Abstract
Aims
Endothelin-1 (ET-1[1–21]) is an extremely potent vasoconstrictor in the human skin microcirculation and is generated from larger precursor peptides. The aims of the present study were to assess the vasoactive effects of these precursors as well as endothelin blockade in the human skin microcirculation, in vivo.
Methods
Six healthy volunteers received intradermal injections of a range of doses of big ET-1[1–38], ET-1[1–31], ET-1[1–21], BQ-123 (ETA receptor antagonist), BQ-788 (ETB receptor antagonist), phosphoramidon [endothelin converting enzyme (ECE) inhibitor] or saline control (0.9%). Skin blood flow (SBF) was measured using standard laser Doppler flowmetry.
Results
Big ET-1[1–38], ET-1[1–31] and ET-1[1–21] reduced SBF when compared with saline control (P < 0.01 for all). Big ET-1[1–38] and ET-1[1–31] were less potent than ET-1[1–21] as defined by skin vasoconstriction. Phosphoramidon, BQ-123 and BQ-788, given alone, all caused vasodilatation in the human skin microcirculation (P < 0.01 for all).
Conclusions
In the human skin microcirculation, big ET-1[1–38] and ET-1[1–31] are less potent vasoconstrictors than ET-1[1–21]. The effects of big ET-1[1–38] and phosphoramidon suggest the presence of endogenous ECE activity in the skin. In contrast to skeletal muscle resistance vessels, ET-1[1–21] contributes to the maintenance of skin microvascular tone through both ETA and ETB receptor-mediated vasoconstriction.
Keywords: antagonists, converting enzyme inhibitors, endothelins
Introduction
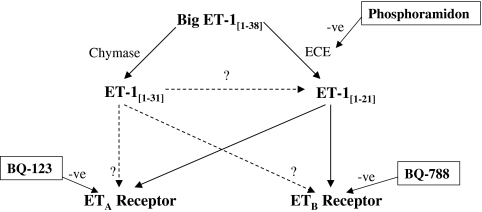
Endothelin-1 [1–21] (ET-1) is a potent vasoconstrictor in several vascular beds including the skin microcirculation [1, 2]. It is formed by the action of endothelin converting enzyme (ECE) on its inactive precursor ET-1[1–38] (big ET-1) (Figure 1). There are two distinct endothelin receptors subtypes; ETA and ETB. Both are present on vascular smooth muscle cells mediating vasoconstriction, while ETB is also present on endothelial cells mediating vasodilatation. Thus, the cardiovascular effects of ET-1[1–21] will depend, in part, on the balance of action at these two receptors, and in particular the balance between the vasodilatating and vasoconstricting actions of the ETB receptor.
Figure 1.
Pathway of endothelin processing. Agents in boxes are enzyme inhibitors/receptor antagonists
The endothelin system may be involved in the pathophysiology of several cardiovascular diseases, including hypertension [3], renal failure [4], pulmonary hypertension [5], and chronic heart failure (CHF) [6]. Blockade of the system remains an area of major interest. In particular, whether selective or dual receptor antagonism or ECE inhibition will provide the best treatment strategy remains unclear. Initial efforts have been focused mainly on endothelin receptor blockade. Endothelin receptor antagonists have benefits in patients with primary pulmonary hypertension [7], although results in patients with CHF have so far been disappointing [8]. Clinical development of ECE inhibitors continues but there are, as yet, no fully published data on their clinical efficacy.
Recently, ET-1[1–31], a new derivative of big ET-1, has been identified in humans. It is generated following the cleavage of big ET-1 at the Tyr31–Gly32 bond by human chymase [9] and may represent an active intermediary in an alternative pathway of ET-1 production (Figure 1). In cultured human coronary artery smooth muscle cells [10, 11] and human mesangial cells [12], ET-1[1–31] increases intracellular calcium. ET-1[1–31] causes vasoconstriction in isolated porcine coronary arteries [13], monkey trachea [14], human umbilical arteries [15] as well as human coronary and mammary arteries [16]. Data suggest that ET-1[1–31] may be cleaved to ET-1[1–21] for its biological activity in cultured bronchial smooth muscle cells [17] and in both guinea pig [18] and human arteries [16]. However, to date there have been no in vivo clinical studies to assess the cardiovascular effects of ET-1[1–31].
