Abstract
Aims
FTY720 is a sphingosine-1-phosphate receptor agonist that redirects lymphocytes from the circulation to lymph nodes without impairing lymphocyte function. It is being developed as an immunomodulator for the prevention of acute rejection after organ transplantation. This study was performed to provide guidance on administration with respect to meals and to measure pharmacologic responses in healthy subjects.
Methods
In this randomized, two-period, crossover study, 14 healthy subjects received placebo on day −1 of each period with baseline circadian measurements of lymphocyte count and heart rate. Subjects subsequently received a single 1 mg oral dose of FTY720 on day 1 under fasting conditions and after a high fat meal. Blood FTY720 concentrations, lymphocyte count, and supine heart rate were assessed over an 8 day period after each FTY720 dose. The effect of food on FTY720 pharmacokinetics was assessed by standard bioequivalence testing.
Results
Both the peak concentration (0.65 ± 0.17 vs 0.64 ± 0.18 ng ml−1) and total exposure (AUC 149 ± 65 vs 139 ± 43 ng ml−1 h) did not differ significantly between fasting and fed states, respectively. The corresponding fed/fasting ratios and 90% confidence intervals were 1.00 (0.86, 1.17) for Cmax and 0.98 (0.86, 1.11) for AUC. Under both treatment conditions peripheral blood lymphocyte count decreased from baseline by 38 ± 9% over the first 2 days postdose and then increased towards predose values over the subsequent week. Whereas a circadian rhythm in supine heart rate was preserved in the presence of FTY720, the heart rate vs time curve was shifted downwards by 10% over the first day postdose and then recovered to prestudy values by days 3–5 postdose. These changes were asymptomatic.
Conclusions
Single 1 mg doses of FTY720 were well tolerated in healthy subjects and elicited a moderate decrease in peripheral blood lymphocyte count and a transient decrease in heart rate consistent with its pharmacological mode of action. FTY720 may be administered without regard to the timing of meals or their fat content.
Keywords: food, FTY720, pharmacodynamics, pharmacokinetics
Introduction
T-lymphocytes play a cardinal role in acute rejection episodes after organ transplantation. Immunosuppressants currently available for long-term prophylaxis either inhibit the activation of T-lymphocytes such as the calcineurin inhibitors cyclosporin and tacrolimus, or inhibit the proliferation of T-lymphocytes such as the antimetabolite mycophenolate mofetil and the antiproliferative sirolimus. Drug development scientists are actively seeking new immunomodulators which interfere in the rejection process via alternative pathways for use in synergistic regimens with currently marketed agents [1]. One such paradigm focuses on agents which alter T-lymphocyte trafficking thereby diverting these cells away from the allograft.
In this context, FTY720 (2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol) is a sphingosine-1-phosphate receptor agonist, which redirects lymphocytes from the circulatory system to lymph nodes and inhibits their egress from the latter sites. At clinically relevant concentrations, it does not impair T-lymphocyte activation, expansion, or memory processes suggesting a mechanism markedly different from that of any classical immunosuppressant [2, 3]. FTY720 is currently being evaluated in renal transplantation, and is administered once daily as a capsule formulation. These trials have yielded preliminary evidence that FTY720 can prevent acute rejection episodes when combined with corticosteroids and either cyclosporin or everolimus [4, 5]. The two main pharmacological effects of FTY720 were a reversible decrease in blood lymphocyte count and a transient decrease in heart rate after initiating therapy [6, 7].
To provide guidance to clinicians and patients about the timing of FTY720 administration with respect to meals, we performed a crossover single-dose study in healthy subjects to determine the effects of a high fat meal on its absorption. It was hypothesized that dietary fat could affect the oral absorption of FTY720 since it is a structural and functional analogue of sphingosine and may share the same absorption pathways as dietary fats which include the sphingolipid family [8]. Additional objectives of this study were to characterize the effect of FTY720 on blood lymphocyte counts and heart rate in healthy subjects.
Methods
Study design and population
This was a randomized, two-period, crossover study in 14 healthy men. The protocol was approved by the Landesärztekammer Rheinland-Pfalz, Neuenstadt, Germany and the study was performed at the Institut für Klinische Pharmakologie, Grünstadt, Germany. Subjects, all of whom gave written informed consent, were 28.6 ± 5.2 years old (range 20–39) and weighed 81.0 ± 7.6 kg (range 68–95). They were judged eligible for the study based on their past medical history, a physical examination, vital signs, standard clinical laboratory parameters, and an electrocardiogram performed at a screening visit.
