Abstract
Aim
A person with Type A personality is an ‘aggressor’ compared with the rarely harried Type B. Although debrisoquine hydroxylase (CYP2D6) capacity has been associated with personality, no study has specifically investigated its association with personality Type A and B. Therefore the aim of this research was to study the impact of CYP2D6 on Type A and B personality.
Methods
Type A and B personality questionnaires were administered to 48 healthy patients undergoing elective orthopaedic surgery. After obtaining informed consent, patients were genotyped for the various CYP2D6 alleles by allele-specific polymerase chain reaction. Based on the genotypes, patients were grouped as extensive metabolizer (EM)1 (normal) (CYP2D6*1/*1), EM2 (intermediate) (CYP2D6*1/*4, CYP2D6*1/*5, CYP2D6*1/*9 and CYP2D6*1/*10) and EM3 (slow) (CYP2D6*4/*10, CYP2D6*5/*10, CYP2D6*10/*10 and CYP2D6*10/*17). χ2 was used to determine the relationship between the groups and personality types.
Results
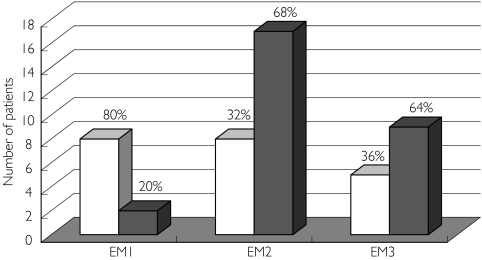
The percentages of patients who were of the EM1, EM2 and EM3 groups were 20.8%, 52.1% and 27.1%, respectively. There was a significant difference (P = 0.032) between the three groups in terms of personality type, in which EM1 showed a tendency to be of personality Type A while EM2 and EM3 tended to be of personality Type B.
Conclusion
The study suggests that there is a relationship between CYP2D6 activity and Type A and B personality.
Keywords: CYP2D6, personality Type A and B, polymerase chain reaction
Introduction
A number of studies in Caucasian populations have shown a relationship between genotype and the extent of the metabolism of CYP2D6 substrate, debrisoquine [1]. Individuals lacking significant metabolism [poor metabolizers, (PM)] are almost always homozygous for null alleles, whereas individuals homozygous for CYP2D6*10 have slower rates of metabolism than individuals homozygous for CYP2D6 wild type who are not phenotypically PMs [2]. Extensive metabolizers (EMs) are individuals who are homozygous or heterozygous for the wild type and exhibit normal enzyme activity, while ultrarapid metabolizers (UMs) are carriers of duplicated or multiduplicated active genes [3, 4]. Variations of drug metabolism among ethnic groups also exist [5]. Care must therefore be taken when interpreting results from different ethnic groups.
It has also been shown that there are significant personality differences between EM and PM of debrisoquine among healthy volunteers living in Sweden [6] and Spain [7] when assessed using the Karolinska Scales of Personality inventory. It was postulated that debrisoquine hydroxylase also metabolizes endogenous substance(s) such as biogenic neurotransmitter amines important for the central nervous function [6]. There is now considerable evidence that debrisoquine hydroxylase is also present and functions in the human brain [8–10]. It was hypothesized that PMs of debrisoquine have low activity of this enzyme in the brain as well as in the liver, which may decrease the metabolism of an endogenous substance in the central nervous system (CNS), causing personality differences from the EMs [7].
The Karolinska Scales of Personality inventory contains 135 statements that measure differences in personality variables such as somatic anxiety, impulsiveness and psychasthenia [10]. Compared with this inventory, the Personality Type A and B questionnaire is short and is much simpler to administer [11]. No study has so far attempted to determine the relationship between debrisoquine hydroxylation capacity and Type A and B personality, and to our knowledge this is the first to do so.
Methods
Selection of subjects
Forty-eight (39 males and nine females) unrelated patients were selected based on the study's inclusion and exclusion criteria. Written informed consent was obtained from each patient after a full explanation of the study. Their ages ranged between 18 and 48 (mean ± SD =24.73 ± 8.86 years). There were 44 Malays, three Chinese and one Indian.