The aims of this study (Fig. 1) were to investigate the in vivo vascular effects of ET-1[1–21], its precursors big ET-1[1–38] and ET-1[1–31], and blockade of endogenous ET-1 activity by BQ-123 (a selective ETA receptor antagonist) [19], BQ-788 (a selective ETB receptor antagonist) [20] and inhibition of ET-1 generation by phosphoramidon (an ECE inhibitor) in the human skin microcirculation.
Methods
Subjects
Six healthy men (age range 20–30 years), with no risk factors for vascular disease, participated in each study. Written informed consent was obtained and studies were performed with the approval of the local research ethics committee and in accordance with the Declaration of Helsinki. No subject was taking regular medication and all avoided medication for 1 week before each study. All subjects abstained from alcohol for 24 h and from food, caffeine and tobacco for at least 12 h before each study.
Skin blood flow measurement
Skin blood flow was assessed using standard laser Doppler skin flowmetry (2 channel, MBF 3D; Moor Instruments Ltd, Axminster, UK) at baseline and every 2 min for the first 10 min and then every 5 min up to 60 min. Voltage output from the Doppler flowmeter was calibrated with standard flux solution (Moor Instruments Ltd) and transferred to a Macintosh personal computer (Classic II; Apple Computer Inc., Cupertino, CA, USA) with a MacLab analogue-to-digital converter and ‘CHART’ software (v.3.28; AD Instruments, Castle Hill, Australia). Signals were averaged over 20 s at each time point.
Study drugs
ET-1[1–31] (Peptide Institute, Osaka, Japan), and big ET-1[1–38], ET-1[1–21], BQ-123, BQ-788 and phosphoramidon (Clinalfa, Laufelfingen, Switzerland) were dissolved in physiological saline (0.9%; Baxter Healthcare Ltd, Thetford, UK), which was also used as the vehicle control. Phosphoramidon was poorly soluble, allowing a limited dose range to be examined.
Study protocol
Subjects rested recumbent in a quiet room maintained at a constant temperature of 22–24 °C for 15 min to allow stabilization of skin blood flow. Four sites for injection were identified and marked on the volar aspect of each forearm. Care was taken to avoid underlying veins (demonstrated by high baseline Doppler signals) and arteries (demonstrated by pulsatile Doppler signals). A laser probe holder was attached to the skin using adhesive tape to reduce probe movement during the study. All study drugs were administered by 10 µl intradermal injection [0.33-mm (29.5 SWG) needle; Becton Dickinson, Dublin, Ireland]. Following dose-ranging pilot studies, subjects received, in random order, either saline control or study drug over a range of concentrations; big ET-1[1–38] (0.1–30 pmol), ET-1[1–31] (1 pmol to 0.3 nmol), ET-1[1–21] (1 amol to 1 pmol), BQ-123 (0.1–30 nmol), BQ-788 (0.1–30 nmol) and phosphoramidon (0.1–10 nmol). The maximum dose of phosphoramidon was limited by solubility.
Data handling and statistical analysis
Results are expressed in arbitrary perfusion units (PU). Intradermal injection of saline placebo causes an increase in laser Doppler signal [1] and therefore all results are presented as placebo corrected mean ± SEM. Area under the curve (AUC) for the response between 0 and 30 min was used to determine potency. Potency was estimated as the dose required to cause a significant vasoconstriction in the skin compared with saline placebo. Statistical difference was tested by anova with repeated measures over time and paired Student's t-test with correction for repeated measures (Excel 5.0; Microsoft Ltd, Seattle, WA, USA). A P-value <0.05 was considered to be statistically significant.
Results
Intradermal drug delivery was well tolerated by all volunteers. Transient discomfort occurred at some injection sites but was unrelated to injectate and did not persist for longer than 10 s. This discomfort was not associated with impaired tissue viability and did not appear to affect responses.
