored by the University of North Carolina in collaboration with GlaxoSmithKline. The authors would like to gratefully thank and acknowledge Christine Haller (Division of Clinical Pharmacology, University of California, San Francisco, CA, USA), and Ivan Berlin (Department of Pharmacologie, Groupe Hospitalier Universitaire, Paris France) for the use of their data. The authors would also like to thank Dr. Erin Heinzen for her assistance in data analysis. Dr. N. Seth Berry is currently a Research Fellow at the Center for Drug Development Science, Georgetown University Medical Center, Washington, D.C.
Appendix
Statistical evaluation (i.e. AIC) of selected models used to evaluate the systolic blood pressure data. Models were tested on pooled data. Agonist-reverse agonist model described the data, statistically and graphically, better than other evaluated models
References
- 1.Blanck HM, Khan LK, Serdula MK. Use of non-prescription weight loss products: results from a multistate survey. JAMA. 2001;286:930–5. doi: 10.1001/jama.286.8.930. [DOI] [PubMed] [Google Scholar]
- 2.Green GA, Uryasz FD, Petr TA, Bray CD. NCAA study of substance use and abuse habits of college student- athletes. Clin J Sport Med. 2001;11:51–6. doi: 10.1097/00042752-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Kanayama G, Gruber AJ, Pope HG, Jr, Borowiecki JJ, Hudson JI. Over-the-counter drug use in gymnasiums: an underrecognized substance abuse problem? Psychother Psychosom. 2001;70:137–40. doi: 10.1159/000056238. [DOI] [PubMed] [Google Scholar]
- 4.Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ, Rhodes SL, Jungvig L, Gagne J. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA. 2003;289:1537–45. doi: 10.1001/jama.289.12.1537. [DOI] [PubMed] [Google Scholar]
- 5.Geiger JD. Adverse events associated with supplements containing ephedra alkaloids. Clin J Sport Med. 2002;12:263. doi: 10.1097/00042752-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med. 2000;343:1833–8. doi: 10.1056/NEJM200012213432502. [DOI] [PubMed] [Google Scholar]
- 7.Samenuk D, Link MS, Homoud MK, Contreras R, Theohardes TC, Wang PJ, Estes NA., 3rd Adverse cardiovascular events temporally associated with ma huang, an herbal source of ephedrine. Mayo Clin Proc. 2002;77:12–16. doi: 10.4065/77.1.12. [DOI] [PubMed] [Google Scholar]
- 8.Bent S, Tiedt TN, Odden MC, Shlipak MG. The relative safety of ephedra compared with other herbal products. Ann Intern Med. 2003;138:468–71. doi: 10.7326/0003-4819-138-6-200303180-00010. [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson TA, Shahan DR. Greenwood Village, CO.; DRUGDEX System. MICROMEDIX. [Google Scholar]
- 10.Gurley BJ, Gardner SF, White LM, Wang PL. Ephedrine pharmacokinetics after the ingestion of nutritional supplements containing Ephedra sinica (ma huang) Ther Drug Monit. 1998;20:439–45. doi: 10.1097/00007691-199808000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Berlin I, Warot D, Aymard G, Acquaviva E, Legrand M, Labarthe B, Peyron I, Diquet B, Lechat P. Pharmacodynamics and pharmacokinetics of single nasal (5 mg and 10 mg) and oral (50 mg) doses of ephedrine in healthy subjects. Eur J Clin Pharmacol. 2001;57:447–55. doi: 10.1007/s002280100317. [DOI] [PubMed] [Google Scholar]
- 12.Boozer CN, Nasser JA, Heymsfield SB, Wang V, Chen G, Solomon JL. An herbal supplement containing Ma Huang-Guarana for weight loss: a randomized, double-blind trial. Int J Obes Relat Metab Disord. 2001;25:316–24. doi: 10.1038/sj.ijo.0801539. [DOI] [PubMed] [Google Scholar]
- 13.Boozer CN, Daly PA, Homel P, Solomon JL, Blanchard D, Nasser JA, Strauss R, Meredith T. Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int J Obes Relat Metab Disord. 2002;26:593–604. doi: 10.1038/sj.ijo.0802023. [DOI] [PubMed] [Google Scholar]
- 14.Dingemanse J, Guentert T, Gieschke R, Stabl M. Modification of the cardiovascular effects of ephedrine by the reversible monoamine oxidase A-inhibitor moclobemide. J Cardiovasc Pharmacol. 1996;28:856–61. doi: 10.1097/00005344-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Haller CA, Jacob P, Benowitz NL. Pharmacology of ephedra alkaloids and caffeine after single-dose dietary supplement use. Clin Pharmacol Ther. 2002;71:421–32. doi: 10.1067/mcp.2002.124523. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs I, Pasternak H, Bell DG. Effects of ephedrine, caffeine and their combination on muscular endurance. Med Sci Sports Exerc. 2003;35:987–94. doi: 10.1249/01.MSS.0000069916.49903.70. [DOI] [PubMed] [Google Scholar]
