Abstract
Aims
Fosfluconazole is a phosphate prodrug of fluconazole (FLCZ). This study was conducted to investigate the effect of renal impairment on the pharmacokinetics of fosfluconazole and FLCZ, and to assess the safety and toleration of fosfluconazole following a single intravenous bolus injection of fosfluconazole in subjects with normal and impaired renal function.
Methods
In an open, parallel-group, two-centre study, subjects with normal and impaired renal function received a single 1000-mg bolus intravenous injection of fosfluconazole. Subjects were categorized as Normal (> 80 ml min−1), Mild (51–80 ml min−1), Moderate (30–50 ml min−1) or Severe (< 30 ml min−1) impairment group according to their Cockcroft and Gault creatinine clearance (CLcr) values. Concentrations of fosfluconazole and FLCZ were determined in plasma and urine samples taken up to 240 h and 48 h postdose, respectively.
Results
Fosfluconazole plasma concentrations were very similar across the four groups, and there was no apparent relationship between any of the fosfluconazole pharmacokinetic parameters with increasing renal impairment. The conversion of fosfluconazole to FLCZ was unaffected by the degree of renal impairment. Only small amounts of fosfluconazole were excreted in the urine suggesting almost complete conversion to FLCZ. FLCZ concentrations were still detected in plasma after 240 h postdose and remained higher at the later sampling times in subjects in the Moderate and Severe groups. The area under the plasma concentration vs. time curve between time zero and infinity (AUC), the terminal elimination phase half-life (t1/2) and the mean residence time (MRT) of FLCZ all increased with the degree of renal impairment. The ratios (95% confidence interval) for AUC (Renal impairment group/Normal group) were 112.8% (89.5, 142.1), 240.6% (128.2, 451.4) and 355.1% (259.3, 486.3) for the Mild, Moderate and Severe impairment groups, respectively. There was a linear relationship between CLcr with AUC, t1/2, MRT and the total plasma clearance of FLCZ (CL/F). Both the amount excreted over 48 h in the urine and the renal clearance of FLCZ decreased with an increase in renal impairment. The adverse events reported were mild to moderate in intensity, and there was no observed relationship with impairment group. There were no severe or serious adverse events, and in general fosfluconazole was well tolerated.
Conclusions
The pharmacokinetics of fosfluconazole, including its efficient conversion into FLCZ, were unaffected by renal impairment. For FLCZ, there was a significant linear relationship between CLcr and AUC, t1/2, MRT and CL/F, with AUC, t1/2 and MRT increasing and CL/F decreasing as renal impairment increased. The dose adjustment used for FLCZ (half normal dose for patients with CLcr at ≤50 ml min−1) can be applied to fosfluconazole as well. There were no safety concerns for any subject in this study, and fosfluconazole and FLCZ were well tolerated by all the treatment groups.
Keywords: fluconazole, fosfluconazole, pharmacokinetics, renal impairment, safety
Introduction
Fluconazole (FLCZ, Figure 1) is an antifungal agent which is efficacious in the treatment of patients with serious systemic fungal infections [1–19]. FLCZ is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug [20]. The pharmacokinetics of FLCZ are markedly affected by reduction in renal function [21, 22]. The current intravenous (i.v.) dosage form requires the administration of a high-volume infusion which is undesirable in critically ill patients in whom fluid overload must be avoided. There is, consequently, a need for a small-volume high-dose formulation of FLCZ.
Figure 1.
Chemical structures of fluconazole and fosfluconazole
Fosfluconazole (Figure 1) is a phosphate prodrug of FLCZ which is highly soluble (> 100 mg ml−1 in the proposed vehicle) compared with FLCZ (4 mg ml−1) [23]. In vitro, fosfluconazole is at least 25-fold less potent than FLCZ against single isolates of Candida species and Cryptococcus neoformans, but in vivo has similar efficacy to FLCZ in experimental models of fungal disease. These observations are consistent with efficient conversion of fosfluconazole to FLCZ in vivo. The hydrolysis rate of fosfluconazole to FLCZ in tissue was significantly faster than chemical hydrolysis in solution and has been shown to be mediated by phosphatase enzymes. Fosfluconazole is rapidly converted in vitro to FLCZ in homogenates of kidney, lung and liver of rat, dog and human. It is known that phosphatases are found in high concentrations in liver, lung and kidney [24]. However, in vivo, fosfluconazole has insufficient lipophilicity to readily cross cell membranes passively, and therefore is most likely to be efficiently hydrolysed in tissues where phosphatases are expressed extracellularly, for example the brush border membranes in the kidney proximal tubule [25, 26].
