Abstract
Aims
Moxonidine is an I1-imidazoline receptor agonist that reduces blood pressure by inhibition of central sympathetic activity. The effects of the drug under physical and mental stress have not been studied in detail.
Methods
We investigated the effects of 0.4 mg moxonidine orally on sympathetic activity, blood pressure and heart rate in a double-blind, placebo-controlled crossover study in 12 healthy volunteers. The subjects underwent physical exercise test using bicycle ergometry and a mental stress test using an adaptive reaction test device. Potential association of parameters with the GNB3 C825T polymorphism was also assessed.
Results
Under resting conditions, moxonidine decreased plasma noradrenaline (NA: −66.1 ± 12 pg ml−1; P < 0.01 vs placebo) and adrenaline (A: −18.8 ± 6 pg ml−1; P < 0.05 vs placebo). Physical exercise evoked a significant increase in plasma NA and A (NA: 760 ± 98 pg ml−1; A: 97 ± 9 pg ml−1; P < 0.001 vs baseline), which was significantly reduced after pretreatment with moxonidine (NA: 627 ± 68 pg ml−1; P < 0.05 vs placebo; A: 42.8 ± 4 pg ml−1; P < 0.01 vs placebo). Maximal physical exercise capacity was not limited by moxonidine (NS). During the mental stress test, increases in NA (placebo: 146 ± 24 pg ml−1, moxonidine: 84 ± 26 pg ml−1; P < 0.01 vs placebo) and A (placebo: 22.8 ± 9 pg ml−1, moxonidine: 8.0 ± 8 pg ml−1; P < 0.01 vs placebo) were significantly reduced after pretreatment with moxonidine. Increases in blood pressure during mental stress were significantly lower after pretreatment with moxonidine (P < 0.05 vs placebo). There was no association of the response to moxonidine with GNB3 genotypes (NS).
Conclusions
Moxonidine decreases total sympathetic tone under basal conditions as well as during physical exercise and mental stress without limiting absolute exercise capacity. Thus, moxonidine appears suitable for the treatment of patients with high SNS activity and hypertension induced by physical or mental stress. As the drug does not reduce exercise capacity, it may be considered as an alternative to β-adrenoceptor blockers in selected patients.
Keywords: mental stress, moxonidine, physical exercise, sympathetic activity
Introduction
The sympathetic nervous system (SNS) is an important regulator of cardiovascular function. Its activity is determined by psychological, neuronal and humoral factors [1, 2]. Activation of neurohumoral systems as well as impairment of local regulatory mechanisms plays a significant role in the pathogenesis and prognosis of cardiovascular diseases [3].
Centrally acting antihypertensive drugs such as clonidine, guanfacine and α-methyldopa have been widely used in the past as effective central sympatholytic antihypertensive drugs [4]. However, due to their unpleasant side-effects these drugs are no longer used as first line therapy in hypertension.
The newly developed central antihypertensives, i.e. moxonidine and rilmenidine, act mainly on imidazoline-1 receptors and less so on central α2-adrenoreceptors in an agonistic fashion. We have previously shown, that moxonidine under resting conditions not only reduces plasma catecholamine concentrations, but importantly central SNS activity as assessed by the measurement of muscle sympathetic nerve activity (MSA) using microneurography [5].
Central SNS overactivation due to physical or mental stress may contribute to unwanted effects of the SNS on the heart, the vessels and the kidney leading to myocardial and vascular hypertrophy, hypertension and proteinuria [6–8].
It has been shown that antihypertensive drugs act differently on SNS activity under resting conditions. Pure vasodilators mostly stimulate central SNS activity, whereas ACE inhibitors and central sympatholytics reduce central SNS activity [5, 9, 10]. Only very few data are available on the effects of antihypertensive drugs under physical and mental stress. Indeed, β-adrenoceptor blockers, peripheral α-adrenoceptor blockers, diuretics and DHP calcium channel blockers increase plasma noradrenaline during exercise [11–13], whereas ACE inhibitors and Ang-II receptor antagonists have no effect on stimulated SNS activity [9, 14]. Effects of antihypertensive drugs on SNS activity during mental stress are not systematically studied; we found no effect of the DHP calcium antagonist nifedipine on MSA during mental stress [10].