Effect of endothelin agonists
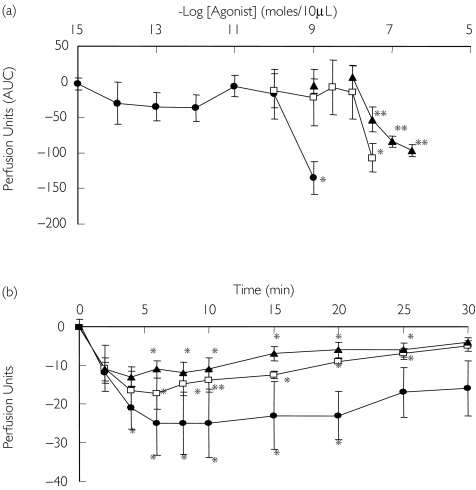
Big ET-1[1–38], ET-1[1–31] and ET-1[1–21] caused vasoconstriction that was visually evident on the skin, causing an area of marked pallor. Big ET-1[1–38] and ET-1[1–31] were ∼30-fold less potent than ET-1[1–21] (Figure 2a). Compared with control, sustained reduction in blood flow was caused by big ET-1[1–38] (30 pmol; maximum decrease 25 ± 8 PU, P = 0.04), ET-1[1–31] (0.3 nmol; maximum decrease 13 ± 3 PU, P = 0.04) and ET-1[1–21] (1 pmol; maximum decrease 17 ± 4 PU, P = 0.003) (Figure 2b). At these concentrations, vasoconstriction was sustained and was still visibly present at 24 h, although the duration of response beyond 60 min was not formally assessed.
Figure 2.
(a) Dose–response (AUC) to big ET-1[1–38] (0.1–30 pmol) (u), ET-1[1–31] (1 pmol to 0.3 nmol) (▴), and ET-1[1–21] (1 amol to 1 pmol) (•). (b) Effect of maximum dose of endothelin agonist on skin blood flow; ET-1[1–38] (30 pmol), ET-1[1–31] (0.3 nmol) and ET-1[1–21] (1 pmol). *P < 0.05; **P < 0.01 vs. placebo
Effect of endothelin blockade
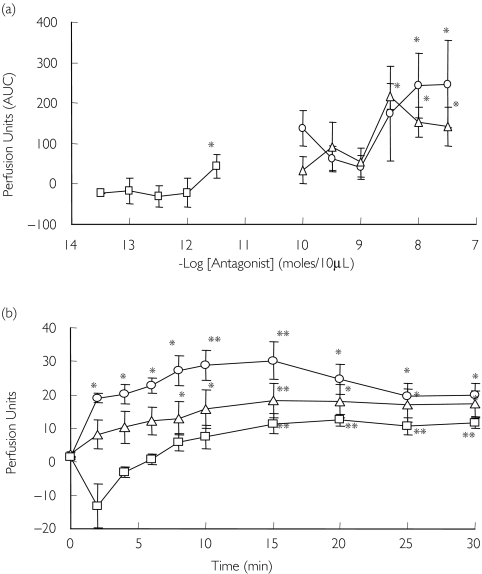
BQ-123 and BQ-788 caused vasodilatation (Figure 3a). Compared with control, a sustained increase in blood flow was caused by BQ-123 (300 nmol; maximum increase 30 ± 5 PU, P = 0.002) and BQ-788 (300 nmol; maximum increase 18 ± 5 PU, P = 0.004) (Figure 3b). Compared with control, phosphoramidon caused a small increase in blood flow at the highest dose (10 pmol; maximum increase 11 ± 2 PU, P = 0.009; Figure 3a, b).
Figure 3.