In the previous clinical studies in healthy volunteers [27], fosfluconazole was rapidly and almost completely converted to FLCZ with only minor amounts excreted in the urine, had a volume of distribution at the higher doses which was similar to the extracellular volume (0.2 l kg−1), and was eliminated with a terminal half-life of approximately 2.3 h. There was apparent dose proportionality in FLCZ pharmacokinetics. Bolus i.v. injections of fosfluconazole were well tolerated at doses of up to 2000 mg in healthy subjects. It is anticipated that fosfluconazole will be used for the treatment of systemic fungal infections in patients who may have impaired renal function. It was therefore necessary to investigate whether the conversion of fosfluconazole to FLCZ is influenced by renal impairment to allow dosing recommendations to be made.
In this study, subjects with normal and impaired renal function received a 1000-mg bolus i.v. injection of fosfluconazole to investigate the effect of renal impairment on the pharmacokinetics, safety and toleration of fosfluconazole and FLCZ.
Methods
Subjects
Subjects who gave written informed consent underwent an examination. Male and female subjects aged 18–75 years were eligible for inclusion in the study, provided that they weighed 55–100 kg and were within the permitted weight range for their height and frame according to Quetelet's index [28] (normal subject 18–28 kg m−2, subject with renal impairment 18–32 kg m−2).
Female subjects had to be either postmenopausal (2 years after the last period) or surgically sterilized, and have a negative pregnancy test result immediately prior to dosing.
Subjects were allocated to a renal impairment group on the basis of CLcr calculated from serum creatinine concentration during the screening period using the Cockcroft and Gault equation [29]. Subjects without renal impairment (CLcr of >80 ml min−1) were classified as ‘Normal’. Subjects with ‘Mild’ impairment, with ‘Moderate’ impairment and with ‘Severe’ impairment were to have a CLcr of 51–80 ml min−1, 30–50 ml min−1, and <30 ml min−1. The investigator discussed with sponsor any CLcr results that were at the borderline of the ranges for different groups prior to subject inclusion. Subjects with renal impairment had various types of chronic renal dysfunction with the exception of nephrotic syndrome.
Volunteers were excluded if evidence of clinically significant disease (which was not related to underlying renal disease for subjects with renal impairment) or clinically significant allergies (especially drug hypersensitivity) were observed. In addition, volunteers were excluded if they had received any experimental drug within the past 4 months; had evidence of drug abuse; were HIV+; were hepatitis B surface antigen-positive. Hepatitis B core antibody results should also have been negative, but a positive result could be accepted at the discretion of the investigator. Women who drank more than 21 units of alcohol per week or men who drank more than 28 units per week were excluded. Subjects with renal impairment were excluded if they had received a renal transplant; were clinically nephrotic; were on dialysis; had liver function test results which were >1.2 times the upper end of the reference range; were taking drugs which are known to interact with FLCZ. The concurrent administration of all usual chronically administered drugs used to treat renal failure was allowed.
Study design
This was an open, parallel-group study in subjects with normal and impaired renal function. All subjects received a single 1000-mg bolus i.v. injection of fosfluconazole.
The study was performed at two centres, the Aster Clinical Research Centre (Paris, France) and Apex GmBH (Munich, Germany), in compliance with the ethical principles originating from the revised Declaration of Helsinki (South Africa, 1996). The clinical study protocol was approved prior to the start of the study, by a local Independent Ethics Review Committee, CCPPRB Pitié Salpetrière (Paris, France), Bayerische Landesärztekammer (Munich, Germany) and Unabhängige Ethikkomission Schwaben (Danube, Germany).
Drug administration
Fosfluconazole was supplied as 100 mg ml−1 solutions in 10-ml ampoules and administered manually as a bolus i.v. injection via an indwelling cannula inserted into a forearm vein of the subjects. The dose was given over a maximum duration of 2 min while the subjects were semirecumbent.
