Abstract
The World Medical Association's Declaration of Helsinki was first adopted in 1964. In its 40-year lifetime the Declaration has been revised five times and has risen to a position of prominence as a guiding statement of ethical principles for doctors involved in medical research. The most recent revision, however, has resulted in considerable controversy, particularly in the area of the ethical requirements surrounding placebo-controlled trials and the question of responsibilities to research participants at the end of a study. This review considers the past versions of the Declaration of Helsinki and asks the question: How exactly has the text of the Declaration changed throughout its lifetime? Regarding the present form of the Declaration of Helsinki we ask: What are the major changes in the most recent revision and what are the controversies surrounding them? Finally, building on the detailed review of the past and present versions of the Declaration of Helsinki, we give consideration to some of the possible future trajectories for the Declaration in the light of its history and standing in the world of the ethics of medical research.
Keywords: justice, Nuremberg Code, placebo-controlled trial, publication bias, research ethics, World Medical Association
Introduction
The Declaration of Helsinki (DoH) is, indisputably, a remarkable document. In less than 2000 words, the World Medical Association (WMA) spells out a set of ethical guidelines for physicians and other participants in medical research. At the recent Scientific Session held in association with the WMA's annual assembly, various independent experts on research ethics confirmed the central role of this document. At this meeting the DoH was described as the ‘cornerstone’ document pertaining to medical research ethics [1] and as ‘the most widely recognized source of ethical guidance for biomedical research’ [2]. Yet the DoH's guideline statements are not without controversy; and even more so since the most recent revision at the 16th Annual Assembly of the WMA in Edinburgh in October 2000.
In this paper we review the past and outline the present form of the text of the DoH. The major changes in the Edinburgh (2000) revision are outlined, along with some of the controversies to which they have given rise. Finally, we consider the possible future trajectories for this important document. Throughout this article we focus on the text that emerges at each stage of the process. The process leading to each revision is now extensively documented by the WMA at its own website [3]. We aim, through this review, to familiarize the reader with the current content of the DoH and an historical understanding of how the Declaration has changed with each revision. In so doing our hope is that awareness of the ethical issues for doctors participating in medical research will be heightened and that more will be encouraged to join the debate to ensure that this document remains an important guiding set of principles for many years to come.
We recognize that there is a major issue in modern philosophy regarding whether the meaning of a text is inherent in the author's intent or in the reader's interpretation [4]. Philosophers Hans-Georg Gadamer and Paul Ricoeur essentially take the position that the text has a mediating function seeking to fuse the horizons of understanding of author and reader [5]. Since, for the most part, researchers and others seeking to implement the guiding principles of the DoH have not attended WMA meetings and have no easy means of access to the ‘intent’ behind the text as it emerges, we consider it essential to take a stance akin to that of Gadamer and Ricoeur. Thus our emphasis is on the text which emerges rather than the debate which leads to the text. In this instance, however, the ‘author’, the WMA, is able to monitor both changing events in medical research and readers' response to and interpretation of the DoH and the Declaration can be modified accordingly. This was explicitly stated in the 1975 version of the DoH: ‘[the recommendations] should be kept under review in the future’ (see Appendix 2). Although the Edinburgh (2000) amendment saw this statement removed, in this sense, at least, the DoH can be conceived of as a ‘living document’.
Declaration of Helsinki: past
The British Medical Journal announced the emergence of the DoH in its 18 July 1964 edition with the following words: ‘A draft code of ethics on human experimentation was published in the British Medical Journal of 27 October 1962. … A revised version was accepted as the final draft at the meeting of the World Medical Association in Helsinki in June 1964. … It is to be known as the Declaration of Helsinki’ [6] (emphasis ours). Attached to this inconspicuous announcement was the just over 700 words of the text of the original DoH. There seemed little indication at the time of how important this document would become in the context of research ethics.
One of the darkest episodes in the history of medical research – the horrific experiments carried out by doctors on concentration camp victims in Nazi Germany – was exposed at the Nuremberg trials of 1947. Emerging from the Nuremberg trials was a code of ethics setting out ‘standards to which physicians must conform when carrying out experiments on human subjects’. The original DoH is seen as having its roots in the Nuremberg Code (see Appendix 1). Fluss identifies 12 markers of ethical research within the Nuremberg Code [7]. He points out that, of these, 10 markers appear in the original DoH and two markers are abandoned. The Nuremberg requirement that ‘The voluntary consent of the human subject is absolutely essential’ is changed and the DoH allowed consent to be given by the ‘legal guardian’ in cases of ‘legal incapacity’. The other abandoned ‘marker’ was the statement ‘During the course of the experiment the human subject should be at liberty to bring the experiment to an end if he has reached the physical or mental state where continuation of the experiment seems to him to be impossible’. This somewhat confusing statement was eliminated in the original DoH and appears to be covered most closely by the sentence: ‘The investigator or the investigating team should discontinue the research if in his or their judgement it may, if continued, be harmful to the individual’. This is, of course, in addition to the subject or subject's legal guardian's freedom to withdraw consent at any time [8].
The original DoH also states ‘In the field of clinical research a fundamental distinction must be recognized between clinical research in which the aim is essentially therapeutic for a patient, and clinical research the essential object of which is purely scientific and without therapeutic value to the person subjected to the research’ [8]. This led to the fundamental structure of the document. The paragraphs of the original and the first four revisions of the DoH are grouped under the headings ‘Introductory statements’, ‘I. Basic principles’, ‘II. Clinical research combined with professional care’ and ‘III. Non-therapeutic clinical research’. This structure persisted until the Edinburgh (2000) revision when it was substantially revised, and we return to this issue under ‘Declaration of Helsinki: present’.
First revision: Tokyo (1975)
The first revision to the DoH was adopted by the WMA at its 29th annual assembly in Tokyo (1975). This document was drafted by three Scandinavian professors of medicine [9].
The document was extensively revised from the 1964 version. Arguably the single most important addition in terms of the ensuing conduct of medical research was the requirement that independent committees review research protocols. Another major development was a significant elaboration of the requirements for informed consent. These requirements were also moved to the section entitled ‘Basic Principles’ (see Appendix 2; paragraphs I.9–I.11). Additional considerations regarding informed consent are presented in the section pertaining to ‘Medical Research Combined with Clinical Care’. These changes coincided with a simplification of the consent requirements for ‘non-therapeutic’ research wherein it is now simply stated ‘The subjects should be volunteers’ (paragraph III.2). Since the elaborated principles in the section ‘Basic Principles’ apply both to the ‘Clinical’ and to the ‘non-therapeutic’ category of research, there was no net loss of protection for subjects.
Table 1 outlines summary statements of the most important changes which took place in the 1975 revision. Appendix 2 gives the full text of the 1975 DoH. In addition to the major changes in content, there was a revision of the overtly sexist language in the 1964 version. The phrase ‘fully qualified medical man’ was changed to ‘medically qualified person’ (see paragraph I.3) and the use of the pronoun ‘his’ in reference to ‘doctor’ in the 1964 version was changed to ‘his or her’.
Table 1.
Key changes in the Tokyo (1975) revision of the Declaration of Helsinki
| Introduction | |
| 3rd, 4th and 5th paragraphs | Nature and purpose of medical research |
| 6th paragraph | Respect for environment and for animals used in research |
| 7th paragraph | Keep Declaration under review |
| Basic Principles | |
| I.2 | Independent committee review of research protocols |
| I.5 | Interests of human subject must prevail over interests of science and society |
| I.8 | Obligations regarding accuracy in publishing |
| I.9–I.11 | Enhanced requirements for informed consent |
| I.12 | Protocol must declare that requirements of Declaration of Helsinki adhered to |
| Medical Research Combined With Professional Care (Clinical Research) | |
| II.2 | Best current therapy should be comparator arm |
| II.3 | Assurance of access to best proven methods |
| II.4 | Refusal of research participation not to affect doctor–patient relationship |
| II.5 | When doctor considers it is essential not to obtain informed consent* |
| Non-therapeutic Biomedical Research Involving Human Subjects (Non-clinical Biomedical Research) | |
| III.2 | Less detail regarding consent (most of detail moved to Basic Principles section) |
| III.4 | Well-being of subject takes precedence over interests of science and society (see I.5) |
This is the only paragraph from the 1975 (and subsequent minor revisions) completely removed at the Edinburgh (2000) revision. (N.B. These are listed under the numbering system of the paragraphs in the Declaration with the exception of the ‘Introduction’ section, which is not numbered.)
The revision which took place in 1975 was even more extensive, as a proportion of the starting document, than the Edinburgh (2000) revision. Almost nothing was removed from the 1964 version and much was added. The result was an almost doubling in the length of the document. Given the relatively minor revisions of 1983, 1989 and 1996 (see below), it is effectively the 1975 version of the DoH which became the guiding document for the ethics of research involving human subjects for a quarter of a century.