The G protein β3 subunit 825T allele was shown in several studies to be significantly associated with an increased risk for hypertension [15–17]. Efficacy of antihypertensive treatment may depend on the pharmacogenetic response to cardiovascular drugs [18]. Therefore, we investigated the effects of moxonidine on exercise and mental stress-induced SNS activation in normotensive healthy volunteers in a double-blind, placebo-controlled crossover study. As a secondary endpoint, we assessed the association of the acute response to 0.4 mg moxonidine or placebo with the carrier status of the GNB3825T allele.
Methods
Study design
We performed a double-blind, placebo controlled crossover study in 12 young healthy subjects assessing the effects of 0.4 mg moxonidine on exercise and mental stress-induced sympathetic activation (for demographic characteristics see Table 1). The subjects were free of any cardiovascular diseases based on medical history and a physical examination before the study. Written informed consent was obtained from all subjects. The ethics committee of the University Hospital Essen, Germany, approved the study.
Table 1.
Major demdographic and safety parameters of the subjects
| Age (years) | 24.2 ± 4.5 |
| Sex (male: female) | 12/0 |
| Body mass index (kg m−2) | 22.3 ± 1.3 |
| Genotype (825T allele) CC vs CT/TT | 6 : 6 |
| Haemoglobin (g dl−1) | 15.1 ± 1.1 |
| Hematocrit (%) | 44.4 ± 3 |
| Sodium (mmol l−1) | 140.5 ± 2 |
| Potassium (mmol l−1) | 3.9 ± 0.3 |
| Creatinine (mg dl−1) | 0.8 ± 0.2 |
| GOT (U l−1) | 20.4 ± 5 |
| Gamma GT (U l−1) | 11 ± 3 |
Procedure
The subjects were investigated in the salt replete state. On the study day, the subjects were positioned in a supine position, a peripheral venous access was inserted in the forearm 40 min after resting in the quiet conditions of a climatized room, blood was drawn to assess basal parameters (catecholamines) as well as basal haemodynamics (heart rate, blood pressure). Thereafter, either placebo or 0.4 mg moxonidine were administered orally. After 3 h of resting conditions plasma catecholamines and basal haemodynamics were reassessed. Subsequently, a bicycle exercise-test was performed starting with 50 watt increasing to 100, 150 and 200 watt every 2 min. At the end of each step, plasma catecholamines, heart rate, and blood pressure were reassessed. After the exercise test, a resting period of 60 min was allowed, and during this phase the subjects were again in the supine position. Then basal concentrations of plasma catecholamines, heart rate and blood pressure were again assessed. Next a mental stress test was performed using an adaptive reaction tester. This device forces the subject to push four different buttons (red, blue, yellow, green) according to the corresponding LED. The device is programmed to guarantee a fault rate of approximately 50%, thus leading to a well-defined and reproducible mental stress (Basler Art, Fa. Dr Maus, Germany).
Seven days later, the same procedure was performed this time administering 0.4 mg moxonidine or placebo according to the double-blind, placebo controlled crossover study design.
During the whole observation period, vital parameters (heart rate and blood pressure) were monitored every 15 min for safety reasons. On the study day, all subjects were studied under the same conditions, i.e. in the morning (09.00 h), after a light breakfast. After micturition to avoid any stimulation of sympathetic nerve activity through bladder distension, subjects were asked to resume the supine position [19].
We chose the period of 180 min after drug administration based on previously published literature on the pharmacokinetics and dynamics of moxonidine as well as on the basis of our own previous experience, which showed a significant effect of moxonidine on MSA after more than 120 min [5, 9, 10].
Heart rate was calculated from the mean RR-interval min−1 of an ECG. Blood pressure was assessed noninvasively using a mercury manometer (Dinamap Compact T, Fa. Critikon Germany) assessing Korotkow phase I for systolic and IV for diastolic blood pressure.