Pharmacokinetic sampling
Blood samples (5 ml) for assay of fosfluconazole and FLCZ were collected in heparinized tubes at predose and at 5, 15, 30 and 45 min and 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 16, 24, 30, 36, 48, 72, 96, 120, 168 and 240 h postdose. An additional 5 ml of blood were taken at 15 min and 1 h postdose for protein binding analysis. Within 1 h of collection the blood samples were centrifuged at 1500 g at 4 °C for 10 min. The resulting plasma was stored in screw-capped polypropylene tubes on ice before being frozen at −70 °C or below.
Subjects emptied their bladders immediately prior to dosing. A 10-ml aliquot of the predose sample was retained for pharmacokinetic analysis. Urine collections were then made from 0–4, 4–8, 8–24 and 24–48 h following the start of the i.v. injection. Urine volumes were measured and recorded. Samples within each collection period were well mixed, and a 10-ml aliquot taken for fosfluconazole and FLCZ analysis. Aliquots were stored at −70 °C or below within 30 min of completion of the collection.
Drug assay
Plasma, urine and ultrafiltrate concentrations of fosfluconazole and FLCZ were determined at Maxxam Analytics Inc. (Mississauga, Ontario, Canada). Plasma samples for protein binding were supplied to Quintiles Scotland Ltd (Edinburgh, UK) for ultrafiltration.
The analytical procedure involved solid-phase extraction of the analytes with separation by liquid chromatography, followed by atmospheric pressure ionization and tandem mass spectrometric detection (API LC/MS/MS). Urine samples were diluted with plasma (1 : 9).
The lower limit of quantification was 0.2 µg ml−1 in plasma and ultrafiltrates and 1.0 µg ml−1 in urine, while the upper limit of the calibration curve was 15 µg ml−1 for fosfluconazole and FLCZ in plasma and ultrafiltrates, and 100 µg ml−1 in urine.
During the study the overall method imprecision values for the analysis of plasma quality control samples (coefficient of variation, CV%) were 9.2, 7.3 and 8.7% for fosfluconazole and 6.3, 5.7 and 10.7% for FLCZ at target fosfluconazole and FLCZ concentrations of 0.60, 6.00 and 12.0 µg ml−1, respectively. The inaccuracy (bias) of the assay at all concentrations ranged from −4.2 to 16.7% for fosfluconazole and −4.2 to 6.7% for FLCZ.
The overall method imprecision values for the analysis of urine quality control samples (CV%) were 11.5, 10.0 and 6.3% for fosfluconazole and 9.8, 10.8 and 5.1% for FLCZ at target fosfluconazole and FLCZ concentrations of 3.00, 30.0 and 80.0 µg ml−1, respectively. The inaccuracy (bias) of the assay at all concentrations ranged from −6.1 to 4.7% for fosfluconazole and −7.0 to 1.6% for FLCZ.
The overall method imprecision values for the analysis of plasma protein binding and ultrafiltrate quality control samples (CV%) were 5.5, 5.2 and 6.2% for fosfluconazole and 9.2, 5.7 and 7.3% for FLCZ at target fosfluconazole and FLCZ concentrations of 0.60, 6.00 and 12.0 µg ml−1, respectively. The inaccuracy (bias) of the assay at all concentrations ranged from −8.0 to 13.0% for fosfluconazole and −11.8 to 2.8% for FLCZ.
Pharmacokinetic analysis
The pharmacokinetic analysis of fosfluconazole and FLCZ plasma concentrations was performed by noncompartmental methods. The maximum observed plasma concentration (Cmax) and the first time to Cmax (Tmax) of FLCZ were taken directly from the recorded plasma concentration vs. time curve. The terminal elimination phase half-life (t1/2) was calculated as ln2/kel, where kel is the terminal rate constant, calculated by ordinary least squares linear regression, using points in the linear terminal portion of the log concentration vs. time curve. The area under the plasma concentration vs. time curve between time zero and infinity (AUC) was calculated as AUCt + (Ct/kel), where AUCt is the area under the plasma concentration vs. time curve between time zero and time t of the last quantifiable concentration, calculated by the linear trapezoidal method, and Ct is the last quantifiable concentration at time t, estimated by linear regression analysis. The mean residence time (MRT) was estimated by AUMC/AUC, where AUMC is the area under the first-moment of the concentration vs. time curve with extrapolation to infinity. The total plasma clearance (CL) was calculated as Dose/AUC. For FLCZ this was expressed as CL/F (F was the fraction of fosfluconazole converted to FLCZ). The volume of distribution at steady state (Vss) of fosfluconazole was estimated as CL × MRT. The amount of FLCZ excreted unchanged in the urine to 48 h (Ae48) was calculated as the concentration in the urine × volume and is also reported as a percentage recovery of the dose administered [Ae48 (%) = 100 × Ae48/dose]. This is reported as Ae (%) for fosfluconazole. Renal clearance (CLR) of FLCZ was calculated as Ae48/AUC48, where AUC48 is the area under the concentration vs. time curve from time zero to 48 h postdose.