Second revision: Venice (1983)
Given the extensive nature of the revision in 1975, it could be argued that the very minor changes of 1983 hardly warrant the term revision. However, it is the practice of the WMA in respect of the DoH to list all amendments in the preamble to the Declaration with no indication whether the amendment was major or minor. This practice has only been varied with the addition of the Note of Clarification to paragraph 29 in 2002 which is mentioned in the preamble (see Appendix 3) but not described as a revision, since the text of the actual paragraphs of the Declaration did not change.
In 1983 there were four fairly minor changes to the text of the DoH [10]: the word ‘doctor(s)’ was changed to ‘physician(s)’ in the 16 instances where the word occurred in the 1975 version. In the ‘Introduction’, the quotation from the Introduction from the International Code of Medical Ethics changed slightly as the wording of this code had changed. Also in the ‘Introduction’, the Latin phrase a forteriori was changed to ‘especially’ in the statement ‘In current medical practice most diagnostic, therapeutic or prophylactic procedures involve hazards. This applies especially to biomedical research’. Finally, in the ‘Basic Principles’ section, the requirement that where a minor is able to give ‘a consent’ that such consent should be sought was added to paragraph I.11 dealing with situations of legal incapacity for consent.
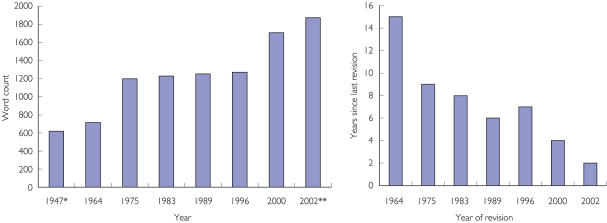
Since nothing was removed from the document, these minor revisions led to an increase in the length of the document, which now comprised just over 1200 words (see Figure 1).
Figure 1.
Word count for each revision of Declaration of Helsinki and years since last revision. *Nuremberg Code, **Includes Note of Clarification
Third revision: Hong Kong (1989)
This revision requires a fairly careful reading to see where any difference at all occurs. The only change in wording which occurs is in paragraph I.2 under the section ‘Basic Principles’. Previously the Declaration required that experimental protocols ‘should be transmitted to a specially appointed independent committee for consideration, comment and guidance’. This was considerably elaborated in 1989. Protocols were now to be ‘transmitted for consideration, comment and guidance to a specially appointed committee independent of the investigator and the sponsor provided that this independent committee is in conformity with the laws and regulations of the country in which the research experiment is performed’ [11].
Given the requirement, as already stipulated in the introduction, that ‘physicians are not relieved [by the DoH] from criminal, civil and ethical responsibilities under the laws of their own countries’, it has to be questioned whether the additional requirements in paragraph I.2 are unnecessarily repetitive. It should be acknowledged that such repetition is not without precedent. From the Tokyo (1975) revision reference to national legislation is made in the paragraphs referring to informed consent. It could be argued that the use of repetition stresses the need for reference to national legislation in the instances in which it occurs.
Overall, the effect of the minor revision in 1989 added 29 words to the length of the DoH (Figure 1).
Fourth revision: Somerset West, South Africa (1996)
As in 1983 and 1989, the actual changes to the text were minimal. However, the nature of the small textual change provided a seed out of which grew a much larger debate. In 1996, at the 48th General Assembly [11, 12], the WMA adopted the following addition (shown in italics) to paragraph II.3 in the section pertaining to ‘Medical Research Combined with Clinical Care (Clinical Research)’:
‘II.3 In any medical study, every patient – including those of a control group, if any – should be assured of the best proven diagnostic and therapeutic method. This does not exclude the use of inert placebo in studies where no proven diagnostic or therapeutic method exists’. [Italics ours]
This occurred in the context of rising disquiet about the use of placebo controls in studies of materno–fetal HIV transmission. It is the first time the DoH makes reference to any specific type of research methodology, i.e. the placebo-controlled trial. A careful reading of paragraph II.3 without the addition would appear to have the same requirement on researchers, but for the first time the DoH refers specifically to placebo. It is the addition of this specific requirement that meant that the Food and Drug Administration of the USA chose to continue to refer to the 1989 version of the DoH in its regulations [13]. This brings us neatly to the present version of the DoH with its attendant controversies.
The Declaration of Helsinki: present
We do not outline every detail of the textual changes, since only three of the 32 paragraphs are completely unchanged, while eight are completely new [14]. Also, since our focus is on the text of the Declaration, the events surrounding the eventual Edinburgh (2000) amendment are not reviewed here. They are described in detail by Human and Fluss in documents readily accessed at the WMA website and the interested reader is directed there [15, 16].
We single out for comment the revised structure of the document, the most controversial of the new paragraphs –19, 29 and 30 – and four other paragraphs (1, 6, 9, 27) which, although they have not yet given rise to significant debate in the literature, are striking changes in the way the document addresses aspects of medical research ethics. The text of the DoH, Edinburgh (2000) revision is appended to this paper (Appendix 3). Since we have described above all of the (very minor) changes that took place in 1983, 1989 and 1996, the interested reader can, by referring to these and the two full versions appended, see all of the changes in the Edinburgh (2000) revision.
A restructured document
In all versions up to the 2000 revision the following structure applied to the document: there was an Introduction (where the paragraphs were not numbered) followed by numbered paragraphs under the headings of ‘Basic Principles’, ‘Medical Research Combined with Professional Care (Clinical Research)’ and ‘Non-therapeutic Biomedical Research Involving Human Subjects (Non-clinical Biomedical Research)’ (see Appendix 2; the 1975 version of DoH illustrates this structure).
The 2000 version of the DoH is completely restructured. There is now a section headed ‘Introduction’ comprising paragraphs 1–9 which sets out the scope of the document and some of the underlying principles. Although many of the statements in the ‘Introduction’ were present in previous versions of the Declaration, they have been re-ordered to present a more logical sequence of reasoning. Arguably one of the most important statements is the requirement in paragraph 5 that ‘In medical research on human subjects, considerations related to the well-being of the human subject should take preference over the interests of science and society’. By the end of the ‘Introduction’ the document has very clearly set up the dilemma that gives rise to the need for clear thinking about research ethics. On the one hand, it would be unethical not to challenge current methods in medical practice (paragraph 6) through research. On the other hand, it is wrong to simply use people as a means to an end (paragraph 5), particularly vulnerable people (paragraph 8). Having described this ethical tension in the ‘Introduction’, the DoH then seeks in the next two sections to articulate the guiding principles for deciding what research meets the ethical standards required and what does not.
After the ‘Introduction’, there follow paragraphs 10–27 under the all-encompassing heading ‘Basic Principles for All Medical Research’. Finally, there are an additional five paragraphs (28–32) under the heading ‘Additional Principles for Medical Research Combined with Medical Care’. It is in this section that we find the controversial paragraphs 29 and 30.
This is a major logical re-framing of how the DoH categorizes different types of research involving human subjects. The pre-2000 versions of the Declaration effectively dichotomized research into therapeutic (potentially benefiting the subject directly) and nontherapeutic (no direct benefit to subject). In the Edinburgh (2000) revision the new category of ‘Medical Research Combined with Medical Care’ is recognized as a subset of ‘all medical research involving human subjects’.
There is no longer any specific section dealing with ‘Non-therapeutic’ research, which is often viewed as synonymous with ‘healthy volunteer’ research. There is specific reference to ‘healthy volunteers’ in three paragraphs of the Edinburgh (2000) revision. Paragraph 16 explicitly states that participation of healthy volunteers as research subjects is permissible. Were this not stated, then a certain way of interpreting paragraph 19 may lead to the conclusion that such research was now proscribed. In paragraph 18 healthy volunteers are identified as a group where the importance of prior weighing of the importance of research against its risks and burdens is especially important. Finally, Paragraph 8 in the ‘Introduction’ lists ‘those who will not benefit personally from the research’ among those groups that are vulnerable and in need of special protection.
This revision of how research is categorized has been strongly supported by Levine [17] as removing a previously illogical distinction. It must be of concern, however, that there is no longer a section of the DoH dealing with research where there is no potential benefit to the participants. Such groups do present some differences in methods of recruitment and such participants are often paid for their participation in research. These issues need further consideration and debate.