Patients included were those going for elective orthopaedic surgery such as removal of implant and open reduction internal fixation in Hospital Universiti Sains Malaysia. The questionnaires were administered preoperatively. Subjects were otherwise healthy as assessed by medical history and physical examination. Those with head injuries or those who could not understand instructions regarding the study protocol were excluded. The study was approved by the university's Research and Ethics Committee and complies with the Declaration of Helsinki.
Experimental protocols
The Type A and B personality questionnaire based on the short rating scale of Bortner [11] was used. The questionnaire was translated into the national language (Bahasa Malaysia) by the researchers and then translated back into English by an independent person. The Bahasa Malaysia translated version was first validated using 30 patients. These results were not included in the study. When patients were found to be illiterate (two cases), the questionnaire was read out and filled in by the researcher according to the answers given. The same researcher was used for all subjects in order to avoid bias.
Blood (2 ml) was collected from each patient into EDTA tubes (Eppendorf, Westbury, NY, USA) for genotyping. Each sample was shaken gently for adequate mixing with the anticoagulant before being immediately transported on ice to the laboratory. The samples were stored at −20 °C until DNA extraction (within 5 days).
DNA extraction
Genomic DNA was isolated from peripheral lymphocytes of each patient. The blood was transferred into a 50-ml sterile conical propylene tube (Becton Dickinson, San Jose, CA, USA) before the addition of 2 ml of cold sterile lysis buffer (0.64 m sucrose, 0.02 m Tris–HCl, 2% Triton-X100). The tubes were centrifuged at 3500 r.p.m. (2200 g) for 15 min at 4 °C. The supernatant was decanted and the pellet washed with Tris–EDTA (TE). This procedure was repeated twice and each pellet was resuspended in 2 ml saline–EDTA. Then, 100 µl of 20% SDS and 12.5 µl of RNase-A (10 mg ml−1) (Clontech, Palo Alto, CA, USA) were added to each tube before incubation at 37 °C for 1 h. Next, 12.5 µl of proteinase-K (20 mg ml−1) (Clontech) were added to the tubes before overnight incubation at 37 °C. DNA was then ethanol precipitated and resuspended in 200 µl TE buffer.
Genotyping
Polymerase chain reaction (PCR) was performed to detect *3 and *4 alleles according to the method described by Heim and Meyer [12], with slight modifications. Genotyping for C188/T mutation was performed using two primer sets 9/10 and 9/10B according to the modified method of Johansson et al. [13]. The assays for CYP2D6*5 and the duplication gene were based on the methods described by Steen et al. [14], while the assays for the CYP2D6*9 and CYP2D6*17 alleles were based on the methods described by Tyndale et al. [15] and Masimirembwa et al. [16], respectively, with slight modifications.
Based on the genotypes, patients were grouped into three main classes: EM1 (CYP2D6*1/*1), EM2 (CYP2D6*1/*4, CYP2D6*1/*5, CYP2D6*1/*9 and CYP2D6*1/*10) and EM3 (CYP2D6*4/*10, CYP2D6*5/*10, CYP2D6*10/*10 and CYP2D6*10/*17). Allele frequency was also calculated among the patients sampled [17]. Only alleles that are commonly found among Asian subjects were studied [18, 19].
Statistics
A 2 × 3 χ2 table was used to determine the relationship between patients' personality type and the various genotype groups. χ2 was also used to assess the difference between sex, race, smoking status and fracture types among the groups. When more than 20% of the cells in the χ2 table had expected frequency <5, Fisher's exact test was used. anova was used to compare the three groups in terms of age and body weight. A P-value of <0.05 was considered to be statistically significant. Data were analysed using SPSS (Version 10.0, Stigmastat, SPSS Science, Chicago, IL, USA) on an IBM-compatible computer.
Sample size calculation
The formula for single proportion sample size was used to determine the appropriate sample size (n) for the study [20].
Using the formula:
where p = proportion of EM among Asians reported by previous studies [18, 19] = 0.98, Δ = 95% confidence interval width of ±5% = 0.05, when α= 0.05 and β= 0.80 (80% power to detect differences), zα = 1.960.