Moxonidine (0.4 mg orally) or placebo was supplied and blinded by the hospital pharmacy (Universitätsklinik Essen, Germany) in a neutral capsule.
Data are given as mean ± SEM. The significance of differences was calculated by analysis of variance for repeated measures (anova; 95% confidence intervals); a P value of = 0.05 was taken for statistical significance.
Assays
Plasma catecholamines and renin were determined by HPLC as described previously [20, 21]. Immediately after drawing the blood, the samples were stored on ice and cool-centrifuged as described [21].
Results
Safety parameters
Basal laboratory values (haemoglobin, haematocrit, creatinine, liver enzymes) were within normal ranges (Table 1). Except for a dry mouth in four healthy volunteers after intake of moxonidine there were no adverse effects after administration of either placebo or moxonidine.
Plasma catecholamines
Resting conditions
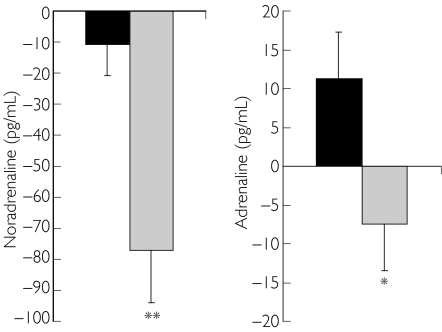
Under resting conditions plasma noradrenaline and adrenaline concentrations decreased significantly vs placebo (NA: P < 0.01 vs placebo; Figure 1, left panel; A: P < 0.05 vs placebo; Figure 1, right panel).
Figure 1.
Changes in plasma noradrenaline (left panel) and adrenaline (right panel) under resting conditions after administration of either placebo or 0.4 mg moxonidine. */**P < 0.05/ < 0.01 vs baseline. Placebo (▪), moxonidine ( )
)
Bicycle exercise
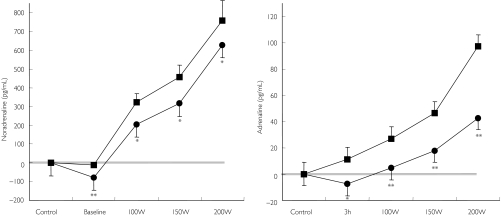
Bicycle exercise induced a significant increase in plasma noradrenaline and plasma adrenaline concentrations (P < 0.001 vs baseline). Stimulated plasma noradrenaline concentrations were significantly lower after pretreatment with moxonidine compared with placebo (P < 0.05 vs placebo; Figure 2, left panel). Similarly, during physical exercise, adrenaline plasma concentrations were significantly lower after pretreatment with moxonidine compared with placebo (P < 0.05 vs placebo; Figure 2, right panel). Maximal physical exercise capacity did not differ between placebo and moxonidine treatment (NS).
Figure 2.
Changes in plasma noradrenaline (left panel) and adrenaline (right panel) during bicycle exercise testing after administration of either placebo or 0.4 mg moxonidine. */**P < 0.05/ < 0.01 vs baseline. W = Watt. Placebo (▪), moxonidine (•)
Mental stress test
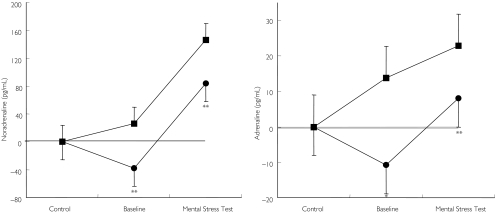
Mental stress evoked a significant increase in plasma noradrenaline and a marked increase in adrenaline (P < 0.01 vs baseline). After the mental stress test, noradrenaline plasma concentrations were significantly lower after pretreatment with moxonidine compared with placebo (P < 0.01, Figure 3, left panel). Similarly, adrenaline plasma concentrations were markedly reduced after pretreatment with moxonidine compared with placebo (P < 0.01 vs placebo, Figure 3, right panel).
Figure 3.