(a) Dose–response (AUC) to BQ-123 (0.1–30 nmol) (○), BQ-788 (0.1–30 nmol) (▵), phosphoramidon (0.1–10 nmol) (□). (b) Effect of maximum dose of endothelin blockade on skin blood flow; BQ-123 (30 nmol), BQ-788 (30 nmol), phosphoramidon (10 nmol). *P < 0.05; **P < 0.01 vs. placebo
Discussion
In the human skin microcirculation, we have confirmed that ET-1[1–21] is a potent vasoconstrictor and shown for the first time that ET-1[1–31] and big ET-1[1–38] also cause skin vasoconstriction in vivo. Our results suggest that there is ECE activity in the skin as demonstrated by the vasoconstriction following intradermal administration of big ET-1[1–38] and vasodilatation with ECE inhibition. In addition, we have confirmed that selective blockade of the ETA receptor caused skin microvasculatory vasodilatation [2] and shown directly that selective blockade of the ETB receptor also results in vasodilatation. In contrast to observations in resistance vessels, this suggests that ETB receptors in the skin contribute to ET-1-mediated vasoconstriction not only in arterial disease [2] but also in healthy blood vessels.
The novel finding that ET-1[1–31] is a vasoconstrictor is of interest, and the first evidence of its vasoactive properties in vivo in man. If ET-1[1–31] is converted to ET-1[1–21] by a non-ECE pathway and if this contributes importantly to ET-1 generation, then specific receptor blockade may offer greater functional inhibitory activity than ECE inhibition. Alternatively, ET-1[1–31] may have vasoconstricting activity of its own at endothelin receptors. Recently, the vasoconstricting effects of ET-1[1–31] have been shown to be mediated via the ETA receptor in rabbit renal resistance vessels [21], an effect which was unaffected by phosphoramidon. Although ET-1[1–31] is less potent than ET-1[1–21] as a vasoconstrictor, local production of ET-1[1–31] may occur specifically in tissues that express human chymase, such as from the mast cells within the shoulder region of coronary atheromatous plaques [22, 23]. Therefore, generation of ET-1[1–31] could contribute to coronary artery spasm at the time of plaque rupture. Indeed, ET-1[1–31] may be cleaved to ET-1[1–21] for its biological activity in cultured bronchial smooth muscle cells [17] and in both guinea pig [18] and human arteries [16].
The fact that big ET-1[1–38] has affinity for the endothelin A receptor which is 1000-fold less than ET-1[1–21] [20], and that big ET-1[1–38] was ∼30-fold less potent than ET-1[1–21], makes it unlikely that big ET-1[1–38] caused a major direct vasoconstrictor action and suggests that there is some conversion of big ET-1 to ET-1[1–21] in the skin. It has previously been demonstrated that big ET-1[1–38] is vasoactive in human arteries [3] but not veins [25], and that this vasoconstriction can be blocked by ECE inhibition [26]. The presence of ECE activity in the skin is further supported in this present study by the vasodilatory effects of phosphoramidon. This is also consistent with preliminary clinical studies where systemic ECE inhibition caused systemic vasodilatation and a reduction in blood pressure [27]. In our study there appeared to be a transient vasoconstriction to phosporamidon, although this was not consistent and did not reach statistical significance. This observation may warrant further investigation.
Endothelin antagonists have been studied in the skin by other groups. The selective ETA receptor antagonist PD147953 and the nonselective endothelin receptor antagonist PD145065 caused vasodilatation and attenuated the vasoconstrictor effects of ET-1[1–21] in the human skin in vivo [1, 28]. BQ-123 is a selective ETA receptor antagonist and has been shown in many vascular beds [29] to be a vasodilator by blocking the effects of ET-1[1–21]. There are few data on the effects of endothelin antagonists in the skin [1, 2, 28]. The present study demonstrates that BQ-123 causes vasodilatation, confirming that ET-1 contributes to the maintenance of basal vascular tone in the skin of healthy volunteers. BQ-788 also caused skin vasodilatation. This result was unexpected, as selective ETB receptor antagonism causes vasoconstriction in skeletal muscle [30] and when given systemically [31]. In addition, previous data on the skin microcirculation have demonstrated that ET-1 mediates vasoconstriction via the ETA receptor with little contribution from the ETB receptor [28, 32]. However, our data indicate that the dominant effect of ET-1[1–21] on the ETB receptor is vasoconstriction in the human skin microcirculation, and that blockade of the ETB receptor results in vasodilatation. It is possible that some of this effect may be a result of displacement of ET-1 from ETB receptors and a reduction of available ETB receptor binding sites, thereby increasing vasoconstriction via the ETA receptor. This finding may require further investigation.