Therefore,
Our sample size of 48 subjects was therefore adequate for the study among Asian subjects.
For comparison, when subjects were Caucasians and the percentage of PMs were higher (7%) [6], the calculated sample size is more, as calculated below:
Results
The number of patients who were EM1, EM2 and EM3 was 10, 25 and 13, respectively (Table 1). No patients were found to be of PM or UM status. Most (81.2%) of the patients were students. There was no significant difference in terms of age, sex, race, body weight, smoking status or fracture types among the three genotype groups.
Table 1.
Reported CYP2D6 genotypes and their groupings
| Genotype | Group | n | Percentage |
|---|---|---|---|
| *4/*10 | EM3 | 5 | 10.42 |
| *5/*10 | EM3 | 3 | 6.25 |
| *10/*10 | EM3 | 4 | 8.33 |
| *10/*17 | EM3 | 1 | 2.08 |
| *1/*5 | EM2 | 1 | 2.08 |
| *1/*9 | EM2 | 1 | 2.08 |
| *1/*4 | EM2 | 1 | 2.08 |
| *1/*10 | EM2 | 23 | 45.83 |
| *1/*1 | EM1 | 10 | 20.83 |
| Total | 49 | 100.00 |
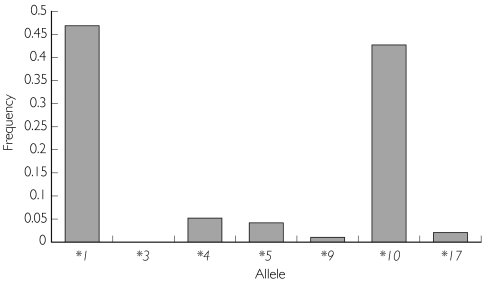
Figure 1 shows the distribution of personality types in the various groups, while Figure 2 shows the calculated allele frequencies. The groups with the ‘intermediate’ (EM2) and ‘slow’ enzyme activity (EM3) were found to be mainly of personality Type B compared with the ‘normal’ type (EM1) (P = 0.032), in which there was a predominance of Type A.
Figure 1.
Distribution of patients who were of Type A and B personalities in their various groupings. (EM1 = CYP2D6*1/*1), (EM2 = CYP2D6*1/*4, CYP2D6*1/*5, CYP2D6*1/*9 & CYP2D6*1/*10) and (EM3 = CYP2D6*4/*10, CYP2D6*5/*10, CYP2D6*10/*10 & CYP2D6*10/*17). Personality Type A (□), personality Type B ( )
)
Figure 2.
Calculated allele frequency among the patients (n = 96)
Discussion
More than 90% of PMs can be diagnosed by using PCR and additional PCR analysis in selected cases [21]. Although CYP2D6*4 is the most common allele occurring in Caucasians [22], CYP2D6*10 has been found to be more common among Japanese and Chinese [2], and among Malays it has been reported to occur at 49.5% [18]. In this study, the percentage occurrence of CYP2D6*10 (45%) is very close to that reported mainly because most (86.3%) of our subjects were also Malays. From the calculated allele frequency, it is possible that Malay subjects have more similarity to the Chinese and Japanese populations compared with Caucasians in terms of debrisoquine hydroxylation capacity.
There has been little information on the frequency of CYP2D6 mutations among South-east Asian populations. The frequency of PMs has been reported to be as low as 1% in these populations [23] and is about 2% in other studies [18, 19]. As there was no patient found to be of PM or UM status in our study, a comparison of the personalities of EMs with these groups could not be carried out. A different personality type was nevertheless observed within the Ems, with the ‘normal’ group having personality that is largely Type A and the ‘intermediate’ and ‘slow’ metabolizer groups showing a preponderance of Type B personality. The Spanish group [7] also found differences in personality type within the EM envelope indicating that there is a direct relationship between debrisoquine hydroxylase activity and personality type.