Paragraph 29: The benefits, risks, burdens and effectiveness of a new method should be tested against those of the best current prophylactic, diagnostic, and therapeutic methods. This does not exclude the use of placebo, or no treatment, in studies where no proven prophylactic, diagnostic or therapeutic method exists (See Appendix 3for Note of Clarification)
As already mentioned, the 1996 version of the DoH is the first version of the DoH to mention specifically the use of placebo in trials. Paragraph II.2 from the 1996 version stated ‘The potential benefits, hazards and discomfort of a new method should be weighed against the best current diagnostic and therapeutic methods’. This has been changed to the wording seen in the first sentence of paragraph 29 (above). The sentence which then followed in the 1996 version (and which formed the first sentence of paragraph II.3) stated ‘In any medical study, every patient – including those of a control group, if any – must be assured of the best proven diagnostic and therapeutic method’ has been eliminated. Finally, in the 2000 revision very little is changed in the actual sentence referring to placebo which is the second sentence in paragraph 29 (above); the words ‘inert placebo’ from the 1996 version are changed to ‘placebo, or no treatment’. In a careful reading of the two versions, however, it appears that very little has changed in the overall ethical guidance with respect to placebo use. Therefore, what is surprising is that the outcry following the 2000 revision far exceeded the response to the 1996 revision.
The overall effect of paragraph 29 would seem to rule out use of placebo wherever proven treatment exists. As mentioned, this raised such a cry of protest that the WMA took the unprecedented step of issuing, in 2001, a Note of Clarification to Paragraph 29. The Note of Clarification was formally adopted as part of the DoH in 2002, although the WMA has not described this as a ‘revision’ since the actual text has not been modified – only ‘clarified’!
However, the Note of Clarification certainly seems to modify the requirements and represents the first occasion where the WMA have issued explanatory text indicating the intent behind a specific paragraph. One of the best summaries with respect to placebo use in trials is that of Emanuel and Miller [18], who define three broad positions: placebo orthodoxy, active-control orthodoxy and the ‘middle ground’ (see Table 2 for definitions). It would appear that the Note of Clarification moves the stance of the DoH from what appears to be active-control orthodoxy towards the ‘middle ground’. The debate in the literature over the ethics of placebo controls has raged for at least the past decade between the proponents of ‘active-control orthodoxy’ such as Rothman, Michels and Weijer [19–21] and those supporting ‘placebo orthodoxy’ such as Levine [22] and Temple [23].
Table 2.
Emanuel and Miller's three ethical positions with respect to placebo-controls [18]
| Active-control orthodoxy | Placebo orthodoxy | Middle ground |
|---|---|---|
| ‘Whenever an effective intervention …exists, it must be used in the control group … placebo controls areinappropriate because the clinicallyrelevant question is … whether [a newdrug] is better than standard treatment’ | ‘When effective treatments exist, there mustbe compelling methodological reasons toconduct a placebo-controlled trial’ | ‘Without a placebo group to ensurevalidity, the finding that there is nodifference between the investigationaland standard treatments can bemisleading or uninterpretable’ |
The Note of Clarification lists two situations where placebo is acceptable: where there is a scientifically compelling reason, or where the condition under study is minor and the subject at no increased risk of serious or irreversible harm. These two situations are linked by the word ‘or’ which has been questioned by Macklin [2]. She asserts that the connector should be ‘and’ (i.e. both conditions must be fulfilled). The risk otherwise is that scientifically compelling reasons could be used to justify an increased risk of serious harm through use of placebo and this is argued to be inappropriate. This would be in line with the introductory principle of paragraph 5 that ‘considerations related to the well-being of the subject should take preference over the interests of science and society’. The counterarguments are both that valuable research may be prevented [24] and that placebo-controlled trials often require a much smaller sample size and follow-up time and therefore expose fewer people to any risks inherent in the research [18].
A further issue with respect to paragraph 29 has been the interpretation of the words ‘best current’ as the standard of comparator arm. Does this mean best in existence or best available in a local context? The Note of Clarification does not address the issue. The UK Nuffield Council on Bioethics argues the issue extensively, recognizing that ‘The Declaration of Helsinki (2000) is the primary source of guidance on which the majority of other guidance draws’ [25]. Their conclusion regarding the interpretation of ‘best proven’ is that ‘the minimum standard of care that should be offered [in the control arm] is the best intervention available as part of the national public health system’.
There is still considerable discussion around the circumstances in which placebo control is ethically acceptable. It seems clear that for some serious conditions where there is often ‘one chance’ at cure – such as many forms of cancer – placebo-controls should be ruled out. At the other end of the scale, except for the most extreme adherents to ‘active-control orthodoxy’, minor and self-limiting conditions seem to present little problem regarding placebo use. It must be remembered that paragraph 29 refers to ‘proven’ treatment, not ‘active’ treatment. Just because a pharmaceutical agent is shown to have pharmacological ‘activity’ does not mean it has been properly ‘proven’ to be superior to placebo. Indeed, such proof may never be forthcoming in some conditions where placebo response is either high or greatly variable. Symptoms of chronic stable angina, for example, can show a highly variable placebo response [26] and this condition is selected by Emanuel and Miller [18] as an example where a well-designed placebo-controlled trial should be satisfactory on ethical grounds provided patients are well monitored for worsening symptoms, that appropriate ‘rescue’ or ‘escape’ medication is available, and participants are fully aware of their right to withdraw from the trial at any time.
In the middle of these extremes are many clinical scenarios where the issue of whether placebo-controlled research is acceptable or whether serious or irreversible harm is risked needs to be undertaken on a ‘disease-by-disease’ basis. Among the conditions which have given rise to recent debate in this regard are hypertension [27], depression [28], schizophrenia [29] and postmenopausal osteoporosis [30]. Taking osteoporosis as one example, Brody and colleagues [31] have pointed out that there are groups of patients in whom placebo-controlled trials clearly do not violate paragraph 29. They specifically identify as suitable for placebo-controlled trials: ‘competent, well-informed patients [who] refuse approved therapies for sound reasons’, situations where ‘there is a reasonable basis for substantial disagreement or lack of consensus among professionals about whether approved treatments are better than placebos’, or ‘subjects are refractory to known effective agents’. It should be noted, however, that this approach may introduce biases.
A person consenting to participate in any blinded randomized controlled trial is effectively agreeing not to be given information that most individuals would want to receive; that is, to know what treatment they are receiving at any one time. This agreement not to know such information is not unique to trials using placebo-controls. Placebo-controls are not deemed unethical in and of themselves by paragraph 29. What is called into question is the potential harm to research participants who may not receive otherwise available proven treatments during the course of a placebo-controlled study.
The issue of placebo-control, probably more than any other, highlights the need for delicate considerations to balance ethical tensions which often exist between research which seeks to obtain answers as efficiently as possible (and there is nothing inherently wrong with that) and the well-being of participants in research. The DoH, particularly in paragraph 11 but also in other places throughout the document, affirms that unless research constitutes ‘good science’ it is unethical. However, as already mentioned, paragraph 5 places an ethical onus on the doctor never to sacrifice the interests of the individual in the interests of science and society. At the same time paragraph 6 (and others) place an ethical duty on doctors to undertake research. Taking any of the paragraphs to an extreme while ignoring the other paragraphs risks either endangering the well-being of participants or placing catastrophic barriers in the way of medical advance, which has the potential also to rebound to harm the individuals. The process of independent ethical review (paragraph 13) and adequate informed consent (paragraphs 22–26) must serve to protect the participants. Ethics committees are charged with deciding what kind of control group is ethically justified in individual protocols and ought to do so in full appreciation of the ethical tensions described above.
So, despite the adoption of the note of clarification, there is considerable work to be done in clarifying in what circumstances placebo-controlled studies are ethically acceptable. It would be useful to see evidence-based guidelines like those developed for mood disorders [32] undertaken for a wide variety of conditions. This would greatly assist those designing research protocols and ethics committees in their required assessment of the risks and benefits (paragraphs 16–19). Of course, such guidelines, to be useful, would need to be frequently updated to take into account medical advances.
Even after carefully thought out debate it is likely that there will still be those who would wish to see the Declaration interpreted in a way that would place greater restriction on use of placebo [33]. As Macklin cautions, ‘Two paragraphs (29 and 30) … remain controversial and would still be controversial if changed to meet criticisms’ [2].
Paragraph 30: At the conclusion of the study, every patient entered into the study should be assured of access to the best proven prophylactic, diagnostic and therapeutic methods identified by the study
In the most recent edition of their highly successful textbook, The Principles of Biomedical Ethics, Beauchamp and Childress make the following observation: ‘Until the 1990s, the paradigm for ethical analysis focused on the risks and burdens of research (emphasis theirs) … and on the need to protect potential and actual research subjects from harm, abuse, and exploitation…. However, a paradigm shift recently occurred … As a result, justice as fair access to research (both participation in research and access to the results of research) became as important as protection from exploitation’ [34]. The most recent revision to the DoH, in particular paragraph 30 but also reflected in paragraph 19 (see below), would seem to bear this out. Nicholson asserts regarding paragraph 30 that ‘this is potentially the most far-reaching of all the changes to the Declaration’. Concerns about the implications of paragraph 30 have led to the WMA assembling a Workgroup to consider either an amendment to the paragraph or the addition of a note of clarification. The report of the Workgroup was presented to the Council meetings which preceded the most recent WMA General Assembly (10–14 September 2003 in Helsinki) and it was decided that no amendment or clarification would be undertaken but that the Workgroup's deliberations would be continued and consultations widened [35, 36]. Although this decision has drawn criticism [37], we argue that it represents a ‘sensible and measured’ approach to the situation [38].
