Abstract
Aims
There have been case reports of taste disturbance for the angiotensin II receptor blockers losartan and valsartan, but not for candesartan. This study was undertaken to examine whether candesartan causes taste disturbance.
Methods
Candesartan cilexetil (4 mg day−1) or vehicle was given to healthy volunteers (n = 8) for 7 days in a randomized, double-blind, placebo-controlled, cross-over design with a 2-week washout period. Clinical gustometry using the filter-paper disc test and electrogustometry were sequentially performed before and at the end of each trial. Serum and salivary zinc concentrations were also measured.
Results
Detection thresholds of four basic tastes (sweet, salty, sour and bitter) determined by the paper disc test were significantly (P < 0.05 in all tests) worsened (i.e. score of test increased) after repeated dosing of the drug, although the subjects did not notice such changes. The mean ± SEM (and 95% CI) scores of the four tastes at just before the seventh dosing of candesartan or vehicle was 3.38 ± 0.32 (3.02, 3.74) and 2.63 ± 0.18 (2.18, 3.08) for sweetness, 3.63 ± 0.38 (4.49, 2.77) and 2.63 ± 0.26 (3.27, 1.98) for salt, 4.01 ± 0.42 (3.04, 4.98) and 2.61 ± 0.32 (3.35, 1.87) for sourness, 4.01 ± 0.38 (3.22, 4.80) and 2.99 ± 0.33 (2.24, 3.74) for bitterness, for candesartan and placebo, respectively. Electrogustometry confirmed the candesartan-related taste disturbance. Serum and salivary zinc concentrations were not influenced by candesartan.
Conclusions
These data suggest that candesartan subclinically reduces taste sensitivity after repeated dosing in healthy subjects. Because similar events are reported for losartan and valsartan in case reports, this adverse effect might be a class effect of angiotensin-II receptor blockers (ARBs).
Keywords: adverse reactions, angiotensin-II receptor blockers, dysgeusia, gustometry
Introduction
Taste disturbance is caused by several endogenous and exogenous factors, about 20% of which are drug-related [1–3]. Antihypertensive drugs have been identified as potential causes of taste disturbance [2, 3]. Fifty percent of patients with taste disturbance have low serum zinc concentrations and some antihypertensive drugs complex with or chelate zinc. Zinc metalloprotein is present in salivary fluid and is important in the maintenance of taste cells.
Captopril, an ACE inhibitor, induces taste disturbance. The captopril molecule contains a thiol-group (–SH) and has been shown to form chelates with zinc [2–4]. However, other ACE inhibitors without the thiol-radical have been reported to cause taste disturbance. The ACE enzyme is a zinc-dependent enzyme. Because ACE inhibitors, even without thiol groups, need zinc for reaction, the inhibition of ACE by the drug may affect the zinc of the ACE protein in the salivary gland cells, and subsequently may alter taste.
Angiotensin-II receptor blockers (ARBs) are relatively new but widely used antihypertensive agents [5]. ARBs inhibit angiotensin II-mediated effects and induce haemodynamic changes similar to ACE inhibitors. Moreover, taste disturbance is also reported with the ARBs losartan and valsartan [6–9]. To our knowledge, there have been no case reports on candesartan cilexetil (candesartan), another ARB. Therefore, it is interesting to examine whether taste disturbance is a class effect of ARBs. To address this issue, candesartan or placebo was given orally for 7 days in a randomized, double-blind, cross-over design to healthy subjects. Recognition thresholds for four basic tastes (sweet, salty, sour and bitter) by filter paper disc test and detection threshold for electrogustometry were determined at the end of each period.
Methods
Study design
Eight healthy men (29–46 years) were enrolled in this study. All subjects gave written informed consent. They were non-smokers and did not receive any medications throughout the study. Before the initiation of the study, no otolaryngeal disorders were detected in these subjects. The study was of a randomized, double-blind, placebo-controlled, cross-over design with a 2-week washout period. On day 1 (observation period), the detection thresholds for tastes were determined and salivary fluid was obtained at 09.00 h, 12.00 h, 15.00 h and 21.00 h. Subjects took candesartan cilexetil (Blopress®, Takeda Pharmaceutical Co. Ltd, Tokyo, Japan, 4 mg, of powder plus lactose, wrapped in a wafer) or placebo (100 mg of lactose, wrapped in a wafer) at 09.00 h for 7 days from day 2 to day 8. On day 8, an evaluation test and samplings of salivary fluid and blood were performed at 09.00 h, 12.00 h, 15.00 h and 21.00 h for the measurement of zinc and candesartan concentrations. On the day of the taste evaluations, subjects took their breakfast before 07.00 h. For lunch, a similar light meal (sandwich with milk) was served to all subjects just after the evaluation at 15.00 h. Subjects were prohibited from taking any other food or drink (except distilled water) until the end of the study at 21.00 h. After the washout period, an identical protocol was repeated in a cross-over fashion (days 23–30). The protocol was approved by the Ethics Committee of Jichi Medical School.