Protein binding was calculated as
The fraction of fosfluconazole unbound (fu) was calculated as
where ppb0.25 and ppb1 are the percentages of protein bound at 15 min and 1 h.
The clearance of unbound drug (CLu) was calculated as CL/fu.
CLcr was calculated at screening from the Cockcroft and Gault equation as shown:
where G = 0.85 (females) or 1.00 (males).
The measured CLcr was calculated from serum creatinine (from the 48 h blood sample), urine creatinine and urine volume (from the 24–48 h postdose sample) as shown:
Safety assessments
All observed or subjective adverse events were ascertained by nonleading questioning by the investigator and were recorded. Classification by body system was according to the Coding Symbol Thesaurus of Adverse Reaction Terms (COSTART) publication.
Blood and urine samples for laboratory safety tests were collected at prestudy screening, predose, 48 and 168 h postdose and at follow-up 21 days after dosing. Plasma from the 5, 15, 30 min, 1, 2, 4, 8 and 24-h samples was aliquoted for calcium and phosphate measurements. Laboratory test abnormalities were evaluated for clinical significance against sponsor-defined criteria based on normal ranges.
Supine blood pressure (after 5 min supine), supine pulse rate and a 12-lead ECG was taken at screening, predose, 1, 2, 4, 8, 48 and 168 h postdose and at follow-up.
Statistical analysis
Linear regression models were used to assess the relationship between CLcr and FLCZ (AUC, CL/F, Cmax, Tmax, t1/2 and MRT) or fosfluconazole (CL, CLu, Vss and MRT) pharmacokinetic parameters. Separate analyses were conducted using the Cockcroft and Gault CLcr values and the measured CLcr values. The fosfluconazole parameters AUC, CL, Vss, t1/2 and MRT were subjected to one-way analysis of variance (anova) fitting impairment group as a factor. Group comparisons were carried out for the FLCZ parameters AUC, Cmax, Tmax, t1/2, and MRT using t-tests with unequal group variances (as the assumption of equal variances across the groups was violated). The differences between the renally impaired group means and the normal group mean were calculated, along with 95% confidence intervals (CI). AUC and Cmax were log-transformed prior to analysis, giving differences between the subject groups as ratio estimates when back-transformed.
All statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC, USA), version 6.09 [30].
Results
Demographics
A summary of the demographic characteristics is presented in Table 1. Twenty-five subjects (15 males and 10 females) entered the study and all subjects completed the study and were analysed for pharmacokinetics and safety. There were six subjects in the Normal, Moderate and Severe groups, respectively, and seven subjects allocated to the Mild group. In the Severe group it was not possible to recruit any female subjects. Three subjects having borderline Cockcroft and Gault CLcr values of 52, 53 and 52 ml min−1 were included in the Moderate Group. A further subject having a Cockcroft and Gault CLcr of 30 ml min−1 was accepted into the Severe category. The possibility of accepting subjects having borderline CLcr values into one group instead of another was discussed in the Protocol, and all allocations to the different impairment groups were made at screening. Two were black (one female in the Mild group and one male in the Severe group) and 23 were white. Hypertension was present in four subjects in the Moderate group, and all six subjects in the Severe group, compared with only one subject in each of the Normal and Mild groups. One subject in the Normal group, four subjects in the Mild group and all subjects in the Moderate and Severe groups received concomitant medications during the course of the study.
Table 1.