Changes in noradrenaline (left panel) and adrenaline (right panel) during mental stress test after administration of either placebo or 0.4 mg moxonidine. */**P < 0.05/ < 0.01 vs baseline. Placebo (▪), moxonidine (•)
Blood pressure
Resting conditions
Under resting conditions there was no significant reduction of systolic and diastolic blood pressure vs baseline (NS vs placebo).
Bicycle exercise
Systolic and diastolic blood pressure increased significantly during physical exercise (P < 0.01, Table 2). Moxonidine had no significant effect on either systolic or diastolic blood pressure under stimulated conditions (NS vs placebo).
Table 2.
Effects of moxonidine vs placebo on heart rate and blood pressure under resting conditions, and after physical exercise and mental stress
| HR (beats min−1) | Placebo Systolic BP (mm Hg) | Diastolic BP (mm Hg) | HR (beats min–1) | Moxonidine Systolic BP (mm Hg) | Diastolic BP (mm Hg) | |
|---|---|---|---|---|---|---|
| Baseline | 65 ± 6 | 118 ± 11 | 75 ± 6 | 63 ± 8 | 118 ± 10 | 76 ± 9 |
| Maximal physical exercise | 145 ± 16## | 177 ± 29## | 91 ± 11# | 153 ± 15# | 176 ± 21## | 85 ± 9# |
| Mental stress test | 83 ± 5# | 128 ± 8# | 81 ± 8# | 83 ± 9# | 111 ± 11* | 71 ± 5* |
BP, blood pressure. Data are mean ± SEM.
P < 0.05 vs placebo.
P < 0.05/
< 0.01 vs baseline.
Mental stress test
Mental stress test increased systolic and diastolic blood pressure significantly (P < 0.01, Table 2). There was a significant reduction of systolic and diastolic blood pressure after pretreatment with moxonidine compared with placebo during mental stress (P < 0.05, Table 2).
Heart rate
Resting conditions
Under resting conditions heart rate did not differ significantly vs baseline (Table 2).
Bicycle exercise
Physical exercise induced a marked increase in heart rate (P < 0.01 vs baseline). There was no significant effect of moxonidine on heart rate during exercise (NS, Table 2).
Mental stress test
Mental stress increased heart rate (P < 0.05 vs baseline). During mental stress there was no significant effect of moxonidine on heart rate (NS, Table 2).
Association of the effects of moxonidine with the GNB3825T allele
There were no statistically significant associations of the GNB3825T allele with changes in noradrenaline, adrenaline, blood pressure, and heart rate during physical exercise or mental stress, although carriers of the 825T allele tended to have a stronger reduction in noradrenaline plasma concentrations during physical exercise after pretreatment with moxonidine (not shown).
Discussion
This double-blind, placebo-controlled crossover study demonstrates for the first time, that the I1-imidazoline receptor agonist moxonidine not only reduces significantly exercise-induced sympathetic activation without affecting exercise capacity, but also blunts sympathetic activation due to mental stress. The significant reduction in the concentrations of plasma adrenaline after administration of moxonidine during physical exercise and, more so, during mental stress, indicates, that moxonidine affects also sympathetic outflow to the suprarenal gland.
The SNS is an important regulator of the circulation and the heart [1]. Both exercise and mental stress evoke a marked activation of SNS activity [11, 22], which might be an additional risk factor in certain disease states including coronary artery disease, impaired left ventricular function and diabetes mellitus [7, 23–25].
In the present study we used 0.4 mg moxonidine which is a well established, effective dose for the treatment of mild to moderate hypertension [26, 27]. Moxonidine has a high (94%) bioavailability and reaches its peak plasma concentration after less than 1 h [5, 28]. We have previously shown that central sympatholytic effects of moxonidine are achieved within 150 min after oral administration, when plasma concentrations are already falling [5]. Thus, plasma concentrations are not an ideal indicator of drug effects. This can be explained by the fact, that the drug rapidly diffuses into the CNS, where it exerts its sympatholytic effects [28, 29].