Endothelin receptor antagonists have proven benefits in patients with primary pulmonary hypertension [7] and reduce blood pressure in hypertensive patients [33]. To date, clinical trials investigating the potential benefits of endothelin receptor antagonists in CHF have been disappointing [8]. ECE inhibitors inhibit endothelin production and provide a potential alternative to endothelin receptor antagonism. Unlike receptor blockade, which can increase plasma ET-1[1–21] concentrations, ECE inhibitors have the advantage that they block ET-1[1–21] production, thus reducing plasma ET-1[1–21] concentrations and leaving clearance receptors unblocked. However, the effects of raised plasma concentration of ET-1[1–21], associated with ETB receptor blockade [30], may not be relevant if ETA receptors are also blocked. However, if there are clinically significant non-ECE pathways of production with vasoactive intermediary peptides such as ET-1[1–31], then ECE inhibition may be a less attractive treatment strategy.
While the doses of peptides used in these experiments are known, the concentration when injected into the skin cannot be measured, due to the unknown volume of distribution in the skin. However, as the same volume of injectate was used for each dose, comparing the relative potencies of each peptide is probably justified. Furthermore, true potency may vary depending on whether compounds are full or partial agonists. Highly selective antagonists of each receptor were not available to us. Although BQ-788 might have blocked ETA receptor function, its 100-fold greater affinity for the ETB receptor [34] compared with the ETA receptor, and the equal potency of BQ-123 and BQ-788 in this study, suggest that BQ-788 does indeed mediate vasodilatory effects via blockade of the ETB receptor. Another limitation is that phosphoramidon is not only an inhibitor of ECE but also an inhibitor of neutral endopeptidase (NEP). However, whether in health or cardiovascular disease [3, 35, 36], the inhibition of NEP tends to cause vasoconstriction and therefore if anything lead to an underestimate of the vasodilator effects of selective ECE inhibition.
In summary, the discovery of a vasoactive intermediary endothelin peptide may have importance for the further development of endothelin blockers as clinical therapies. The skin microcirculation provides an opportunity to investigate the vasoactive properties of a compound, in vivo, in a relatively safe manner due to the small doses which are administered. However, further studies are required to determine whether ET-1[1–31] is vasoactive in other vascular beds. In particular it will be of interest to note whether ET-1[1–31] causes vasoconstriction in resistance blood vessels.
Acknowledgments
S.J.L. was supported by a British Heart Foundation Junior Research Fellowship (FS/98040).
References
- 1.Wenzel RR, Noll G, Luscher TF. Endothelin receptor antagonists inhibit endothelin in human skin microcirculation. Hypertension. 1994;23:581–6. doi: 10.1161/01.hyp.23.5.581. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel RR, Duthiers N, Noll G, Bucher J, Kaufmann U, Luscher TF. Endothelin and calcium antagonists in the skin microcirculation of patients with coronary artery disease. Circulation. 1996;94:316–22. doi: 10.1161/01.cir.94.3.316. [DOI] [PubMed] [Google Scholar]
- 3.Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;334:852–4. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- 4.Goddard J, Johnston NR, Hand MF, Cumming AD, Rabelink TJ, Rankin AJ, Webb DJ. Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure. A comparison of selective and combined Endothelin receptor blockade. Circulation. 2004. (in press) [DOI] [PubMed]