Type A behaviour is characterized by a feeling of a chronic sense of time urgency, excessive competitive drive, impatience and hostility [24]. Those with Type B personalities are the opposite. They are rarely harried and have low or no desire to obtain a wildly increasing number of things in an ever-decreasing amount of time [24]. Compared with the Personality Type A and B questionnaire by Bortner as used in this study, the Karolinska Scales of Personality Inventory used by both the Spanish [7] and the Swedish [6] groups measure a different type of personality. Using the inventory, the Spanish group has found that the EM1 group are less prone to anxiety (low scores in psychic anxiety) and are more successfully sociable (higher scores in the socialization scale) [7]. This implies high vitality, alertness, efficiency and ease of decision-making [6]. The Swedish group, on the other hand, found opposite results [6]. However, our study, which measures personality Type A and B, is more similar to the Swedish group in which EM1 is found to be more prone to anxiety (having Type A personality).
In this study we chose to measure personality Type A and B rather than to use the long Karolinska Scales of Personality Inventory, because the short rating scale is simple and easy to administer without being too taxing for patients. We also found the results among our Malaysian subjects to be no different from the Swedish group that assessed personality using the Karolinska Scales of Personality Inventory.
CYP2D6 is highly polymorphic. Even though our calculated sample size of 48 was adequate as confirmed by the statistically positive result, a larger sample size may be warranted to substantiate the association between personality Type A and B with the CYP2D6 gene.
In conclusion, this study suggests that there exists a relationship between CYP2D6 activity and personality Type A and B, with the EM1 group showing a tendency to be of personality Type A, while EM2 and EM3 tend to be of personality Type B. The association between CYP2D6 and a dopamine transporter [8] suggests a role of the dopamine system in the brain as part of the relationship between personality Type A and B and debrisoquine hydroxylation capacity. However, further studies are warranted to confirm this association.
Acknowledgments
We thank Dr Jennie Wong of the National University of Singapore for providing positive control for CYP2D6*5, Professor Inger Johansson of Karolinska Institutet, Sweden for providing positive control for CYP2D6*10, and Dr rer. nat. Ulrich Griese of the Dr Margarete Fischer-Bosch-Institut für Klinische Pharmakologie Auerbachstr for providing positive controls for CYP2D6*3 and *4. Special thanks to Professor Hans Van Rostenberghe for helping to edit the manuscript. The study was supported by a grant from the Ministry of Science, Technology and Environment, the Government of Malaysia.
References
- 1.Daly AK, Armstrong M, Monkman SC, et al. Genetic and metabolic criteria for the assignment of debrisoquine 4-hydroxylation phenotypes. Pharmacogenetics. 1991;1:33–41. doi: 10.1097/00008571-199110000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Yokota H, Tamura S, Furuya H, et al. Evidence for a new variant CYP2D6 allele, CYP2D6J, in a Japanese population associated with lower in vivo rates of sparteine metabolism. Pharmacogenetics. 1993;3:256–63. doi: 10.1097/00008571-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Meyer UA, Zanger UM. Molecular mechanisms of genetic polymorphisms of drug metabolism. Annu Rev Pharmacol Toxicol. 1997;37:269–96. doi: 10.1146/annurev.pharmtox.37.1.269. [DOI] [PubMed] [Google Scholar]
- 4.Ingelman-Sundberg M, Oscarson M, McLellan RA. Polymorphic human cytochrome P450 enzymes, an opportunity for individualized drug treatment. Trends Pharmacol Sci. 1999;20:342–9. doi: 10.1016/s0165-6147(99)01363-2. [DOI] [PubMed] [Google Scholar]
- 5.Kalow W. Interethnic variation of drug metabolism. Trends Pharmacol Sci. 1991;12:102–7. doi: 10.1016/0165-6147(91)90516-u. [DOI] [PubMed] [Google Scholar]