Few studies have been published assessing the effects of cardiovascular drugs on exercise-stimulated SNS activity. We have previously shown that the peripheral α-adrenoceptor blocker doxazosin increases exercise-induced SNS activity [30]. Indeed, in the ALLHAT-trial, the doxazosin group had a higher incidence of heart failure when compared with the diuretic group [31]. Possibly, the increased sympathetic tone due to the α-adrenoceptor blocker may have contributed to this effect [30]. A study comparing the effects of nifedipine on exercise-induced SNS activation found a similar increase of plasma noradrenaline when compared with a β-adrenoceptor blocker [32]. ACE-inhibitors and the AT-1-receptor antagonist losartan did not significantly modulate exercise-induced increases in plasma noradrenaline when compared with placebo in a double-blind, placebo controlled trial [30]. Furthermore, we have assessed the effects of the DHP calcium-antagonist nifedipine on mental stress induced SNS activity and found no changes in MSA when compared with placebo as assessed by microneurography [10]. However, DHP calcium antagonists acutely stimulate basal SNS activity, whereas during chronic administration, at least with some Calcium Antagonists (i.e. lercanidipine), central SNS activity is not stimulated [33].
The findings of the present study can be attributed to a central sympatholytic effect of the drug and demarcates the drug from all other antihypertensive drugs including the β-adrenoceptor blockers [13, 34, 35]. Indeed, β-adrenoceptor blockers do not reduce plasma catecholamine concentrations during exercise but may even increase them [11]. Although it can be argued that the receptors mediating the effects of the catecholamines are blocked, it is not clear, whether increased peripheral catecholamine concentrations can still stimulate other adrenoceptors including β3-receptors in adipose tissue. In the present study, moxonidine reduced plasma noradrenaline and adrenaline under resting conditions as well as under physical and mental stress; relative increases in the catecholamine plasma concentrations were similar to placebo. Thus, moxonidine does not reduce the relative sympathetic response to the stimuli, but decreases total sympathetic tone.
Pharmacogenetic aspects in the therapy of cardiovascular drugs gain more and more relevance in terms of response, efficacy and, thus, costs. In the present study, we assessed the association of the response to moxonidine with the GNB3825T allele as a secondary endpoint. We found no differences between the alleles, although carriers of the 825T allele tended to have a greater reduction in noradrenaline plasma concentrations. However, this study assessed only acute effects of a single dose of moxonidine. Thus, the present data cannot be extrapolated to chronic therapy with moxonidine in hypertension and cardiovascular disease.
In our study, exercise capacity after administration of moxonidine was comparable with the administration of placebo. In contrast, exercise capacity is reduced on β-adrenoceptor blocker treatment [36–38]. This may make moxonidine the drug of choice in physically active hypertensives bothered by the exercise limitations during β-adrenoceptor blocker therapy.
Our study assessed the acute effects of a single-dose of moxonidine in healthy volunteers. Potentially the drug effects are different during chronic administration under pathopyhsiological conditions. However, several studies have shown that moxonidine effectively reduces blood pressure even after many weeks of therapy [39–41].
In summary, moxonidine reduces both exercise- and mental stress-induced SNS activation without affecting exercise capacity. This makes moxonidine a favourable antihypertensive drug for hypertensive patients with enhanced SNS activity and/or patients bothered by the exercise limitations of β-adrenoceptor blockers. Furthermore, the sympatholytic properties under basal and stimulated conditions make moxonidine favourable for combination therapy with peripheral vasodilators, which otherwise may further stimulate SNS activity.
Acknowledgments
The study was supported by the Deutsche Forschungsgemeinschaft (DFG, grant No. WE 1772/3–2 and 3–3), a grant from SOLVAY, Hannover (Germany) and a grant from the OERTEL Foundation.