The debate centres around the issue of what happens to patients in a trial once the trial is over. Capron has characterized this as an example of the larger question ‘who owes what to whom and why?’ [39] In contrast to paragraph 29, where the critical question has been characterized as ‘are participants worse off in the trial than they were before the trial?’, the question here is ‘are participants worse off after the trial than they were during the trial?’. Those who see paragraph 30 as imposing too great a burden on researchers emphasize the benefits which accrue to patients during a trial where there was no access to treatment beforehand and assert that nothing is lost (compared with the pretrial situation) if, at the end of the trial, the status quo resumes and access is lost. In contrast, those supporting paragraph 30 as it is emphasize the additional trauma and distress caused to patients who, after treatment for a duration of the trial, learn what is possible for them, only to be deprived of access when the status quo resumes post trial. They argue that these patients are, indeed, worse off after the trial than they were before. There is no easy way towards consensus on this and the WMA press release regarding the DoH following the 2003 General Assembly noted ‘sharp differences of opinion over how to protect human participants in medical research’ [35].
Other major changes in the Edinburgh (2000) revision
Paragraphs 29 and 30 have given rise to the greatest controversy. It is arguable that they may have overshadowed debate about other paragraphs which have changed significantly. Space does not permit elaboration in detail of every change in the 2000 revision, so we focus on significant changes introduced through paragraphs 1, 6, 9, 19 and 27.
Paragraph 1: ‘The World Medical Association has developed the Declaration of Helsinki as a statement of ethical principles to provide guidance to physicians and other participants in medical research involving human subjects. Medical research involving human subjects includes research on identifiable human material or identifiable data’
Paragraph 1 outlines first of all the raison d'etre of the DoH. Although this statement has not changed from the earlier versions, it has been moved to become the opening statement of the DoH. However, the second sentence for the first time explicitly declares that the provisions of the DoH apply to identifiable human tissue and identifiable data.
Overall this paragraph has evoked little comment, although Riis has raised two concerns [40]. First, he considers that anonymized research should also be covered by the Declaration because of the possible harms associated with ‘group stigmatization’. Second, he notes that there is ‘brief mention of “human material” and “data” without including statements applicable to epidemiological and large-scaled genetics research’. Certainly the explicit inclusion of identifiable material and data has taken place without any considerations of the possibility of different requirements for consent later in the document, and this requires further consideration.
Paragraph 6: ‘The primary purpose of medical research involving human subjects is to improve diagnostic and therapeutic procedures and the understanding of the aetiology and pathogenesis of disease. Even the best proven prophylactic, diagnostic and therapeutic methods must continuously be challenged through research for their effectiveness, efficiency, accessibility and quality’
The first sentence is not new to the 2000 revision of the DoH but the second sentence of paragraph 6 is entirely new. This places a distinct ethical burden on physicians to challenge current methods through research. The choice of the four criteria by which existing methods are to be challenged (effectiveness, efficiency, accessibility and quality) is not further justified nor are the actual criteria defined. However, to any readers who would see documents such as the DoH as placing obstacles in the way of research, paragraphs such as this explicitly describe the very real ethical tension which exists and which is described as balancing ‘the protection of, and respect for, research patients and healthy volunteers with the necessary freedom of research to facilitate scientific progress as a public good’ [40].
Paragraph 9: ‘Research investigators should be aware of the ethical, legal and regulatory requirements for research on subjects in their own countries as well as applicable international requirements. No national ethical, legal or regulatory requirement should be allowed to reduce or eliminate any of the protections for human subjects set forth in this Declaration’
To understand the sea-change which this statement represents we need to consider the paragraph which was included in all previous versions of the DoH: ‘It must be stressed that the standards as drafted are only a guide to physicians all over the world. Physicians are not relieved from criminal, civil and ethical responsibilities under the laws of their own countries’. From previously being seen as guidance which did not in any way supersede national regulations, the DoH has recast itself as a minimum set of international standards ‘binding’ physicians worldwide.
It is perhaps very surprising that this paragraph has not given rise to greater controversy. The issue of the relationship between law and ethics is complex. However, it is noteworthy that the WMA in 2003 issued their own statement on the matter: ‘In some cases the law mandates unethical conduct. The fact that a physician has complied with the law does not necessarily mean that the physician has acted ethically. When the law is in conflict with medical ethics, physicians should work to change the law. In circumstances of such conflict, ethical responsibilities supersede legal obligations’ [41]. This statement by the WMA applies broadly to the relationship between ethics and the law and is not limited to observation of the DoH. This statement of course gives no guidance to the physician in the situation where two ethical codes conflict. What should a physician of devout religious persuasion do, for example, if he or she believes that something in a secular ethical code is not in harmony with an ethical code mandated by their faith? However, it is noteworthy that the Declaration of Helsinki itself has remained relatively free of any objections to it on the grounds that it clashes with other codes of ethics.
Paragraph 19: ‘Medical research is only justified if there is a reasonable likelihood that the populations in which the research is carried out stand to benefit from the results of the research’
This is another statement which projects the concerns of the DoH into the realm of social justice. There are those who argue that this is not an appropriate role for the DoH [13] and others who argue strongly that the DoH should play a major role in combating what have been described as ‘double standards’ in the world of medical research [2]. Issues surrounding this debate have been discussed under ‘Paragraph 30’ above. Although not giving rise to the same degree of controversy as paragraphs 29 and 30, there was sufficient debate about this paragraph to warrant calls for a Note of Clarification and documentation was prepared in this regard [42]. It was, however, decided by the WMA Council in May 2003 not to proceed with a Note of Clarification to paragraph 19.
Paragraph 27: ‘Both authors and publishers have ethical obligations. In publication of the results of research the investigators are obliged to preserve the accuracy of the results. Negative as well as positive results should be published or otherwise publicly available. Sources of funding, institutional affiliations and any possible conflicts of interest should be declared in the publication. Reports of experimentation not in accordance with the principles laid down in this Declaration should not be accepted for publication’
Of the four sentences in this paragraph, the first and the last were present in previous versions and will not be discussed further. The third sentence, requiring disclosure of potential conflicts of interests, has parallels in paragraphs 13 and 22. The overall result is that such potential conflicts must be disclosed to: (i) the committee undertaking independent review, (ii) the patient when informed consent is sought, and (iii) any research publication. Although the question of what constitutes a conflict of interest is not fully defined, there seems little objection to the inclusion of these requirements in the DoH.
The requirement to make negative results available also seems to raise little objection, but should be recognized for the important advance that it is. As pointed out by Godlee, ‘Negative results are just as important to scientific understanding, if less exciting for researchers and editors, as positive studies’. She asks ‘What has publication bias to do with ethics?’ and answers ‘it gives only part of the picture and so distorts our views on what is the best treatment for patients’ [43].
There is now, within the DoH, a recognition that the publication bias which results from the propensity to publish ‘positive’ results at the expense of ‘negative’ results has the potential to harm patients and thus carries with it ethical obligations. The difficulty however, remains that publications seek to maintain their readership and that publishing positive results which may change the course of medical practice is widely perceived as more interesting than negative results which would tend to favour the status quo. It is possible that the internet may provide at least a partial solution and that negative results which would otherwise be unpublished may be made publicly accessible through the World Wide Web. The issue of electronic ‘open access publishing’ has recently been debated [44]. One point of contention surrounds who pays for such publication, and the recently launched Public Library of Science charges authors for publication. Lacking completely in the debate in this recent article, however, is what effect these changes may have on the publication of negative results and avoidance of publication bias. Therefore, it still remains unclear whether the aspirations of paragraph 27 will be achieved in practical terms.
Other changes
As pointed out above, the 2000 revision of the DoH left very few paragraphs unchanged. The changes not commented on in detail are listed in Table 3. The fact that we have not commented in detail is not an indication that the changes are considered unimportant, but rather that their introduction seems to have caused little controversy. Our discussion therefore now proceeds to consideration of possible future trajectories for the DoH.
Table 3.