Samplings of saliva and blood
Spontaneously salivated salivary fluid was collected into a special polyethylene tube after gargling with distilled water three times. The plastic tubes were incubated with 1 m HCl for 30 min and washed with distilled water before use to remove any metals. Blood samples were taken from the cubital vein and serum was transferred into the special tubes after centrifugation. Both salivary fluid and serum were stored at −80 °C until assayed.
Assay
Total zinc concentration was measured by atomic absorption spectrophotometry [10]. Salivary and serum concentrations of candesartan and M-II, an active metabolite, were measured by high-performance liquid chromatography [11]. Detection limits for candesartan and M-II were 1 and 2 ng ml−1, respectively.
Evaluation for taste disturbance
Two types of gustometries were performed in each subject.
1 Semi-quantitative clinical gustometry using a filter-paper disc
Semi-quantitative clinical gustometry using filter-paper discs (the filter-paper disc method; Taste disc®, Sanwa Chemical Laboratory, Nagoya, Japan) were performed. This semiquantitative method is routinely used for the evaluation of dysgeusia in the clinical setting [12–14]. In brief, recognition thresholds for four basic tastes (sweet, salty, sour and bitter) were evaluated using the same chemical solutions (sucrose, NaCl, tartaric acid and quinine, respectively). The solutions were sequentially diluted with distilled water into five grades. Concentration number 1 is the lowest and 5 is the highest (0.3, 2.5, 10, 20 and 80% for sucrose, 0.3, 1.25, 5, 10 and 20% for NaCl, 0.02, 0.2, 2, 4 and 8%, for tartaric acid, 0.001, 0.02, 0.1, 0.5 and 4% for quinine). Subjects were asked to gargle with distilled water several times just before each test. A small droplet of each solution was added to filter paper (8 mm diameter), which was placed on the left side of the tongue 2 cm from the tip (i.e. locus for left chorda tympani nerve) of the subjects for 1 s. The test was started from concentration number 1 and gradually increased. Subjects selected a single answer from the following list; sweet, salty, sour, bitter, unidentifiable taste, or no taste. Thresholds for basic tastes were determined by their answer. The order of testing for the four basic tastes was randomly chosen. The test was performed by the same person (ST) throughout the study. Mean thresholds for normal volunteers were less than 3 [13, 15]. We have confirmed that the mean changes among three continuous examinations were −0.17 ± 0.05, −0.22 ± 0.07, −0.14 ± 0.05 and −0.19 ± 0.05, for sweet, salty, sour and bitter, respectively, in healthy subjects (n = 8). Thus, the reproducibility of the test was acceptable.
2 Electrogustrometer
The electrogustometry routinely used for the evaluation of hypogeusia in the oto-rhino-laryngology clinic was performed according to the method of Tomita et al. [12, 13, 15] using commercially available equipment (TR-06®, Rion Co., Ltd, Tokyo, Japan). In brief, a single type stimulation rod was placed on the left side of the tongue 2 cm from the tip (i.e. locus for left chorda tympani nerve) and the electrical stimuli was pulsed from the lowest power (−8 dB) and gradually increased. The smallest stimulus that the subjects noticed was regarded as the detection threshold. Normal range was less than + 14 dB [12, 13, 15]. The test was performed following the filter disc test after gargling with distilled water. The test was performed by the same person (ST) throughout the study. We have previously confirmed that the mean change among three continuous examinations was + 0.8 ± 0.2 dB in healthy subjects (n = 8). Thus, the reproducibility of the test is acceptable.