Each study period began with a baseline phase on day −2, a single-blind administration of placebo on day −1, and a treatment phase in which a single 1 mg dose of FTY720 was administered under fasting or fed conditions on day 1 with follow-up assessments up to day 8. For the fasting treatment, subjects fasted for 10 h before drug administration and until 4 h after the dose. For the fed treatment, subjects were dosed with FTY720 5 min after consuming a high fat breakfast consisting of two eggs fried in butter, 4 oz hash brown potatoes, two slices of toast with butter, two slices of bacon, and 240 ml of whole milk [9]. The drug was administered with 200 ml water in both treatments. Standard meals were served starting at 4 h after drug administration. Subjects remained at the study centre from the morning of day −2 until the morning of day 4 and returned to the study centre on days 5, 6, and 8 for assessment. The two study periods were separated by 4 weeks. An end-of-study evaluation was performed on day 8 of period 2.
Clinical assessments
Safety assessments included physical examinations, vital signs, clinical laboratory parameters (biochemistry, haematology, urinalysis), and electrocardiograms at baseline, several times during the treatment periods, and at the end-of-study evaluation. Lymphocyte counts and heart rate were monitored frequently during the study. Total lymphocyte counts were determined by flow cytometry from 5 ml blood samples drawn predose and 1, 2, 4, 6, 8, 12, 16, 20 and 24 h after placebo administration on day −1. On day 1, blood samples were obtained −1 and 0 h predose and 1, 2, 4, 6, 8, 12, 16, 20, 24, 48, 72, 96, 120, and 168 h after the FTY720 dose. Lymphocyte subsets were also determined by flow cytometry predose and 4, 8, 12, 24, 48, 72 and 120 h after the FTY720 dose. Subsets included CD20 (B-cells), CD3 (pan T-cells), CD4 (helper T-cells), CD8 (suppressor T-cells), CD16 (natural killer cells), CD14 (monocytes), CD45RO (memory T-cells), and CD45RA (naïve T-cells). After subjects had been resting in a supine position for 3 min, heart rate was determined together with diastolic and systolic blood pressure using a bedside monitoring device with digital readout. Measurements were recorded every 4 h on day −1 after placebo administration and on day 1 after FTY720 administration at 0, 4, 8, 12, 16, 20, and 24 h postdose. Supine heart rate was also recorded at 48, 72, 96, 120 and 168 h postdose.
Blood sampling
Venous blood samples (3 ml) were obtained before FTY720 administration and then at 1, 2, 4, 6, 8, 12, 16, 20, 24, 36, 48, 72, 96, 120 and 168 h postdose. Samples were collected in an EDTA-containing vacuum tube, inverted several times to mix the contents and frozen at −20 °C.
Determination of FTY720
FTY720 was analyzed in whole blood by a validated liquid chromatography method with tandem mass spectrometry (HPLC/MS/MS) in selected reaction monitoring mode using atmospheric pressure chemical ionization (APCI) as an interface. Briefly, 0.5 ml blood was mixed with 0.5 ml of 100 mm NaOH and 5 ml of 75/25 (v/v) t-butylmethylether and dichloroethane. The organic layer was evaporated under nitrogen at 40 °C and 200 µl of 1/1 (v/v) 20 mm aqueous ammonium acetate and methanol were added, vortexed, and centrifuged. The supernatant (0.1 ml) was injected onto a Symmetry Shield RP8 3.5 µm (50 × 4.6 mm) HPLC column at 40 °C. The mobile phase consisted of methanol and 20 mm aqueous ammonium acetate at a gradient of 75/25% from 0 to 4.8 min after injection, 100/0% from 4.9 to 5.5 min, and 75/25% from 5.6 to 6.6 min. The flow rate was 1 ml min−1 from 0 to 4.9 min and 2 ml min−1 from 4.95 to 6.6 min. The APCI conditions were: 30 psi N2 sheath gas pressure, 175 °C capillary temperature, 450 °C vaporizer temperature, and 5 µA corona discharge. Assay performance was judged on the basis of four quality control concentrations ranging from 0.07 to 6.9 ng ml−1. Accuracy ranged from 94.0% to 99.1% and precision coefficients of variation from 8.6% to 12.5%. The lower limit of quantification was 0.07 ng ml−1.