References
- 1.Wenzel RR, Bruck H, Noll G, Schäfers RF, Daul AE, Philipp T. Antihypertensive drugs and the sympathetic nervous system. J Cardiovasc Pharmacol. 2000;35:43–52. doi: 10.1097/00005344-200000004-00006. [DOI] [PubMed] [Google Scholar]
- 2.Converse RL, Jacobsen TN, Jost CM, Toto RD, Grayburn PA, Obregon TM, Fouad-Tarazi F, Victor RG. Paradoxical withdrawal of reflex vasoconstriction as a cause of hemodialysis-induced hypotension. J Clin Invest. 1992;90:1657–65. doi: 10.1172/JCI116037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palatini P, Julius S. Association of tachycardia with morbidity and mortality: pathophysiological considerations. J Hum Hypertens. 1997;11(Suppl 1):S19–S27. [PubMed] [Google Scholar]
- 4.Grassi G, Turri C, Seravalle G, Bertinieri G, Pierini A, Mancia G. Effects of chronic clonidine administration on sympathetic nerve traffic and baroreflex function in heart failure. Hypertension. 2001;38:286–91. doi: 10.1161/01.hyp.38.2.286. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel RR, Spieker L, Qui S, Shaw S, Luscher TF, Noll G. I1-imidazoline agonist moxonidine decreases sympathetic nerve activity and blood pressure in hypertensives. Hypertension. 1998;32:1022–7. doi: 10.1161/01.hyp.32.6.1022. [DOI] [PubMed] [Google Scholar]
- 6.Amann K, Rump LC, Simonaviciene A, Oberhauser V, Wessels S, Orth SR, Gross ML, Koch A, Bielenberg GW, Van Kats JP, Ehmke H, Mall G, Ritz E. Effects of low dose sympathetic inhibition on glomerulosclerosis and albuminuria in subtotally nephrectomized rats. J Am Soc Nephrol. 2000;11:1469–78. doi: 10.1681/ASN.V1181469. [DOI] [PubMed] [Google Scholar]
- 7.Colucci WS. The effects of norepinephrine on myocardial biology. implications for the therapy of heart failure. Clin Cardiol. 1998;21:120–4. doi: 10.1002/clc.4960211305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philipp T, Distler A, Cordes U. Sympathetic nervous system and blood pressure control in essential hypertension. Lancet. 1978;ii:959–63. doi: 10.1016/s0140-6736(78)92526-6. [DOI] [PubMed] [Google Scholar]
- 9.Noll G, Wenzel RR, de Marchi S, Shaw S, Luscher TF. Differential effects of captopril and nitrates on muscle sympathetic nerve activity in volunteers. Circulation. 1997;95:2286–92. doi: 10.1161/01.cir.95.9.2286. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel RR, Allegranza G, Binggeli C, Shaw S, Weidmann P, Luscher TF, Noll G. Differential activation of cardiac and peripheral sympathetic nervous system by nifedipine: role of pharmacokinetics. J Am Coll Cardiol. 1997;29:1607–14. doi: 10.1016/s0735-1097(97)00095-8. [DOI] [PubMed] [Google Scholar]
- 11.Eliasson K, Kahan T, Hylander B, Hjemdahl P. Responses to mental stress and physical provocations before and during long term treatment of hypertensive patients with β-adrenoceptor blockers or hydrochlorothiazide. Br J Clin Pharmacol. 1987;24:1–14. doi: 10.1111/j.1365-2125.1987.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkner B, Onesti G, Lowenthal DT, Affrime MB. The use of clonidine monotherapy in adolescent hypertension. Chest. 1983;83:425–7. doi: 10.1378/chest.83.2_supplement.425. [DOI] [PubMed] [Google Scholar]
- 13.Heidbreder E, Schafferhans K, Kirsten R, Heidland A. Effect of diuretics and calcium antagonists on circulatory parameters and plasma catecholamines during mental stress. Eur J Clin Pharmacol. 1983;25:19–22. doi: 10.1007/BF00544008. [DOI] [PubMed] [Google Scholar]