Statistics
All data are expressed as the mean ± SEM. Statistical analysis was performed by analysis of variance. Fisher's Protected Least Significance Difference (PLSD) test was used as a posthoc test. These analyses were performed using StatView 5 for Windows (SAS Institute Inc, NC). P < 0.05 was regarded as significant.
Results
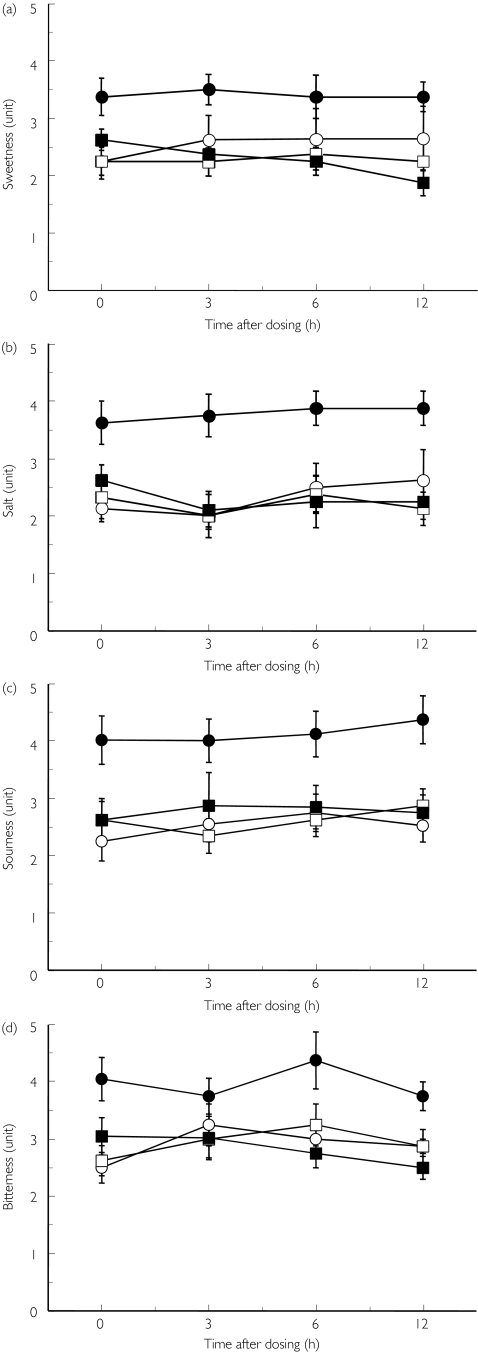
All subjects completed the protocol without any complaints of taste disturbance. Mean blood pressure 24 h before final dosing of the drug was not different between candesartan and placebo (108.5 ± 6.5 and 112.5 ± 4.2 mmHg, respectively, P = 0.08). Figure 1a–d shows the recognition thresholds for tastes using filter-paper disc. During the observation periods (day 1 and day 23), the thresholds of four tastes were within the normal range in all subjects and did not differ significantly between day 1 and day 23. The detection thresholds of four different tastes were significantly (P < 0.05) worsened after the repeated treatment with candesartan, but not with placebo. Significant (P < 0.05) differences were observed at every observation point between the two trials. Mean ± SEM (and 95% CI) scores of four tastes at 24 h after the sixth dosing (i.e. just before the last dosing) of candesartan or vehicle, respectively, were 3.38 ± 0.32 (3.02, 3.74) and 2.63 ± 0.18 (2.18, 3.08) for sweetness, 3.63 ± 0.38 (4.49, 2.77) and 2.63 ± 0.26 (3.27, 1.98) for saltiness, 4.01 ± 0.42 (3.04, 4.98) and 2.61 ± 0.32 (3.35, 1.87) for sourness, 4.01 ± 0.38 (3.22, 4.80) and 2.99 ± 0.33 (2.24, 3.74) for bitterness, respectively. The mean (± SEM) changes of the scores for candesartan and placebo, respectively, were 0.81 ± 0.32 and −0.07 ± 0.21 for sweetness, 0.99 ± 0.39 and 0.07 ± 0.22 for saltiness, 1.35 ± 0.44 and 0.09 ± 0.27 for sourness, 1.02 ± 0.36 and −0.05 ± 0.28 for bitterness. Although the thresholds of sourness in three subjects and bitterness in four subjects were in the abnormal range (i.e. 5 units), the subjects did not notice these changes.