Pharmacokinetic evaluation and statistical analysis
Peak concentration (Cmax), the time of its occurrence (tmax), the truncated area under the concentration-time curve AUC(0,t) by linear trapezoidal summation, the total AUC extrapolated to infinity, and the elimination half-life (t½) were estimated using noncompartmental methods. The method of Pollack & colleagues was applied to derive the apical concentration defined as the mean of all measured concentrations within 20% of the Cmax and the time to the apical concentration derived as the median of all time points associated with these concentrations [10]. Cmax, AUC(0,t), and AUC were log-transformed and tested in an anova model with sequence, subject-within-sequence, period, and treatment as factors. The comparison between treatments was based on the geometric mean parameter ratios (fed/fasting) and the 90% confidence interval. A lack of effect of food was concluded if the 90% confidence interval was contained in the range of 0.80–1.25.
Total lymphocyte and lymphocyte subset counts were plotted against time and inspected for temporal trends. Response parameters were derived from the lymphocyte vs time curves and included the lymphocyte nadir, the time of its occurrence, and the area under the effect curve (AUE). Pertinent comparisons were performed with the anova model described above.
Results
Single doses of FTY720 were well tolerated. There were no adverse events or clinically significant changes in laboratory parameters or electrocardiograms. The effects of FTY720 on lymphocyte counts and heart rate are described below.
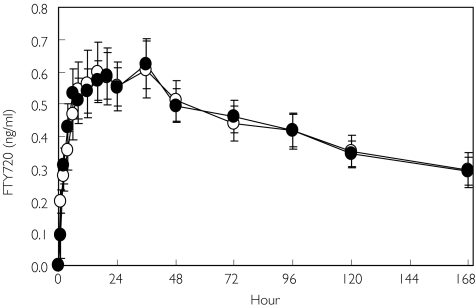
Figure 1 shows the mean concentration-time profile of FTY720 after fasting, and the corresponding pharmacokinetic parameters are summarized in Table 1. Concentrations increased over the first 6 h postdose and remained in a plateau region from 6 to 48 h. Thereafter there was a slow decline in concentrations over the remainder of the week-long blood sampling period. The time to reach the maximum concentration in individual subjects ranged from 12 to 36 h postdose with a median of 28 h. However, the Cmax exhibited only moderate interindividual variability with a coefficient of variation of 26%. Although an absolute maximum concentration could be identified in each subject, the broad plateau region between 6 and 48 h provided a number of close candidate concentrations near Cmax. In all fasting profiles, concentrations contributing to the apical region occurred as early as 6 h postdose and as late as 72 h in individual subjects. The median time that concentrations were in the apical region was 29 h (range 4–66 h), emphasizing the long duration of the plateau. The average apical concentration (0.59 ng ml−1) was close to the absolute Cmax (0.65 ng ml−1). The median time to reach the apical concentration was 20 h and was also close to the tmax of 28 h.
Figure 1.
Mean blood FTY720 concentration profiles in fasting (○) and fed (•) healthy volunteers. Bars represent 95% confidence intervals
Table 1.
Pharmacokinetic parameters for FTY720 given to healthy subjects in the fasting or fed state
| Parameter | Fasting | Fed |
|---|---|---|
| tmax (h) | 28 (12–36) | 36 (12–36) |
| Cmax (ng ml−1) | 0.65 ± 0.17 | 0.64 ± 0.13 |
| tapical (h) | 20 (16–26) | 18 (12–30) |
| Capical (ng ml−1) | 0.59 ± 0.16 | 0.58 ± 0.11 |
| AUC(0,t) (ng ml−1 h) | 72 ± 17 | 72 ± 14 |
| AUC (ng ml−1 h) | 149 ± 65 | 139 ± 43 |
| CL/F (l h−1) | 7.9 ± 3.2 | 7.8 ± 2.5 |
| Vz/F (l) | 1738 ± 711 | 1621 ± 291 |
| t½ (days) | 7.0 ± 2.8 | 6.5 ± 2.1 |
Data are mean ± SD except for time parameters which are median (range).tmax is time to reach peak,Cmax is peak blood concentration,tapical is time to reach apical concentration,Capical is apical concentration, AUC is area under the concentration-time curve, CL/F is apparent oral clearance,Vz/F is apparent distribution volume, t½ is half-life.