Other significant changes to the text of the Declaration of Helsinki in the 2000 revision (see Appendix 2 for full text of Declaration of Helsinki)
| Paragraph number | Subject of the changes |
|---|---|
| 8 (new paragraph) | Research on people from vulnerable groups |
| 13 (modified paragraph) committees | Ethics committees have the right to monitor research; disclosure of potential conflicts of interest to ethics |
| 16 (modified paragraph) | Design of all studies to be publicly available |
| 21 (modified paragraph) | Explicit mention of protection of confidentiality of information about the patient |
| 22 (modified paragraph) | Provisions where consent cannot be obtained in writing |
| 25 (modified paragraph) | ‘Consent’ changed to ‘assent’ with respect to research involving children |
| 26 (new paragraph) | Provisions where consent from subject not possible |
| 31 (modified paragraph) | Requirement to fully inform patient what aspects of their care relate to the research |
| 32 (new paragraph) | Use of unproven techniques to save life or re-establish health should be made the object of research and the results recorded and published where appropriate |
The Declaration of Helsinki: future
There is little doubt that the influence of the DoH remains a central guide to research practice. This is illustrated, at least in part, by the use of the Declaration by other important documents pertaining to research ethics [45]. The Council for the International Organizations of Medical Sciences (CIOMS) guidelines on research ethics, for example, include the full DoH as an appendix and make extensive reference to the DoH in the text. In the longer term, it may be that the influence becomes ‘diluted’ by the confusing proliferation of international guidelines, codes of practice and other instruments such as those recently developed by CIOMS, by the International Conference on Harmonization (ICH) and by the Council of Europe. However, none of the above is really of the same genre of document as the DoH. Each is much lengthier, and attempts to cover questions of what to do in particular practical situations. The DoH, on the other hand, seeks to articulate a basic set of principles, to function as a code of ethics.
Therefore, it could be argued that the main influence of the DoH is not so much in answering specific questions about certain ethical protocols – although some of its paragraphs are certainly useful in that regard – but rather the DoH is part of the foundation on which these more detailed guidelines have been drafted.
There are a number of other trends which need consideration in terms of the future of the DoH. Probably the most important underlying question, however, is ‘from where does the DoH draw its authority?’ We consider four possible sources for this authority.
The World Medical Association (WMA)
One possible answer is that it draws its authority from being a Declaration of the WMA. This is the largest global grouping of doctors and as such there may be legitimacy in the claim that it is an authoritative body for making statements about the collective views of the medical profession.
However, one historical observation would seem to undermine any argument that this explains the authority of the DoH. Arguably the Declaration's period of greatest acceptance as an authoritative document dates in the period from the late 1970s (after the 1975 amendment had been widely promulgated) to the mid–late 1990s when increasing calls for modification to the DoH began to be voiced. However, this was a period of considerable internal turmoil for the WMA. In the 1980s, several countries (the so-called ‘Toronto Group’), including the UK, withdrew from the WMA over ongoing objections to the refusal of the South African Medical Association to condemn apartheid. The events of history have allowed reconciliation of this rift and all of the breakaway countries had rejoined the WMA by 1995 [46].
This, we believe, calls into question any conclusion that the DoH's authority rests solely, or even largely, on the nature of its ‘author’. It may even be that as the WMA strengthens and enlarges it will be more difficult to obtain consensus on documents such as the DoH, and particularly on difficult paragraphs such as 29 and 30.
The Declaration's succinctness
Although there is also clear evidence of a trend toward the DoH becoming longer (see Figure 1), there is no doubt that the Declaration – still less than 2000 words in length – is one of the most succinct documents encapsulating the principles guiding research ethics in existence. It can be read from beginning to end in less than 10 minutes.
On the one hand, the increasing complexity of research issues means that it is hardly surprising that a lengthening has occurred. On the other hand, the question must be asked: How much has its succinctness helped to establish its authority? If this is a major basis of the DoH's influence then the increasing length of the document, and the use of ‘clarifications’, must be a matter of great concern.
The Declaration's long-standing pre-eminence
There is an apparent tendency toward the DoH being changed more frequently (see Figure 1). However, it must be recognized that only two of the revisions (1975 and 2000) were more than minor in nature. This means that the period between extensive revisions is 11 (from 1964 to 1975) and 25 (from 1975 to 2000) years, respectively. Therefore the DoH, essentially in its 1975 form, had a quarter of a century to become embedded in the medical research community, and this may contribute significantly to the position it has come to occupy. On the other hand, there is recognition of the need to update the document to recognize the changing world of biomedical research [15]. Finding the correct balance between the need to modernize the document and the necessity to allow the text to become familiar within the medical research community will be important to maintaining the status of DoH.
It should be pointed out that the delegates to the World Medical Assembly are well aware of these trends toward lengthening of the document and more frequent changes. A previously published version [47] of Figure 1 was presented during the President's opening address of the Scientific Session of the most recent World Medical Assembly in Helsinki [48].
The Declaration has successfully articulated more broadly accepted principles
Did the DoH achieve its authority because it accurately articulated deeply held and broadly based ethical principles regarding the ethics of medical research? Almost like an ancient religious text, where commentaries debate the meaning of individual words, the DoH is the subject of almost a word-by-word analysis. Consider Article 29, where an enormous amount of ink has been spilled over the meaning of ‘best current’. The Nuffield Council Document on ‘Research in Developing Countries’ devotes an entire chapter to what is effectively a debate about the true interpretation of this phrase [49].
If this is the basis of the Declaration's authority then the relevant question is whether the Edinburgh (2000) revision represents a superior expression of these deeply and widely held values to that of its predecessors.
Only time will tell what is the correct answer regarding the future of the DoH. However, it is worth reflecting on the following: when controversies arise, such as those surrounding paragraphs 29 and 30, there really are only three broad reasons which may underline such controversies.
First, if the wording of the document is at odds with the true underlying ethical principles then they must be better articulated, i.e. better ‘word-smithing’ is the way forward. Second, it may be that there really is no universal consensus about the ethical issues at stake, in which case some kind of ‘agreement to differ’ would be the only way to achieve a consensus document.
A third possible reason for a flurry of controversy over the wording needs to be considered. Has the document shone an uncomfortable light on practices which are questionable ethically? In this last regard, bioethicist H. Tristram Englehardt [50] speaks of the potential offensiveness of ethics. Aspects of his discussion could be paraphrased along these lines; to say someone is in the wrong factually has the potential to create a certain degree of offence, but to say that someone is in the wrong ethically is to criticise at a much deeper level and may cause a much more profound level of offence. If the reason for the controversy over statements such as paragraph 30 is that the text of the DoH has made parts of the research community feel very uncomfortable about the ethics of certain types of research, then it is important that the guiding principles not be amended or diluted through notes of clarification, but rather it is the behaviour of the research community which needs to change.
Concluding remarks
In compiling this review, we have sought to familiarize readers with the evolving text of the DoH over its nearly half-century of existence. We have raised what we see as important issues regarding its future, but up to now we have avoided one important question. Since time immemorial the medical profession has used codes of ethics to sum up the ethical responsibilities members of the profession take upon themselves in the practice of medicine. Undoubtedly the best known of the ancient codes is the Hippocratic Oath [51]. With respect to ethical codes in medical research the Nuremberg Code and the DoH hold pride of place. The unanswered question is whether the existence of such codes really raises the ethical standards in medical research or whether they are ‘Only words, words; to be led out to battle against other words?’ [52]. The fact that a supposedly rigorous code of medical research ethics existed in Germany from 1931 through to the end of the Second World War [53] raises this question rather starkly and has led Weisstub to caution: ‘We should not be naive about the capacity of codes or legislation to bring unanimity and predictability to the subject’ [54].
Yet there is little doubt that promulgation of the Edinburgh (2000) revision of DoH has sensitized the medical research community to many important issues once again. On the one hand, some may question the value of a document that aspires to such a high ethical standard. On the other hand, it must also be of considerable interest to note the responses of a researcher or an organization to these aspirations. A very interesting question which deserves much greater consideration is to ask just what is revealed when the response to the text is to seek loopholes and ask ‘what can I get away with?’, as opposed to ‘How can I seek to achieve these aspirational standards in my research?’.
Acknowledgments
R.V.C.'s post is supported by an educational grant from Johnson & Johnson Ltd.
Appendix 1: The Nuremberg Code (1947)
The judgement by the war crimes tribunal at Nuremberg laid down 10 standards to which physicians must conform when carrying out experiments on human subjects.
The voluntary consent of the human subject is absolutely essential. This means that the person involved should have legal capacity to give consent, should be so situated as to be able to exercise free power of choice, without the intervention of any element of force, fraud, deceit, duress, overreaching, or other ulterior form of constraint or coercion; and should have sufficient knowledge and comprehension of the elements of the subject matter as to enable him to make an understanding and enlightened decision. This latter element requires that before the acceptance of an affirmative decision by the experimental subject there should be made known to him the nature, duration, and purpose of the experiment; the method and means by which it is to be conducted; all inconveniences and hazards reasonably to be expected; and the effects upon his health or person which may possibly come from his participation in the experiment. The duty and responsibility for ascertaining the quality of the consent rests upon each individual who initiates, directs, or engages in the experiment. It is a personal duty and responsibility which may not be delegated to another with impunity.
The experiment should be such as to yield fruitful results for the good of society, unprocurable by other methods or means of study, and not random and unnecessary in nature.