Figure 1.
Detection thresholds for tastes using filter-paper disc after repeated dosing of candesartan. Four basic tastes (sweet (a), salty (b), sour (c) and bitter (d)) were evaluated by using chemical solutions before (day 1 + day 23) and at the end of each treatment (day 8 + day 30). Mean ± SEM, n = 8. Candesartan: before (○), after (•); Placebo: before (□), after (▪)
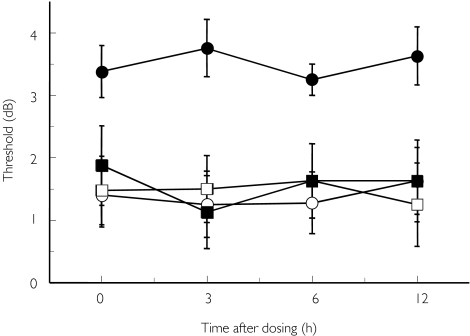
Detection threshold using an electrogustometer is shown in Figure 2. Compared with the observation period, the threshold was significantly (P < 0.05) worsened after repeated treatment with candesartan, but not with placebo. However, all values remained within the normal range. Mean ± SEM (and 95% CI) scores of threshold at 24 h after the thirteenth dosing (i.e. just before the last dosing) of candesartan or vehicle were +2.75 ± 0.84 (4.72, 0.73) and −0.25 ± 1.28 (2.02, −2.04), respectively.
Figure 2.
Detection threshold using electrogustometer after repeated dosing of candesartan before (day 1 + day 23) and at the end of each treatment (day 8 + day 30). Mean ± SEM, n = 8. Candesartan: before (○), after (•); Placebo: before (□), after (▪)
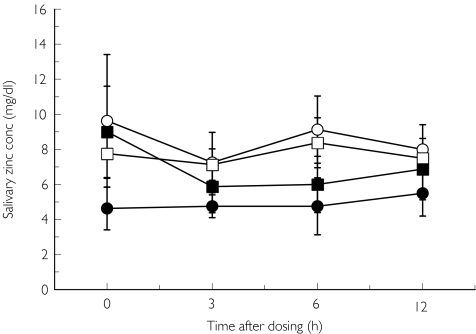
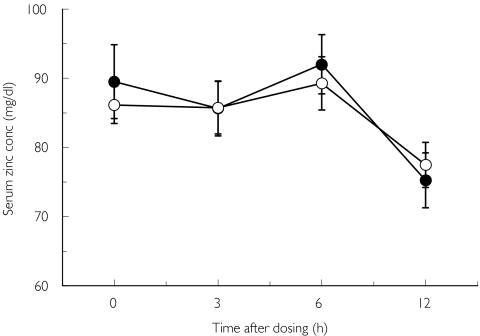
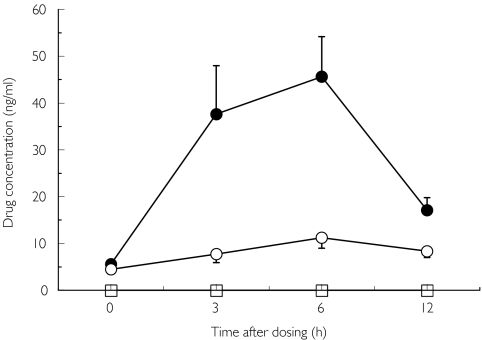
Salivary zinc concentration before (day 1, day 23) and at the end of repeated treatment (day 8, day 30) with candesartan and placebo are shown in Figures 3a and b. There were no significant differences in this parameter between the two trials. No significant differences were observed in serum zinc concentrations between the candesartan and placebo trials (Figure 4). Serum, but not salivary candesartan and M-II were detected after the final dosing of the drug (Figure 5).
Figure 3.
Salivary zinc concentration before (day 1 + day 23) and at the end (day 8 + day 30) of the treatment. Mean ± SEM, n = 8. Candesartan: before (○), after (•); Placebo: before (□), after (▪)
Figure 4.
Serum zinc concentration at the end of each treatment. Mean ± SEM, n = 8. Candesartan (•), placebo (○)
Figure 5.