- 6.Bertilsson L, Alm C, Carreras DLC, et al. Debrisoquine hydroxylation polymorphism and personality. Lancet. 1989;ii:555. doi: 10.1016/s0140-6736(89)90094-9. [DOI] [PubMed] [Google Scholar]
- 7.Llerena A, Edman G, Cobaleda J, et al. Relationship between personality and debrisoquine hydroxylation capacity: suggestion of an endogenous neuroactive substrate or product of the cytochrome P4502D6. Acta Psychiatr Scand. 1993;87:23–8. doi: 10.1111/j.1600-0447.1993.tb03325.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalow W, Tyndale RF. Debrisoquine/sparteine monooxygenase and other P450s in brain. In: Kalow W, editor. Pharmacogenetics of Drug Metabolism. 1. Oxford: Pergamon Press; 1992. pp. 649–56. [Google Scholar]
- 9.Anzenbacher P, Anzenbacerova E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci. 2001;58:737–47. doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegle I, Fritz P, Eckhardt K, et al. Cellular localization and regional distribution of CYP2D6 mRNA and protein expression in human brain. Pharmacogenetics. 2001;11:237–45. doi: 10.1097/00008571-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Schalling D, Asberg M, Edman G, et al. Markers for vulnerability to psychopathology: temperament traits associated with platelet MAO activity. Acta Psychiatr Scand. 1987;76:172–82. doi: 10.1111/j.1600-0447.1987.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 12.Bortner RW. Short rating scale as a potential measure of pattern A behaviour. J Chron Dis. 1969;22:87–92. doi: 10.1016/0021-9681(69)90061-7. [DOI] [PubMed] [Google Scholar]
- 13.Heim M, Meyer UA. Genotyping of poor metabolisers of debrisoquine by allele-specific PCR amplification. Lancet. 1990;336:529–32. doi: 10.1016/0140-6736(90)92086-w. [DOI] [PubMed] [Google Scholar]
- 14.Johansson I, Oscarson M, Yue QY, et al. Genetic analysis of the Chinese cytochrome P450 2D locus: characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol Pharmacol. 1994;46:452–9. [PubMed] [Google Scholar]
- 15.Steen VM, Andreassen OA, Daly AK, et al. Detection of the poor-metaboliser associated CYP2D6(D) gene deletion allele by long-PCR technology. Pharmacogenetics. 1995;5:215–23. doi: 10.1097/00008571-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Tyndale R, Aoyama T, Broly F, et al. Identification of a new variant CYP2D6 allele lacking the codon encoding Lys–281: possible association with the poor metaboliser phenotype. Pharmacogenetics. 1991;1:26–32. doi: 10.1097/00008571-199110000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Masimirembwa C, Persson I, Bertilsson L, et al. A novel mutant variant of CYP2D6 gene (CYP2D6*17) common in a black African population: association with diminished debrisoquine hydroxylase activity. Br J Clin Pharmacol. 1996;42:713–19. doi: 10.1046/j.1365-2125.1996.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teh LK, Ismail R, Yusof R, et al. Heterogeneity of the CYP2D6 gene among Malays in Malaysia. J Clin Pharm Ther. 2001;26:1–7. doi: 10.1046/j.1365-2710.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee EJD, Yeoh PN, Gong NH. Oxidation phenotyping in Chinese and Malay populations. Clin Exp Pharmacol Physiol. 1988;15:889–91. doi: 10.1111/j.1440-1681.1988.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 20.Stolley PD, Strom LB. Sample size calculations for clinical pharmacology studies. Clin Pharmacol Ther. 1986;39:489–90. doi: 10.1038/clpt.1986.85. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Barcelo M, Chow LY, Chiu HF, et al. Genetic analysis of CYP2D6 locus in a Hong Kong Chinese population. Clin Chem. 2000;46:18–23. [PubMed] [Google Scholar]
- 22.Gonzalez FJ, Meyer UA. Molecular genetics of the debrisoquine-sparteine polymorphism. Clin Pharmacol Ther. 1991;50:233–8. doi: 10.1038/clpt.1991.131. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez FJ, Idle JR. Pharmacogenetic phenotyping and genotyping: present status and future potential. Clin Pharmacokinet. 1994;26:59–70. doi: 10.2165/00003088-199426010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Robbins S. Work stress. In: Robbins S, editor. Organisational Behaviour: Concepts, Controversies and Application. 1. Australia: Prentice Hall; 1989. pp. 510–11. [Google Scholar]