- 14.Creager MA, Faxon DP, Weiner DA, Ryan TJ. Haemodynamic and neurohumoral response to exercise in patients with congestive heart failure treated with captopril. Br Heart J. 1985;53:431–5. doi: 10.1136/hrt.53.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R, Sharma AM, Ritz E, Wichmann HE, Jakobs KH, Horsthemke B. Association of a human G-protein beta3 subunit variant with hypertension. Nat Genet. 1998;18:45–8. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 16.Beige J, Hohenbleicher H, Distler A, Sharma AM. G-Protein beta3 subunit C825T variant and ambulatory blood pressure in essential hypertension. Hypertension. 1999;33:1049–51. doi: 10.1161/01.hyp.33.4.1049. [DOI] [PubMed] [Google Scholar]
- 17.Benjafield AV, Jeyasingam CL, Nyholt DR, Griffiths LR, Morris BJ. G-protein beta3 subunit gene (GNB3) variant in causation of essential hypertension. Hypertension. 1998;32:1094–7. doi: 10.1161/01.hyp.32.6.1094. [DOI] [PubMed] [Google Scholar]
- 18.Turner ST, Schwartz GL, Chapman AB, Boerwinkle E. C825T polymorphism of the G protein beta (3)-subunit and antihypertensive response to a thiazide diuretic. Hypertension. 2001;37:739–43. doi: 10.1161/01.hyp.37.2.739. [DOI] [PubMed] [Google Scholar]
- 19.Fagius J, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension. 1989;14:511–7. doi: 10.1161/01.hyp.14.5.511. [DOI] [PubMed] [Google Scholar]
- 20.Hjemdahl P. Catecholamine measurements by high-performance liquid chromatography. Am J Physiol. 1984;247:E13–E20. doi: 10.1152/ajpendo.1984.247.1.E13. [DOI] [PubMed] [Google Scholar]
- 21.Michel MC, Brodde OE, Insel PA. Peripheral adrenergic receptors in hypertension. Hypertension. 1990;16:107–20. doi: 10.1161/01.hyp.16.2.107. [DOI] [PubMed] [Google Scholar]
- 22.Davis SN, Galassetti P, Wasserman DH, Tate D. Effects of gender on neuroendocrine and metabolic counterregulatory responses to exercise in normal man. J Clin Endocrinol Metab. 2000;85:224–30. doi: 10.1210/jcem.85.1.6328. [DOI] [PubMed] [Google Scholar]
- 23.Cohn JN. The sympathetic nervous system in heart failure. J Cardiovasc Pharmacol. 1989;14:S57–S61. [PubMed] [Google Scholar]
- 24.Hsueh WA, Buchanan TA. Obesity and hypertension. Endocrinol Metab Clin North Am. 1994;23:405–27. [PubMed] [Google Scholar]
- 25.Krieger DR, Landsberg L. Mechanisms in obesity-related hypertension: role of insulin and catecholamines. Am J Hypertens. 1988;1:84–90. doi: 10.1093/ajh/1.1.84. [DOI] [PubMed] [Google Scholar]
- 26.Kuppers HE, Jager BA, Luszick JH, Grave MA, Hughes PR, Kaan EC. Placebo-controlled comparison of the efficacy and tolerability of once-daily moxonidine and enalapril in mild-to-moderate essential hypertension. J Hypertens. 1997;15:93–7. doi: 10.1097/00004872-199715010-00010. [DOI] [PubMed] [Google Scholar]
- 27.Mitrovic V, Patyna W, Huting J, Schlepper M. Hemodynamic and neurohumoral effects of moxonidine in patients with essential hypertension. Cardiovasc Drugs Ther. 1991;5:967–72. doi: 10.1007/BF00143521. [DOI] [PubMed] [Google Scholar]
- 28.Trenk D, Wagner F, Jahnchen E, Planitz V. Pharmacokinetics of moxonidine after single and repeated daily doses in healthy volunteers. J Clin Pharmacol. 1987;27:988–93. doi: 10.1002/j.1552-4604.1987.tb05602.x. [DOI] [PubMed] [Google Scholar]