The week-long blood sampling protocol allowed full characterization of the absorption phase and the early portion of the elimination phase. The truncated AUC(0,t) to day 8 represented approximately 52% of the total AUC with the remaining 48% (range 25% to 72%) being extrapolated. The truncated AUC(0,t) exhibited a moderate interindividual variability of 24%. The elimination half-life averaged 7.0 days.
Figure 1 demonstrates that the mean concentration-time profiles of FTY720 were nearly superimposable in the fasting and fed states. Mean peak and apical concentrations were essentially unaltered postprandially. Cmax satisfied bioequivalence criteria with a fed/fasting point estimate of 1.00 and 90% confidence interval of 0.86, 1.17. Inspection of the individual data revealed that 11 subjects (79%) had fed/fasting Cmax ratios within the equivalence interval (0.80–1.25) and two had values marginally outside (0.78 and 1.26). The remaining subject had a very low value for Cmax after fasting administration (0.26 ng ml−1) and a normal value after fed administration (0.65 ng ml−1) yielding a ratio of 2.53. Overall interindividual variability in Cmax was not affected by food (20%) compared with the variability after fasting (26%).
Truncated AUC(0,t) demonstrated equivalence with a fed/fasting point estimate of 1.02 and 90% confidence interval of 0.88, 1.18. Inspection of the individual data indicated that 11 subjects (79%) had fed/fasting AUC(0,t) ratios within the equivalence interval (0.80–1.25) and two had values outside (0.73 and 1.37). The remaining subject had a very low value of AUC(0,t) after fasting administration (31 ng ml−1 h) and a normal value after fed administration (76 ng ml−1 h) yielding a ratio of 2.42. Overall interindividual variability in AUC(0,t) was not affected by food (19%) relative to the variability after fasting (24%). The extrapolated AUC was also unaffected by food (point estimate 0.98 (0.86, 1.11).
Figure 2 shows the mean lymphocyte data over a 24 h period after the two placebo administrations. A circadian rhythm was clearly discernible, whereby lymphocyte counts were dampened during the daytime (08.00–18.00 h) and elevated at night (18.00–0.00 h). In contrast, after FTY720 administration the circadian rhythm was replaced by a decline in lymphocyte count from a predose morning value of 2.3 × 109/l to a nadir of 1.4 × 109/l reached approximately 1 day postdose. The nadir count represented a 38 ± 9% decrease in lymphocytes from the predose value and was consistent among the subjects with an interindividual coefficient of variation of 14%. The nadir was followed by a gradual recovery over the week to reach a value of 1.8 × 109/l by day 8. The between treatment period was sufficiently long to allow lymphocyte recovery (predose count before period 1 was 2.1 ± 0.3 × 109/l and before period 2 was 2.3 ± 0.3 × 109/l. As summarized in Table 2, the same temporal pattern in lymphocyte count was observed under fasting and fed conditions. Inspection of the lymphocyte subset data over time (not shown) indicated that the decline to a nadir and subsequent recovery pattern evident for total lymphocytes was also followed for all subsets with the exception of granulocytes and natural Killer cells (CD16) which did not appear to be effected by FTY720 either under fasting or fed conditions.
Figure 2.
Mean lymphocyte trajectories after placebo (open symbols) and FTY720 (filled symbols). Circles represent the fasting period and squares represent the fed period
Table 2.
Lymphocyte response parameters in healthy subjects given FTY720 in the fasting or fed state
| Parameter | Fasting | Fed |
|---|---|---|
| Predose lymphocyte count (109/l) | 2.3 ± 0.3 | 2.2 ± 0.3 |
| Nadir lymphocyte count (109/l) | 1.4 ± 0.2 | 1.2 ± 0.2 |
| Time of nadir lymphocyte count (h) | 22 (6–48) | 22 (6–168) |
| Day 8 lymphocyte count (109/l) | 1.8 ± 0.3 | 1.8 ± 0.3 |
| AUE(0,168 h) (cells × 109/l × h) | 280 ± 43 | 273 ± 40 |
Values are mean ± SD except for time of nadir which is median (range). AUE is the area under the lymphocyte-time effect curve.