- 5.McCulloch KM, Docherty C, MacLean MR. Endothelin receptors mediating contraction of rat and human pulmonary resistance arteries: effect of chronic hypoxia in the rat. Br J Pharmacol. 1998;123:1621–30. doi: 10.1038/sj.bjp.0701785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei CM, Lerman A, Rodeheffer RJ, et al. Endothelin in human congestive heart failure. Circulation. 1994;89:1580–6. doi: 10.1161/01.cir.89.4.1580. [DOI] [PubMed] [Google Scholar]
- 7.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 8.Coletta A, Thackray S, Nikitin N, Cleland JG. Clinical trials update: highlights of the scientific sessions of the American College of Cardiology 2002: LIFE, DANAMI 2, MADIT-2, MIRACLE-ICD, OVERTURE, OCTAVE, ENABLE 1 and 2, CHRISTMAS, AFFIRM, RACE, WIZARD, AZACS, REMATCH, BNP trial and HARDBALL. Eur J Heart Fail. 2002;4:381–8. doi: 10.1016/s1388-9842(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 9.Nakano A, Kishi F, Minami K, Wakabayashi H, Yutaka N, Kido H. Selective conversion of big endothelins to tracheal smooth muscle-constricting 31 amino acid-length endothelins by chymase from human mast cells. J Immunol. 1997;159:1987–92. [PubMed] [Google Scholar]
- 10.Yoshizumi M, Inui D, Okishima N, et al. Endothelin-1-(1–31), a novel vasoactive peptide, increases [Ca2+]i in human coronary artery smooth muscle cells. Eur J Pharmacol. 1998;348:305–9. doi: 10.1016/s0014-2999(98)00158-7. [DOI] [PubMed] [Google Scholar]
- 11.Inui D, Yoshizumi M, Okishima N, et al. Mechanism of endothelin-1-(1–31)-induced calcium signaling in human coronary artery smooth muscle cells. Am J Physiol. 1999;276:E1067–72. doi: 10.1152/ajpendo.1999.276.6.E1067. [DOI] [PubMed] [Google Scholar]
- 12.Yasuoka H, Yoshizumi M, Inui D, et al. Effect of endothelin-1(1–31) on intracellular free calcium in cultured human mesangial cells. Life Sci. 1999;65:267–72. doi: 10.1016/s0024-3205(99)00509-3. [DOI] [PubMed] [Google Scholar]
- 13.Kishi F, Minami K, Okishima N, et al. Novel 31-amino-acid-length endothelins cause constriction of vascular smooth muscle. Biochem Biophys Res Commun. 1998;248:387–90. doi: 10.1006/bbrc.1998.8980. [DOI] [PubMed] [Google Scholar]
- 14.Takai S, Shiota N, Jin D, Miyazaki M. Chymase processes big-endothelin-2 to endothelin-2-(1–31) that induces contractile responses in the isolated monkey trachea. Eur J Pharmacol. 1998;358:229–33. doi: 10.1016/s0014-2999(98)00622-0. [DOI] [PubMed] [Google Scholar]
- 15.Takeji T, Nakaya Y, Kamada M, et al. Effect of a novel vasoconstrictor endothelin-1(1–31) on human umbilical artery. Biochem Biophys Res Commun. 2000;270:622–4. doi: 10.1006/bbrc.2000.2476. [DOI] [PubMed] [Google Scholar]
- 16.Maguire JJ, Kuc RE, Davenport AP. Vasoconstrictor activity of novel endothelin peptide, ET-1(1–31) in human mammary and coronary arteries in vitro. Br J Pharmacol. 2001;134:1360–6. doi: 10.1038/sj.bjp.0704384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayasaki-Kajiwara Y, Naya N, Shimamura T, Iwasaki T, Nakajima M. Endothelin generating pathway through endothelin 1–31 in human cultured bronchial smooth muscle cells. Br J Pharmacol. 1999;127:1415–21. doi: 10.1038/sj.bjp.0702664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honore JC, Plante M, Bkaily G, Rae GA, D’Orleans-Juste P. Pressor and pulmonary responses to ET-1(1–31) in guinea-pigs. Br J Pharmacol. 2002;136:819–28. doi: 10.1038/sj.bjp.0704782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ihara M, Noguchi K, Saeki T, et al. Biological profiles of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci. 1992;50:247–55. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa K, Ihara M, Noguchi K, et al. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci USA. 1994;91:4892–6. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozawa Y, Hasegawa T, Tsuchiya K, Yoshizumi M, Tamaki T. Effect of endothelin-1(1–31) on the renal resistance vessels. J Med Invest. 2003;50:87–94. [PubMed] [Google Scholar]