- 29.Kirch W, Hutt HJ, Planitz V. Pharmacodynamic action and pharmacokinetics of moxonidine after single oral administration in hypertension patients. J Clin Pharmacol. 1990;30:1088–95. doi: 10.1002/j.1552-4604.1990.tb01850.x. [DOI] [PubMed] [Google Scholar]
- 30.Wenzel RR, Wambach C, Schäfers RF, Daul AE, Michel MC, Siffert W, Philipp T. Doxazosin, but not losartan or enalapril, increases exercise-induced sympathetic activation. Kidney Blood Press Res. 1998;21:336–398. [Google Scholar]
- 31.Lasagna L. Diuretics vs alpha-blockers for treatment of hypertension: lessons from ALLHAT. Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. JAMA. 2000;283:2013–4. doi: 10.1001/jama.283.15.2013. [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara S, Arita M, Ueno Y, Shiotani M, Nakatsu C, Nakamura Y, Hano T, Nishio I, Masuyama Y. [Effects of calcium antagonist, angiotensin-converting enzyme inhibitors and beta-blocker on hemodynamic and sympathetic nerve responses to exercise in essential hypertension] J Cardiol. 1991;21:115–24. [PubMed] [Google Scholar]
- 33.Grassi G, Seravalle G, Turri C, Bolla G, Mancia G. Short-versus long-term effects of different dihydropyridines on sympathetic and baroreflex function in hypertension. Hypertension. 2003;41:558–62. doi: 10.1161/01.HYP.0000058003.27729.5A. [DOI] [PubMed] [Google Scholar]
- 34.Sundlof G, Wallin BG, Stromgren E, Nerhed C. Acute effects of metoprolol on muscle sympathetic activity in hypertensive humans. Hypertension. 1983;5:749–56. doi: 10.1161/01.hyp.5.5.749. [DOI] [PubMed] [Google Scholar]
- 35.Wallin BG, Sundlof G, Stromgren E, Aberg H. Sympathetic outflow to muscles during treatment of hypertension with metoprolol. Hypertension. 1984;6:557–62. doi: 10.1161/01.hyp.6.4.557. [DOI] [PubMed] [Google Scholar]
- 36.Kushwaha SS, Banner NR, Patel N, Cox A, Patton H, Yacoub MH. Effect of beta blockade on the neurohumoral and cardiopulmonary response to dynamic exercise in cardiac transplant recipients. Br Heart J. 1994;71:431–6. doi: 10.1136/hrt.71.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franz IW, Lohmann FW, Koch G. Oxygen uptake and plasma catecholamines during submaximal and maximal exercise after long-term beta-receptor blockade. Int J Sports Med. 1985;6:202–6. doi: 10.1055/s-2008-1025840. [DOI] [PubMed] [Google Scholar]
- 38.Schnabel A, Kindermann W, Salas-Fraire O, Cassens J, Steinkraus V. Effect of beta-adrenergic blockade on supramaximal exercise capacity. Int J Sports Med. 1983;4:278–81. doi: 10.1055/s-2008-1026050. [DOI] [PubMed] [Google Scholar]
- 39.Schäfers RF, Löw-Kröger A, Philipp T. Wirksamkeit und Verträglichkeit des neuen zentralwirksamen Antihypertensivums Moxonidin im Vergleich zu Enalapril. Nieren- Hochdruckkrankheiten. 1994;23:221–4. [Google Scholar]
- 40.Swedberg K, Bergh CH, Dickstein K, McNay J, Steinberg M. The effects of moxonidine, a novel imidazoline, on plasma norepinephrine in patients with congestive heart failure. Moxonidine Investigators. J Am Coll Cardiol. 2000;35:398–404. doi: 10.1016/s0735-1097(99)00565-3. [DOI] [PubMed] [Google Scholar]
- 41.Greenwood JP, Scott EM, Stoker JB, Mary DA. Chronic I1-imidazoline agonism: sympathetic mechanisms in hypertension. Hypertension. 2000;35:1264–9. doi: 10.1161/01.hyp.35.6.1264. [DOI] [PubMed] [Google Scholar]