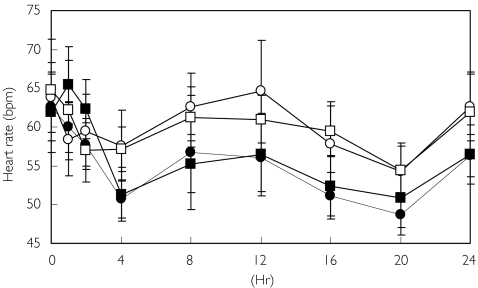
Figure 3 demonstrates that heart rate followed a similar circadian temporal pattern after both placebo and FTY720 treatment. The mean predose heart rates were between 62 and 65 beats min−1 regardless of treatment (Table 3). After placebo administration, the lowest daily heart rate occurred at night about 20 h postdose (04.00 h). The lowest heart rate during the day occurred about 2 h postdose (10.00 h). Nearly all subjects (12 of 14) had a nadir heart rate of less than 60 beats min−1 and 5 of 14 had a nadir of less than 50 beats min−1 after one or both placebo administrations.
Figure 3.
Mean supine heart rate over 24 h after placebo (open symbols) and FTY720 (filled symbols). Circles represent the fasting period and squares represent the fed period
Table 3.
Heart rate response parameters in healthy subjects given FTY720 in the fasting or fed state
| Parameter | Fasting Placebo | FTY720 | Fed Placebo | FTY720 |
|---|---|---|---|---|
| Day 1 predose rate (beats min−1) | 64 ± 8 | 63 ± 7 | 65 ± 11 | 62 ± 9 |
| Morning nadir (beats min−1) | 55 ± 7 | 50 ± 4 | 55 ± 6 | 51 ± 6 |
| Morning time of nadir (h) | 2 (1–4) | 4 (4–8) | 2 (1–8) | 4 (4–8) |
| AUE(0,8 h) (beats min−1 × h) | 477 ± 56 | 443 ± 38 | 474 ± 45 | 454 ± 47 |
| Daily nadir (beats min−1) | 53 ± 6 | 47 ± 3 | 53 ± 6 | 49 ± 6 |
| Daily time of nadir (h) | 16 (1–20) | 20 (4–20) | 4 (2–20) | 10 (4–20) |
| AUE(0,24 h) (beats min−1 × h) | 1435 ± 152 | 1293 ± 115 | 1420 ± 128 | 1317 ± 138 |
| Day 2 morning rate (beats min−1) | – | 56 ± 5 | – | 56 ± 7 |
| Day 3 morning rate (beats min−1) | – | 59 ± 6 | – | 59 ± 7 |
| Day 4 morning rate (beats min−1) | – | 60 ± 8 | – | 63 ± 10 |
| Day 5 morning rate (beats min−1) | – | 61 ± 8 | – | 62 ± 7 |
Values are mean ± SD except for tnadir which is median (range). AUE is area under the heart rate vs time effect curve. Morning nadir taken from the time interval 0–8 h, daily data taken from the interval 0–24 h.
After fasting, the heart rate vs time curve following FTY720 administration compared with placebo (P < 0.01) was shifted downward. Heart rate was decreased by 7% for the morning AUE(0,8 h) and 10% for the full-day AUE(0,24 h). After FTY720 administration, the heart rate nadir was significantly lower for both the full 24 h and the first 8 h compared with placebo (P < 0.001). Morning supine heart rates were obtained at each subsequent visit and recovered to predose values within 2–3 days postdose (Table 3). Heart rate patterns were similar between fasting and fed FTY720 treatments.
Discussion
This study provides the first pharmacodynamic and pharmacokinetic data for FTY720 in healthy subjects. A single 1 mg dose of FTY720 was well tolerated and the only notable effects were on lymphocytes and heart rate. The decrease in total lymphocyte count is related to the ability of FTY720 to redirect lymphocytes from the circulation to the secondary lymphoid tissues. A nearly full recovery of lymphocyte count was observed after 1 week. This is similar to the effect of FTY720 in renal transplant patients in whom the decrease in blood lymphocyte count was not associated with an increased risk of infections [6]. Comparison of these two studies demonstrates that the reduction in lymphocyte counts is FTY720-related and not influenced by cotreatment with cyclosporine or corticosteroids.