Salivary and serum concentrations of candesartan and M-II after repeated dosing of the drug (4 mg, once daily for 7 days). Mean ± SEM, n = 8. Serum candesartan (•), serum M-II (○), salivary candesartan (□). Candesartan and M-II were not detected in salivary fluid at any observation point
Discussion
The filter-paper disc test is semiquantitative, and the detection thresholds for tastes vary slightly among individuals [15]. To avoid such interindividual variation, this study was performed using a randomized, double-blind, placebo-controlled, cross-over design. Detection threshold was also determined by an electrogustometer.
Previous case reports have shown that the patients noticed a metallic taste or burning feeling on the tongue, and lost all four tastes during repeated treatment with losartan [6–8]. Their taste disturbance disappeared within a few weeks after discontinuation of the drug. We evaluated the effect of candesartan on four basic tastes (sweet, salty, sour and bitter) using filter-paper discs. We found that the drug caused subclinical disturbances for these tastes after repeated dosing in healthy subjects. Candesartan-induced taste disturbance was also detected by the electrogustometer. In particular, bitterness and sourness were worsened to a pathological level in almost half the subjects, although they did not notice the disturbance. Based on these findings together with previous observations, we think that taste disturbance is not specific for losartan, but is a class effect of ARBs.
Hypertensive patients noticed the taste disturbance after repeated dosing of losartan and valsartan for 1 week to 3 months [6–9], which indicates that ARB-induced taste disturbance gradually developed. In addition, the present data suggest that this adverse effect persisted for more than 24 h after each dosing of candesartan during repeated treatment. However, the detection thresholds at the observation period (day 1, day 23) of the candesartan and placebo trials did not differ significantly. This indicates that the candesartan-induced taste disturbance was reversible within 2 weeks after the discontinuation of the drug.
A variety of drugs are reported to cause taste disturbance, including thiamazol, d-penicillamine and captopril [16]. Taste disturbance induced by thiamazol and d-penicillamine has been ascribed to the complexing of zinc by these drugs [17] while captopril caused this adverse effect by chelation of zinc [2–4]. Zinc-unrelated taste disturbances have also been reported [2, 3]. In this study, serum and salivary zinc concentrations were not influenced by the repeated dosing of candesartan. Thus, we found no evidence that shows involvement of zinc in candesartan-induced taste disturbance. In salivary fluid, both bound and unbound fractions of zinc were measured. It is therefore uncertain whether the bound fraction of zinc with metalloprotein in the salivary fluid was changed by the drug. Recent advances in molecular biology have identified receptors and ion channels on taste cells. Sweet and bitter taste receptors are the proteins that couple with G-proteins [18, 19]. Coupling and uncoupling to G-protein causes ‘taste-on’ and ‘taste-off’ [2]. The taste receptors have seven transmembrane domains [18, 19]. Angiotenisin II receptor, which is the target molecule of ARB, also belongs to the same category of receptor [20]. It is possible that ARBs are secreted in salivary fluid, which in turn, bind with taste receptors and consequently disturb the sense of sweet and bitter tastes. On the other hand, salt and sour tastes are elicited by ion channels (salt; amiloride-sensitive epithelial Na channel, sour; amiloride-sensitive epithelial Na channels and H+-activated cation channels [18, 19]. In this case, salivary ARBs might plug these channels and disturb salt and sour tastes. Although salivary candesartan and its metabolite were not detected in this study, the above-mentioned hypothesis remains to be determined.
In summary, this study shows that repeated dosing with candesartan subclinically reduces the sensitivity of basic tastes. The effect was reversible after discontinuation of the drug within 2 weeks. Because similar events are reported for losartan and valsartan in the clinical setting, taste disturbance might be a class effect of ARBs. Similar events are reported for ACE-I with unknown mechanisms except for captopril. Further studies are needed to determine the frequency and grade of taste disturbance for each ARB.
Acknowledgments
We thank Takeda Pharmaceutical Co, Ltd, for measurement of candesartan and M-II concentrations. Part of this study was supported by a grant from the Ministry of Health, Welfare and Labor of Japan and the Renal Osteodystrophy Foundation.