- 22.Kaartinen M, Penttila A, Kovanen P. Accumulation of activated mast cells in the shoulder region of human atheroma, the predilection site of atheromatous rupture. Circulation. 1994;90:1669–78. doi: 10.1161/01.cir.90.4.1669. [DOI] [PubMed] [Google Scholar]
- 23.Kovanen P, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92:1084–8. doi: 10.1161/01.cir.92.5.1084. [DOI] [PubMed] [Google Scholar]
- 24.Gray GA, Webb DJ. The endothelin system and its potential as a therapeutic target in cardiovascular disease. Pharmacol Ther. 1996;72:109–48. doi: 10.1016/s0163-7258(96)00101-5. [DOI] [PubMed] [Google Scholar]
- 25.Haynes WG, Strachan FE, Webb DJ. Endothelin ETA and ETB receptors cause vasoconstriction of human and capacitance vessels in vivo. Circulation. 1995;92:357–63. doi: 10.1161/01.cir.92.3.357. [DOI] [PubMed] [Google Scholar]
- 26.Haynes WG, Hand MF, Johnstone HA, Padfield PL, Webb DJ. Direct and sympathetically mediated venoconstriction in essential hypertension: enhanced response to endothelin. J Clin Invest. 1994;94:1359–64. doi: 10.1172/JCI117470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Voogd H, Viskoper R, Taylor S, Harding C, van Kleef E, Essers H. SLV 306, an orally active combined neutral endopeptidase/endothelin converting enzyme inhibitor, lowers blood pressure in essential hypertension. 7th International Conference on Endothelin.2001. [Google Scholar]
- 28.Wenzel RR, Ruthemann J, Bruck H, Schafers RF, Michel MC, Philipp T. Endothelin-A receptor antagonist inhibits angiotensin II and noradrenaline in man. Br J Clin Pharmacol. 2001;52:151–7. doi: 10.1046/j.0306-5251.2001.01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berrazueta JR, Bhagat K, Vallance P, MacAllister RJ. Dose- and time-dependency of the dilator effects of the endothelin antagonist, BQ-123, in the human forearm. Br J Clin Pharmacol. 1997;44:569–71. doi: 10.1046/j.1365-2125.1997.t01-1-00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhaar MC, Strachan FE, Newby DE, et al. Endothelin-A receptor antagonist mediated vasodilation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation. 1998;97:752–6. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- 31.Strachan FE, Spratt JC, Wilkinson IB, et al. Systemic blockade of the endothelin-B receptor increases peripheral vascular resistance in healthy men. Hypertension. 1999;33:581–5. doi: 10.1161/01.hyp.33.1.581. [DOI] [PubMed] [Google Scholar]
- 32.Lipa JE, Neigan PC, Perreault TM, et al. Vasoconstrictor effect of endothelin-1 in human skin: role of ETA and ETB receptors. Am J Physiol. 1999;276:H359–67. doi: 10.1152/ajpheart.1999.276.2.H359. [DOI] [PubMed] [Google Scholar]
- 33.Krum H, Viskoper RJ, Lacourciere Y, et al. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. N Engl J Med. 1998;338:784–90. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 34.Russell FD, Davenport AP. Characterization of the binding of endothelin ETB selective ligands in human and rat heart. Br J Pharmacol. 1996;119:631–6. doi: 10.1111/j.1476-5381.1996.tb15720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spratt JCS, Goddard J, Patel N, et al. Systemic ETA receptor antagonism with BQ-123 blocks ET-A induced forearm vasoconstriction and reduced peripheral vascular resistance in healthy men. Br J Pharmacol. 2001;134:648–54. doi: 10.1038/sj.bjp.0704304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kentsch M, Otter W, Drummer C, Nötges A, Gerzer R, Müller-Esch G. Neutral endopeptidase 24.11 inhibition may not exhibit beneficial haemodynamic effects in patients with congestive heart failure. Eur J Clin Pharmacol. 1996;51:269–72. doi: 10.1007/s002280050196. [DOI] [PubMed] [Google Scholar]