The observed effect of FTY720 on heart rate is consistent with its agonistic properties on sphingosine-1-phosphate receptors in the sinus node and atrial cells of the heart [11]. It was found that the circadian rhythm in heart rate was preserved in the presence of FTY720 which is consistent with intact autonomic control of the heart. No other effects of FTY720 were observed.
FTY720 showed a very slow rate of appearance in blood after oral administration, and concentrations rose to a broad plateau over a 2-day period. In addition to absorption from the gastrointestinal tract, FTY720 may also be absorbed via the lymphatic system as is known for dietary sphingolipids [8, 12]. Despite the prolonged absorption phase, there was only moderate interindividual variation in the peak concentration of the drug. Given the prolonged elimination half-life of FTY720 of about 7 days and the blood concentrations achieved after a 1 mg dose relative to the limit of quantification of the assay, it was not possible to measure directly the major portion of the total AUC. Under these circumstances, regulatory guidance recommends that blood is sampled for a sufficient time to allow gastrointestinal transit and to characterize fully the absorption phase [9]. These conditions were clearly achieved in this study. The peak, early, and total exposure parameters, Cmax, AUC(0,t), and AUC, satisfied standard bioequivalence criteria to demonstrate that food in the form of a high fat meal had no effect on the absorption of FTY720. Characteristics of FTY720 which may explain this lack of effect include its relatively small molecular size, good oral bioavailability (≥ 60% in rats and cynomolgus monkeys), and prolonged absorption.
The pharmacokinetics of single dose FTY720 in these healthy subjects were similar to that observed in stable renal transplant patients [6]. Hence, single dose healthy subject pharmacokinetic data are appropriate for deriving dosage guidelines for patients. Accordingly, these data indicate that FTY720 capsules may be taken without regard to the timing of meals.
References
- 1.Kovarik JM, Burtin P. Immunosuppressants in advanced clinical development for organ transplantation and selected autoimmune diseases. Expert Opin Emerging Drugs. 2003;8:47–62. doi: 10.1517/14728214.8.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann V, Lynch K. FTY720: Targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr Opin Immunol. 2002;14:569–75. doi: 10.1016/s0952-7915(02)00374-6. [DOI] [PubMed] [Google Scholar]
- 3.Pinschewer DD, Ochsenbein AF, Odermatt B, et al. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164:5761–70. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- 4.Fergason RM, Mulgaonkar S, Tedesco H, et al. High efficacy of FTY720 with reduced cyclosporine dose in preventing rejection in renal transplantation. (Abstract) Am J Transplant. 2003;3(Suppl 5):311. [Google Scholar]
- 5.Kahan BD, Tedesco H, Lorber MI, et al. FTY720 combination with everolimus: a novel CNI-free regimen as effective in Afro-American as Caucasians at high risk for DGF. (Abstract) Am J Transplant. 2003;3(Suppl 5):555. [Google Scholar]
- 6.Budde K, Schmouder RL, Brunkhorst R, et al. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J Am Soc Nephrol. 2002;13:1073–83. doi: 10.1681/ASN.V1341073. [DOI] [PubMed] [Google Scholar]
- 7.Budde K, Schmouder RL, Nashan B, et al. Pharmacodynamics of single doses of the novel immunosuppressant FTY720 in stable renal transplant patients. Am J Transplant. 2003;3:846–54. doi: 10.1034/j.1600-6143.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmelz EM, Crall KJ, Larocque R, et al. Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J Nutr. 1994;124:702–12. doi: 10.1093/jn/124.5.702. [DOI] [PubMed] [Google Scholar]
- 9.United States Food and Drug Administration. 2003. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products-general considerations (Revision 1)
- 10.Pollack PT, Freeman DJ, Carruthers SG. Mean apical concentration and duration in the comparative bioavailability of slowly absorbed and eliminated drug preparations. J Pharm Sci. 1988;77:477–80. doi: 10.1002/jps.2600770603. [DOI] [PubMed] [Google Scholar]
- 11.Guo J, MacDonell KL, Giles WR. Effects of sphingosine 1-phosphate on pacemaker activity in rabbit sino-atrial node cells. Eur J Physiol. 1999;438:642. doi: 10.1007/s004249900067. [DOI] [PubMed] [Google Scholar]
- 12.Vesper H, Schmelz EM, Nikolova-Karakashian MN. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr. 1999;129:1239–50. doi: 10.1093/jn/129.7.1239. [DOI] [PubMed] [Google Scholar]