- 17.White LM, Gardner SF, Gurley BJ, Marx MA, Wang PL, Estes M. Pharmacokinetics and cardiovascular effects of ma-huang (Ephedra sinica) in normotensive adults. J Clin Pharmacol. 1997;37:116–22. doi: 10.1002/j.1552-4604.1997.tb04769.x. [DOI] [PubMed] [Google Scholar]
- 18.Persky AM, Ng C, Song MH, Lancaster ME, Balderson DE, Paulik MA, Brouwer KLR. Comparison of the pharmacodynamic responses after increasing doses of ephedrine or a single dose of sibutramine in healthy, overweight volunteers. Int J Clin Pharmacol Ther. (in press) [DOI] [PubMed]
- 19.Pickup ME, May CS, Ssendagire R, Paterson JW. The pharmacokinetics of ephedrine after oral dosage in asthmatics receiving acute and chronic treatment. Br J Clin Pharmacol. 1976;3:123–34. doi: 10.1111/j.1365-2125.1976.tb00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson GR, Beckett AH. Absorption, metabolism, and excretion of the ephedrines in man. II. Pharmacokinetics. J Pharm Sci. 1968;57:1933–8. doi: 10.1002/jps.2600571122. [DOI] [PubMed] [Google Scholar]
- 21.Astrup A, Toubro S. Thermogenic, metabolic, and cardiovascular responses to ephedrine and caffeine in man. Int J Obes Relat Metab Disord. 1993;17(Suppl 1):S41–S43. [PubMed] [Google Scholar]
- 22.Shannon JR, Gottesdiener K, Jordan J, Chen K, Flattery S, Larson PJ, Candelore MR, Gertz B, Robertson D, Sun M. Acute effect of ephedrine on 24-h energy balance. Clin Sci (Lond) 1999;96:483–91. [PubMed] [Google Scholar]
- 23.Vansal SS, Feller DR. Direct effects of ephedrine isomers on human beta-adrenergic receptor subtypes. Biochem Pharmacol. 1999;58:807–10. doi: 10.1016/s0006-2952(99)00152-5. [DOI] [PubMed] [Google Scholar]
- 24.Liu YL, Toubro S, Astrup A, Stock MJ. Contribution of beta 3-adrenoceptor activation to ephedrine-induced thermogenesis in humans. Int J Obes Relat Metab Disord. 1995;19:678–85. [PubMed] [Google Scholar]
- 25.Broadley KJ. Review of mechanisms involved in the apparent differential desensitization of beta1- and beta2-adrenoceptor-mediated functional responses. J Auton Pharmacol. 1999;19:335–45. doi: 10.1111/j.1365-2680.1999.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 26.Chow MJ, Ambre JJ, Ruo TI, Atkinson AJ, Jr, Bowsher DJ, Fischman MW. Kinetics of cocaine distribution, elimination, and chronotropic effects. Clin Pharmacol Ther. 1985;38:318–24. doi: 10.1038/clpt.1985.179. [DOI] [PubMed] [Google Scholar]
- 27.Shi J, Benowitz NL, Denaro CP, Sheiner LB. Pharmacokinetic-pharmacodynamic modeling of caffeine: tolerance to pressor effects. Clin Pharmacol Ther. 1993;53:6–14. doi: 10.1038/clpt.1993.3. [DOI] [PubMed] [Google Scholar]
- 28.Fattinger K, Verotta D, Benowitz NL. Pharmacodynamics of acute tolerance to multiple nicotinic effects in humans. J Pharmacol Exp Ther. 1997;281:1238–46. [PubMed] [Google Scholar]
- 29.Bauer JA, Fung HL. Pharmacodynamic models of nitroglycerin-induced hemodynamic tolerance in experimental heart failure. Pharm Res. 1994;11:816–23. doi: 10.1023/a:1018917522072. [DOI] [PubMed] [Google Scholar]
- 30.Kalman D, Incledon T, Gaunaurd I, Schwartz H, Krieger D. An acute clinical trial evaluating the cardiovascular effects of an herbal ephedra-caffeine weight loss product in healthy overweight adults. Int J Obes Relat Metab Disord. 2002;26:1363–6. doi: 10.1038/sj.ijo.0802061. [DOI] [PubMed] [Google Scholar]
- 31.January B, Seibold A, Whaley B, Hipkin RW, Lin D, Schonbrunn A, Barber R, Clark RB. Beta2-adrenergic receptor desensitization, internalization, and phosphorylation in response to full and partial agonists. J Biol Chem. 1997;272:23871–9. doi: 10.1074/jbc.272.38.23871. [DOI] [PubMed] [Google Scholar]
- 32.Williams BR, Barber R, Clark RB. Kinetic analysis of agonist-induced down-regulation of the beta (2) -adrenergic receptor in BEAS-2B cells reveals high- and low-affinity components. Mol Pharmacol. 2000;58:421–30. doi: 10.1124/mol.58.2.421. [DOI] [PubMed] [Google Scholar]
- 33.Deedwania PC. Hemodynamic changes as triggers of cardiovascular events. Cardiol Clin. 1996;14:229–38. doi: 10.1016/s0733-8651(05)70276-9. [DOI] [PubMed] [Google Scholar]
- 34.Kleinjan KJ, Vukovich MD. Brookings: South Dakota State University; The cardiovascular effects of caffeine and ephedrine during exercise. Department of Health, Physical Education and Recreation, pp. 48. [Google Scholar]
- 35.Curtis BM, O'Keefe JH., Jr Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc. 2002;77:45–54. doi: 10.4065/77.1.45. [DOI] [PubMed] [Google Scholar]
- 36.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 37.Ouellet DM, Pollack GM. A pharmacokinetic-pharmacodynamic model of tolerance to morphine analgesia during infusion in rats. J Pharmacokinet Biopharm. 1995;23:531–49. doi: 10.1007/BF02353460. [DOI] [PubMed] [Google Scholar]