The experiment should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study that the anticipated results justify the performance of the experiment.
The experiment should be conducted as to avoid all unnecessary physical and mental suffering and injury.
No experiment should be conducted where there is an a priori reason to believe that death or disabling injury will occur; except, perhaps, in those experiments where the experimental physicians also serve as subjects.
The degree of risk to be taken should never exceed that determined by humanitarian importance of the problem to be solved by the experiment.
Proper preparations should be made and adequate facilities provided to protect the experimental subject against even remote possibilities of injury, disability or death.
The experiment should be conducted only by scientifically qualified persons. The highest degree of skill and care should be required through all stages of the experiment of those who conduct and engage in the experiment.
During the course of the experiment the human subject should be at liberty to bring the experiment to an end if he has reached the physical or mental state where continuation of the experiment seems to him to be impossible.
During the course of the experiment the scientist in charge must be prepared to terminate the experiment at any stage, if he has probable cause to believe, in the exercise of good faith, superior skill and careful judgement required of him, that a continuation of the experiment is likely to result in injury, disability, or death to the experimental subject.
Appendix 2: Declaration of Helsinki (1975)
Adopted by the 18th WMA General Assembly Helsinki, Finland, June 1964 and amended by the 29th WMA General Assembly, Tokyo, Japan October 1975.
Recommendations guiding medical doctors in biomedical research involving human subjects.
Introduction
It is the mission of the medical doctor to safeguard the health of the people. His or her knowledge and conscience are dedicated to the fulfilment of this mission.
The Declaration of Geneva of the World Medical Association binds the doctor with the words: ‘The health of my patient will be my first consideration’, and the International Code of Medical Ethics declares that ‘Any act or advice which could weaken physical or mental resistance of a human being may be used only in his interest’.
The purpose of biomedical research involving human subjects must be to improve diagnostic, therapeutic and prophylactic procedures and the understanding of the aetiology and pathogenesis of disease.
In current medical practice most diagnostic, therapeutic or prophylactic procedures involve hazards. This applies a fortiori to biomedical research.
Medical progress is based on research which ultimately must rest in part on experimentation involving human subjects. In the field of biomedical research a fundamental distinction must be recognized between medical research in which the aim is essentially diagnostic or therapeutic for a patient, and medical research the essential object of which is purely scientific and without direct diagnostic or therapeutic value to the person subjected to the research.
Special caution must be exercised in the conduct of research which may affect the environment, and the welfare of animals used for research purposes must be respected.
Because it is essential that the results of laboratory experiments be applied to human beings to further scientific knowledge and to help suffering humanity, the World Medical Association has prepared the following recommendations as a guide to every doctor in biomedical research involving human subjects. They should be kept under review in the future. It must be stressed that the standards as drafted are only a guide to physicians all over the world. Doctors are not relieved from criminal, civil and ethical responsibilities under the laws of their own countries.
I Basic principles
Biomedical research involving human subjects must conform to generally accepted scientific principles and should be based on adequately performed laboratory and animal experimentation and on a thorough knowledge of the scientific tradition.
The design and performance of each experimental procedure involving human subjects should be clearly formulated in an experimental protocol which should be transmitted to a specially appointed independent committee for consideration, comment and guidance.
Biomedical research involving human subjects should be conducted only by scientifically qualified persons and under the supervision of a clinically competent medical person. The responsibility for the human subject must always rest with a medically qualified person and never rest on the subject of the research, even though the subject has given her consent.
Biomedical research involving human subjects cannot legitimately be carried out unless the importance of the objective is in proportion to the inherent risk to the subject.
Every biomedical research project involving human subjects should be preceded by careful assessment of predictable risks in comparison with foreseeable benefits to the subject or to others. Concern for the interests of the subject must always prevail over the interest of science and society.
The right of the research subject to safeguard his or her integrity must always be respected. Every precaution should be taken to respect the privacy of the subject and to minimize the impact of the study on the subject's physical and mental integrity and on the personality of the subject.
Doctors should abstain from engaging in research projects involving human subjects unless they are satisfied that the hazards involved are believed to be predictable. Doctors should cease any investigation if the hazards are found to outweigh the potential benefits.
In publication of the results of his or her research, the doctor is obliged to preserve the accuracy of the results. Reports of experimentation not in accordance with the principles laid down in this Declaration should not be accepted for publication.
In any research on human beings, each potential subject must be adequately informed of the aims, methods, anticipated benefits and potential hazards of the study and the discomfort it may entail. He or she should be informed that he or she is at liberty to abstain from participation in the study and that he or she is free to withdraw his or her consent to participation at any time. The doctor should then obtain the subject's freely given informed consent, preferably in writing.
When obtaining informed consent for the research project the doctor should be particularly cautious if the subject is in a dependent relationship to him or her or may consent under duress. In that case informed consent should be obtained by a doctor who is not engaged in the investigation and who is completely independent of this official relationship.
In cases of legal incompetence, informed consent should be obtained from the legal guardian in accordance with national legislation. Where physical or mental incapacity makes it impossible to obtain informed consent, or when the subject is a minor, permission from the responsible relative replaces that of the subject in accordance with the national legislation.
The research protocol should always contain a statement of ethical consideration involved and should indicate that the principles enunciated in the present Declaration are complied with.
II Medical research combined with professional care (clinical research)
In the treatment of the sick person, the doctor must be free to use a new diagnostic and therapeutic measure, if in his or her judgement it offers the hope of saving life, re-establishing health or alleviating suffering.
The potential benefits, hazards and discomfort of a new method should be weighed against the advantages of the best current diagnostic and therapeutic methods.
In any medical study, every patient – including those of a control group, if any – should be assured of the best proven diagnostic and therapeutic method.
The refusal of the patient to participate in a study must never interfere with the doctor–patient relationship.
If the doctor considers it essential not to obtain informed consent, the specific reasons for this proposal should be stated in the experimental protocol for transmission to the independent committee.
The doctor can combine medical research with professional care, the objective being the acquisition of new medical knowledge, only to the extent that medical research is justified by its potential diagnostic or therapeutic value for the patient.
III Non-therapeutic biomedical research involving human subjects (non-clinical biomedical research)
In the purely scientific application of medical research carried out on a human being, it is the duty of the doctor to remain the protector of the life and health of that person on whom biomedical research is carried out.
The subjects should be volunteers – either healthy persons or patients for whom the experimental design is not related to the patient's illness.
The investigator or the investigating team should discontinue the research if in his/her or their judgement it may, if continued, be harmful to the individual.
In research on man, the interest of science and society should never take precedence over considerations related to the well-being of the subject.
Appendix 3: World Medical Association Declaration of Helsinki (2000)
Ethical principles for medical research involving human subjects.
Adopted by the 18th WMA General Assembly Helsinki, Finland, June 1964 and amended by the 29th WMA General Assembly, Tokyo, Japan, October 1975.
35th WMA General Assembly, Venice, Italy, October 1983.
41st WMA General Assembly, Hong Kong, September, 1989.
48th WMA General Assembly, Somerset West, Republic of South Africa, October 1996 and the 52nd WMA General Assembly, Edinburgh, Scotland, October 2000.
Note of Clarification on Paragraph 29 added by the WMA General Assembly, Washington 2002.
A. Introduction
The World Medical Association has developed the Declaration of Helsinki as a statement of ethical principles to provide guidance to physicians and other participants in medical research involving human subjects. Medical research involving human subjects includes research on identifiable human material or identifiable data.
It is the duty of the physician to promote and safeguard the health of the people. The physician's knowledge and conscience are dedicated to the fulfilment of this duty.
The Declaration of Geneva of the World Medical Association binds the physician with the words, ‘The health of my patient will be my first consideration’, and the International Code of Medical Ethics declares that ‘A physician shall act only in the patient's interest when providing medical care which might have the effect of weakening the physical and mental condition of the patient’.
Medical progress is based on research which ultimately must rest in part on experimentation involving human subjects.
In medical research on human subjects, considerations related to the well-being of the human subject should take preference over the interests of science and society.
The primary purpose of medical research involving human subjects is to improve prophylactic, diagnostic and therapeutic procedures and the understanding of the aetiology and pathogenesis of disease. Even the best proven prophylactic, diagnostic, and therapeutic methods must continuously be challenged through research for their effectiveness, efficiency, accessibility and quality.
In current medical practice and in medical research, most prophylactic, diagnostic and therapeutic procedures involve risks and burdens.
Medical research is subject to ethical standards that promote respect for all human beings and protect their health and rights. Some research populations are vulnerable and need special protection. The particular needs of the economically and medically disadvantaged must be recognized. Special attention is also required for those who cannot give or refuse consent for themselves, for those who may be subject to giving consent under duress, for those who will not benefit personally from the research, and for those for whom the research is combined with care.