References
- 1.Zegarelli D. Diseases of oral mucosa. In: Speight TN, Holford N, editors. Avery's drug treatment. Auckland: Adis International; 1997. pp. 608–30. [Google Scholar]
- 2.Henkin RI. Drug-induced taste and smell disorders. Incidence, mechanisms and management related primarily to treatment of sensory receptor dysfunction. Drug Saf. 1994;11:318–77. doi: 10.2165/00002018-199411050-00004. [DOI] [PubMed] [Google Scholar]
- 3.Tomita H, Yoshikawa T. Drug-related taste disturbances. Acta Otolaryngol Suppl. 2002;546:116–21. doi: 10.1080/00016480260046490. [DOI] [PubMed] [Google Scholar]
- 4.Heyneman CA. Zinc deficiency and taste disorders. Ann Pharmacother. 1996;30:186–7. doi: 10.1177/106002809603000215. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan N. Treatment of hypertension: drug therapy. In: Kaplan N, editor. Clinical Hypertension. Baltimore: Williams & Wilkins; 1998. pp. 181–264. [Google Scholar]
- 6.Schlienger RG, Saxer M, Haefeli WE. Reversible ageusia associated with losartan. Lancet. 1996;347:471–2. doi: 10.1016/s0140-6736(96)90047-1. [DOI] [PubMed] [Google Scholar]
- 7.Heeringa M, van Puijenbroek EP. Reversible dysgeusia attributed to losartan. Ann Intern Med. 1998;129:72. doi: 10.7326/0003-4819-129-1-199807010-00023. [DOI] [PubMed] [Google Scholar]
- 8.Ohkoshi N, Shoji S. Reversible ageusia induced by losartan: a case report. Eur J Neurol. 2002;9:315. doi: 10.1046/j.1468-1331.2002.00389.x. [DOI] [PubMed] [Google Scholar]
- 9.Stroeder R, Zeissig L. Angiotensin II-antagonist cGP48933 (valsartan), Ergebnisse einer doppelblinden, plazebo-Kontrolierten Multicenter-Studie. Nieren Hochdruckkrankheiten) 1994;23:217–20. (in German) [Google Scholar]
- 10.Meret S, Henkin R. Simultaneous direct estimation by atomic absorption spectrophotometry of copper and zinc in serum, urine, and cerebrospinal fluid. Clin Chem. 1971;17:369–73. [PubMed] [Google Scholar]
- 11.Stenhoff H, Lagerstrom P, Andersen C. Determination of candesartan cilexetil, candesartan and a metabolite in human plasma and urine by liquid chromatography and fluorometric detection. J Chromatogr B Biomed Sci Appl. 1999;731:411–17. doi: 10.1016/s0378-4347(99)00247-9. [DOI] [PubMed] [Google Scholar]
- 12.Kuga M, Ikeda M. Evaluation of gustatory threshold changes in healthy subjects. Nippon Jibiinkoka Gakkai Kaiho. 1996;99:411–16. doi: 10.3950/jibiinkoka.99.411. [DOI] [PubMed] [Google Scholar]
- 13.Tomita H, Ikeda M, Okuda Y. Basis and practice of clinical taste examinations. Auris Nasus Larynx. 1986;13(Suppl 1):S1–S15. doi: 10.1016/s0385-8146(86)80029-3. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Endo S, Tomita H. Sensitivity of three loci on the tongue and soft palate to four basic tastes in smokers and non-smokers. Acta Otolaryngol Suppl. 2002;546:74–82. doi: 10.1080/00016480260046445. [DOI] [PubMed] [Google Scholar]
- 15.Tomita H, Ikeda M. Clinical use of electrogustometry: strengths and limitations. Acta Otolaryngol Suppl. 2002;546:27–38. doi: 10.1080/00016480260046391. [DOI] [PubMed] [Google Scholar]
- 16.Schiffman S. Taste and smell in disease. New Engl J Med. 1983;308:1275–9. doi: 10.1056/NEJM198305263082107. [DOI] [PubMed] [Google Scholar]
- 17.Erilssen J, Saegaard E, Naess J. Side-effect of thiocarbamide. Lancet. 1975;ii:231–2. doi: 10.1016/s0140-6736(75)91417-8. [DOI] [PubMed] [Google Scholar]
- 18.Gilbertson T, Damak S, Margolskee R. The molecular physiology of taste transduction. Curr Opin Neurobiol. 2000;10:519–27. doi: 10.1016/s0959-4388(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 19.Lindemann B. Receptors and transduction in taste. Nature (Lond) 2001;413:219–25. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- 20.Bockaert J, Pin J. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–9. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]