Research investigators should be aware of the ethical, legal and regulatory requirements for research on human subjects in their own countries as well as applicable international requirements. No national ethical, legal or regulatory requirement should be allowed to reduce or eliminate any of the protections for human subjects set forth in this document.
B. Basic principles for all medical research
It is the duty of the physician in medical research to protect the life, health, privacy, and dignity of the human subject.
Medical research involving human subjects must conform to generally accepted scientific principles, be based on a thorough knowledge of the scientific literature, other relevant sources of information, and on adequate laboratory and, where appropriate, animal experimentation.
Appropriate caution must be exercised in the conduct of research which may affect the environment, and the welfare of animals used for research must be respected.
The design and performance of each experimental procedure involving human subjects should be clearly formulated in an experimental protocol. This protocol should be submitted for consideration, comment, guidance, and where appropriate, approval to a specially appointed ethical review committee, which must be independent of the investigator, the sponsor or any other kind of undue influence. This independent committee should be in conformity with the laws and regulations of the country in which the research experiment is performed. The committee has the right to monitor ongoing trials. The researcher has the obligation to provide monitoring information to the committee, especially any serious adverse events. The researcher should also submit to the committee, for review, information regarding funding, sponsors, institutional affiliations, other potential conflicts of interest and incentives for subjects.
The research protocol should always contain a statement of the ethical considerations involved and should indicate that there is compliance with the principles enunciated in this Declaration.
Medical research involving human subjects should be conducted only by scientifically qualified persons and under the supervision of a clinically competent medical person. The responsibility for the human subject must always rest with a medically qualified person and never rest on the subject of the research, even though the subject has given consent.
Every medical research project involving human subjects should be preceded by careful assessment of predictable risks and burdens in comparison with foreseeable benefits to the subject or to others. This does not preclude the participation of healthy volunteers in medical research. The design of all studies should be publicly available.
Physicians should abstain from engaging in research projects involving human subjects unless they are confident that the risks involved have been adequately assessed and can be satisfactorily managed. Physicians should cease any investigation if the risks are found to outweigh the potential benefits or if there is conclusive proof of positive and beneficial results.
Medical research involving human subjects should only be conducted if the importance of the objective outweighs the inherent risks and burdens to the subject. This is especially important when the human subjects are healthy volunteers.
Medical research is only justified if there is a reasonable likelihood that the populations in which the research is carried out stand to benefit from the results of the research.
The subjects must be volunteers and informed participants in the research project.
The right of research subjects to safeguard their integrity must always be respected. Every precaution should be taken to respect the privacy of the subject, the confidentiality of the patient's information and to minimize the impact of the study on the subject's physical and mental integrity and on the personality of the subject.
In any research on human beings, each potential subject must be adequately informed of the aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks of the study and the discomfort it may entail. The subject should be informed of the right to abstain from participation in the study or to withdraw consent to participate at any time without reprisal. After ensuring that the subject has understood the information, the physician should then obtain the subject's freely given informed consent, preferably in writing. If the consent cannot be obtained in writing, the nonwritten consent must be formally documented and witnessed.
When obtaining informed consent for the research project the physician should be particularly cautious if the subject is in a dependent relationship with the physician or may consent under duress. In that case the informed consent should be obtained by a well-informed physician who is not engaged in the investigation and who is completely independent of this relationship.
For a research subject who is legally incompetent, physically or mentally incapable of giving consent or is a legally incompetent minor, the investigator must obtain informed consent from the legally authorized representative in accordance with applicable law. These groups should not be included in research unless the research is necessary to promote the health of the population represented and this research cannot instead be performed on legally competent persons.
When a subject deemed legally incompetent, such as a minor child, is able to give assent to decisions about participation in research, the investigator must obtain that assent in addition to the consent of the legally authorized representative.
Research on individuals from whom it is not possible to obtain consent, including proxy or advance consent, should be done only if the physical/mental condition that prevents obtaining informed consent is a necessary characteristic of the research population. The specific reasons for involving research subjects with a condition that renders them unable to give informed consent should be stated in the experimental protocol for consideration and approval of the review committee. The protocol should state that consent to remain in the research should be obtained as soon as possible from the individual or a legally authorized surrogate.
Both authors and publishers have ethical obligations. In publication of the results of research, the investigators are obliged to preserve the accuracy of the results. Negative as well as positive results should be published or otherwise publicly available. Sources of funding, institutional affiliations and any possible conflicts of interest should be declared in the publication. Reports of experimentation not in accordance with the principles laid down in this Declaration should not be accepted for publication.
C. Additional principles for medical research combined with medical care
The physician may combine medical research with medical care, only to the extent that the research is justified by its potential prophylactic, diagnostic or therapeutic value. When medical research is combined with medical care, additional standards apply to protect the patients who are research subjects.
The benefits, risks, burdens and effectiveness of a new method should be tested against those of the best current prophylactic, diagnostic, and therapeutic methods. This does not exclude the use of placebo, or no treatment, in studies where no proven prophylactic, diagnostic or therapeutic method exists.
To clarify further the WMA position on the use of placebo-controlled trials, the WMA Council issued, during October 2001, a note of clarification on article 29.
At the conclusion of the study, every patient entered into the study should be assured of access to the best proven prophylactic, diagnostic and therapeutic methods identified by the study.
The physician should fully inform the patient which aspects of the care are related to the research. The refusal of a patient to participate in a study must never interfere with the patient–physician relationship.
In the treatment of a patient, where proven prophylactic, diagnostic and therapeutic methods do not exist or have been ineffective, the physician, with informed consent from the patient, must be free to use unproven or new prophylactic, diagnostic and therapeutic measures, if in the physician's judgement it offers hope of saving life, re-establishing health or alleviating suffering. Where possible, these measures should be made the object of research, designed to evaluate their safety and efficacy. In all cases, new information should be recorded and, where appropriate, published. The other relevant guidelines of this Declaration should be followed.
Note of clarification on paragraph 29 of the WMA Declaration of Helsinki
The WMA is concerned that paragraph 29 of the revised Declaration of Helsinki (October 2000) has led to diverse interpretations and possible confusion. It hereby reaffirms its position that extreme care must be taken in making use of a placebo-controlled trial and that in general this methodology should only be used in the absence of existing proven therapy. However, a placebo-controlled trial may be ethically acceptable, even if proven therapy is available, under the following circumstances:
Where for compelling and scientifically sound methodological reasons it is necessary to determine the efficacy or safety of a prophylactic, diagnostic or therapeutic method; or
Where a prophylactic, diagnostic or therapeutic method is being investigated for a minor condition and the patients who receive placebo will not be subject to any additional risk of serious or irreversible harm.
All other provisions of the Declaration of Helsinki must be adhered to, especially the need for appropriate ethical and scientific review.
1Note of clarification on paragraph 29 of the WMA Declaration of Helsinki. The WMA is concerned that paragraph 29 of the revised Declaration of Helsinki (October 2000) has led to diverse interpretations and possible confusion. It hereby reaffirms its position that extreme care must be taken in making use of a placebo-controlled trial and that in general this methodology should only be used in the absence of existing proven therapy. However, a placebo-controlled trial may be ethically acceptable, even if proven therapy is available, under the following circumstances:
Where for compelling and scientifically sound methodological reasons it is necessary to determine the efficacy or safety of a prophylactic, diagnostic or therapeutic method; or
Where a prophylactic, diagnostic or therapeutic method is being investigated for a minor condition and the patients who receive placebo will not be subject to any additional risk of serious or irreversible harm.
All other provisions of the Declaration of Helsinki must be adhered to, especially the need for appropriate ethical and scientific review.
References
- 1.Crawley FP. The Limits of the Declaration of Helsinki. Address to Scientific Session, World Medical Association General Assembly, September 2003, Helsinki.
- 2.Macklin R. Future challenges for the Declaration of Helsinki: Maintaining Credibility in the Face of Ethical Controversies. Address to Scientific Session, World Medical Association General Assembly, September 2003, Helsinki.
- 3.The World Medical Association Ethics Unit. Declaration of Helsinki. [5 November 2003]; http://www.wma.net/e/ethicsunit.
- 4.Vanhoozer KJ. Is There a Meaning in this Text? Grand Rapids, MI: Zondervan; 1998. pp. 17–29. [Google Scholar]
- 5.Madison GB. Hermeneutics. Gadamer and Ricoeur. In: Popkin RH, editor. Pimlico History of Western Philosophy. London: Pimlico; 1999. pp. 705–12. [Google Scholar]
- 6.Human Experimentation: code of ethics of the World Medical Association. Br Med J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fluss S. How the Declaration of Helsinki developed. Good Clin Prac J. 1999;6:18–21. [Google Scholar]
- 8.World Medical Association. 1964. Declaration of Helsinki.
- 9.Flanigan A. Who wrote the Declaration of Helsinki? JAMA. 1997;277:926. [PubMed] [Google Scholar]
- 10.World Medical Association. 1983. Declaration of Helsinki, 2nd (Venice) amendment.
- 11.World Medical Association. 1989. Declaration of Helsinki, 3rd (Hong Kong) amendment.
- 12.World Medical Association. 1996. Declaration of Helsinki, 4th (Somerset West) amendment.
- 13.Temple R Impact of the Declaration of Helsinki on Medical Research from a Regulatory Perspective. 2003. Address to the Scientific Session, World Medical Association General Assembly, September, Helsinki.
- 14.Nicholson R. Editorial. Bull Med Ethics. 2000;162:1. [PubMed] [Google Scholar]
- 15.Human D, Fluss S The World Medical Association's Declaration of Helsinki: Historical and Contemporary Perspectives. [November 5, 2003]; http://www.wma.net/e/ethicsunit/pdf/draft_historical_contemporary_perspectives.pdf.
- 16.World Medical Association. Chapter 4. [November 5 2003]; The Declaration of Helsinki http://www.wma.net/e/ethicsunit/pdf/chapter_4_decl_of_helsinki.pdf.
- 17.Levine RJ. Some recent developments in the International Guidelines on the Ethics of Research Involving Human Subjects. Ann NY Acad Sci. 2000;918:170–7. doi: 10.1111/j.1749-6632.2000.tb05486.x. [DOI] [PubMed] [Google Scholar]
- 18.Emanuel EJ, Miller FG. Placebo-controlled trials – a middle ground. New Engl J Med. 2001;345:915–9. doi: 10.1056/NEJM200109203451211. [DOI] [PubMed] [Google Scholar]
- 19.Rothman KJ, Michel KB. The continuing unethical use of placebo-controlled trials. New Engl J Med. 1994;331:394–8. doi: 10.1056/NEJM199408113310611. [DOI] [PubMed] [Google Scholar]
- 20.Weijer C, Dickens B, Meslin E. Bioethics for clinicians: 10. Research ethics. Can Med Assoc J. 1997;156:1153–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman KJ. Placebo mania. Br Med J. 1996;313:3–4. doi: 10.1136/bmj.313.7048.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine RJ. The need to revise the Declaration of Helsinki. N Engl J Med. 1999;341:531–4. doi: 10.1056/NEJM199908123410713. [DOI] [PubMed] [Google Scholar]
- 23.Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: Ethical and scientific issues. Ann Intern Med. 2000;133:456–64. doi: 10.7326/0003-4819-133-6-200009190-00014. [DOI] [PubMed] [Google Scholar]
- 24.Neda N Impact of the Declaration of Helsinki on Medical Research in the Developing World. Address to Scientific Meeting, World Medical Association Annual Assembly, September 2003, Helsinki.
- 25.Nuffield Council on Bioethics. The Ethics of Research Related to Healthcare in Developing Countries. London: Nuffield Council; 2002. p. 87. [Google Scholar]
- 26.Bienenfeld L, Frishman W, Glasser SP. The placebo effect in cardiovascular disease. Am Heart J. 1996;132:1207–21. doi: 10.1016/s0002-8703(96)90465-2. [DOI] [PubMed] [Google Scholar]
- 27.Weber MA. The ethics of using placebos in hypertension clinical trials. J Hypertens. 1999;17:5–8. doi: 10.1097/00004872-199917010-00002. (Editorial) [DOI] [PubMed] [Google Scholar]
- 28.Baldwin D, Broich K, Fritze J, Kasper S, Westenberg H, Moller HJ. Placebo-controlled studies in depression: necessary, ethical and feasible. Eur Arch Psych Clin Neurosci. 2003;253:22–8. doi: 10.1007/s00406-003-0400-2. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter WT, Appelbaum PS, Levine RJ. The declaration of Helsinki and clinical trials: a focus on placebo-controlled trials in schizophrenia. Am J Psychiatry. 2003;160:356–62. doi: 10.1176/appi.ajp.160.2.356. [DOI] [PubMed] [Google Scholar]
- 30.Delmas PD, Calvo G, Boers M, et al. The use of placebo-controlled and non-inferiority trials for the evaluation of new drugs in the treatment of postmenopausal osteoporosis. Osteoporos Int. 2002;13:1–5. doi: 10.1007/s198-002-8331-3. [DOI] [PubMed] [Google Scholar]
- 31.Brody BA, Dickey N, Ellenberg SS, et al. Is the use of placebo controls ethically permissible in clinical trials of agents intended to reduce fractures in osteoporosis? J Bone Miner Res. 2003;18:1105–9. doi: 10.1359/jbmr.2003.18.6.1105. [DOI] [PubMed] [Google Scholar]
- 32.Charney DS, Nemeroff CB, Lewis L, et al. National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Arch General Psychiatry. 2002;59:262–70. doi: 10.1001/archpsyc.59.3.262. [DOI] [PubMed] [Google Scholar]
- 33.Michels KB, Rothman KJ. Update on unethical use of placebo in randomised trials. Bioethics. 2003;17:188–204. doi: 10.1111/1467-8519.00332. [DOI] [PubMed] [Google Scholar]
- 34.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 5. New York: Oxford University Press; 2001. pp. 226–7. [Google Scholar]
- 35.World Medical Association. [5 November 2003]; WMA to Continue Discussion on Declaration of Helsinki. Press release September 14 2003. http://www.wma.net/e/press/2003_19.htm.
- 36.Frankish H. WMA postpones decision to amend Declaration of Helsinki. Lancet. 2003;362:963. doi: 10.1016/s0140-6736(03)14398-x. [DOI] [PubMed] [Google Scholar]
- 37.Editorial. One standard, not two. Lancet. 2003;362:1005. [PubMed] [Google Scholar]
- 38.Carlson R, Boyd W, Webb D. One standard, not two. Lancet. 2003;362:1761. doi: 10.1016/S0140-6736(03)14859-3. (letter) [DOI] [PubMed] [Google Scholar]
- 39.Capron AM. Address to the WMA Council Meeting, Divonne-les-Bains, France, May 2003.
- 40.Riis P. Perspectives on the fifth revision of the Declaration of Helsinki. JAMA. 2000;284:3045–6. doi: 10.1001/jama.284.23.3045. [DOI] [PubMed] [Google Scholar]
- 41.World Medical Association. [5 November 2003]; The Law and Medical Ethics. http://www.wma.net/e/press/2003_6.htm.
- 42.World Medical Association. [5 November 2003]; Documentation for the Preparation of Note of Clarification on Paragraph 19 of the Revised Declaration of Helsinki http://www.wma.net/e/ethicsunit/helsinki.htm.
- 43.Godlee F. The ethics of peer review. In: Hudson Jones A, McLellan F, editors. Ethical Issues in Biomedical Publication. Baltimore: Johns Hopkins University Press; 2000. p. 78. [Google Scholar]
- 44.Delamonthe T, Smith R. Open access publishing takes off. Br Med J. 2004;328:1–3. doi: 10.1136/bmj.328.7430.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Idanpaan-Heikkila JE. The Declaration of Helsinki and Other International Ethical Documents Guiding Physicians in the Conduct of Medical Research Address to the Scientific Session of the General Assembly, World Medical Association, Helsinki, 10–14 September 2003.
- 46.Richards T. The World Medical Association: can hope triumph over experience? Br Med J. 1994;308:262–6. doi: 10.1136/bmj.308.6923.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlson RV, Boyd KM, Webb DJ. The Declaration of Helsinki's international guidelines for research ethics: past, present and future. Br J Clin Pharmacol. 2003;55:421–2. [Google Scholar]
- 48.Myllymaki K. Welcome Address to the Scientific Session of the General Assembly World Medical Association, Helsinki, 10–14 September 2003.
- 49.Nuffield Council on Bioethics. The Ethics of Research Related to Healthcare in Developing Countries. London: Nuffield Council; 2002. pp. 85–98. [Google Scholar]
- 50.Englehardt HT. The Foundations of Bioethics. 2. New York: Oxford University Press; 1996. p. 14. [Google Scholar]
- 51.Leven K-H. The invention of Hippocrates: oath, letters and Hippocratic corpus. In: Trohler U, Reiter-Theil S, editors. Ethics Codes In Medicine. Aldershot: Ashgate; 1998. pp. 3–23. [Google Scholar]
- 52.Lewis CS. Till We Have Faces: A Myth Retold. London: Fount; 1956. pp. 319–20. [Google Scholar]
- 53.Sass H-M. Reichsrundschreiben 1931: Pre-Nuremberg German regulations concerning new therapy and human experimentation. J Med Philos. 1983;8:99–111. doi: 10.1093/jmp/8.2.99. [DOI] [PubMed] [Google Scholar]
- 54.Weisstub DN. The ethical parameters of experimentation. In: Weisstub DN, editor. Research on Human Subjects. Oxford: Elsevier Science; 1998. p. 4. [Google Scholar]