Mean (range) demographic characteristics
| Age (years) | Weight (kg) | Height (cm) | CLcr (ml min−1) | |
|---|---|---|---|---|
| Normal | ||||
| Male (n = 4) | 55.3 (46–69) | 78.8 (67–92) | 174.0 (166–182) | |
| Female (n = 2) | 47.0 (46–47) | 70.9 (64–78) | 170.5 (167–174) | |
| Total (n = 6) | 52.6 (46–69) | 93 (82–109) | ||
| Mild | ||||
| Male (n = 3) | 62.1 (60–65) | 74.2 (63–81) | 176.0 (172–180) | |
| Female (n = 4) | 59.8 (50–74) | 64.4 (55–75) | 161.0 (151–174) | |
| Total (n = 7) | 60.8 (50–74) | 64 (55–78) | ||
| Moderate | ||||
| Male (n = 2) | 42.0 (29–54) | 66.2 (58–75) | 170.5 (165–176) | |
| Female (n = 4) | 62.5 (46–74) | 68.1 (59–80) | 162.0 (154–170) | |
| Total (n = 6) | 55.7 (29–74) | 47 (38–53) | ||
| Severe | ||||
| Male (n = 6) | 48.4 (36–63) | 72.9 (61–80) | 178.2 (172–182) | 19 (11–30) |
CLcr, Creatinine clearance calculated from Cockcroft and Gault equation.
Pharmacokinetics
Fosfluconazole
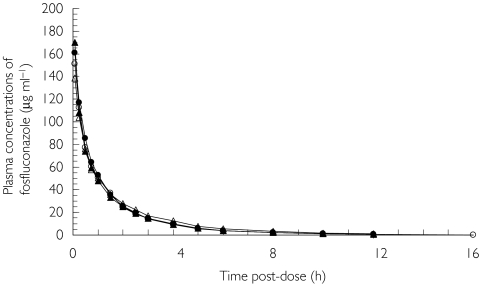
The mean plasma concentration profiles of fosfluconazole are illustrated by group in Figure 2. The maximum recorded concentrations of fosfluconazole occurred at the first sampling time (5 min postdose), and there was no fosfluconazole detected at 24 h or more postdose. Fosfluconazole plasma concentration profiles were very similar across the four groups.
Figure 2.
Mean plasma concentration profiles of fosfluconazole after intravenous injection of fosfluconazole in subjects with normal renal function (○), n = 6; mild renal impairment (▴), n = 7; moderate renal impairment (•), n = 6; and severe renal impairment (Δ), n = 6
The pharmacokinetic parameters AUC, CL, CLu, Vss, t1/2, MRT and Ae for fosfluconazole are summarized in Table 2 and the results of the anova analysis are presented in Table 3. There was no apparent relationship between any of the fosfluconazole pharmacokinetic parameters with increasing renal impairment. Ninety-five percent confidence intervals suggest that no clinically relevant difference is likely to exist between any of the Renal impairment groups and the Normal group.
Table 2.
Mean ± SD fosfluconazole pharmacokinetic parameters after intravenous injection of fosfluconazole in subjects with normal, mild, moderate or severe renal impairment
| Parameter | AUC (µg-h ml−1) | CL (ml min−1 kg−1) | CLu (ml min−1 kg−1) | Vss (l kg−1) | t1/2 (h) | MRT (h) | Ae (%) |
|---|---|---|---|---|---|---|---|
| Normal | 177.4 (173.4) | 1.28 ± 0.230 | 30.48 ± 8.760 | 0.15 ± 0.023 | 2.1 ± 0.48 | 2.0 ± 0.45 | 1.8 ± 1.54 |
| (n = 6) | ± 40.58 | ||||||
| Mild | 175.9 (171.2) | 1.49 ± 0.483 | 27.50 ± 10.657 | 0.17 ± 0.022 | 2.0 ± 0.44 | 2.1 ± 0.50 | 2.5 ± 1.50 |
| (n = 7) | ± 43.09 | ||||||
| Moderate | 180.7 (178.3) | 1.42 ± 0.265 | 28.24 ± 6.630 | 0.15 ± 0.030 | 2.0 ± 0.20 | 1.8 ± 0.36 | 2.0 ± 1.43 |
| (n = 6) | ± 32.79 | ||||||
| Severe | 189.7 (179.0) | 1.37 ± 0.484 | 27.20 ± 14.185 | 0.16 ± 0.025 | 2.0 ± 0.63 | 2.2 ± 0.82 | 0.4 ± 0.34 |
| (n = 6) | ± 70.85 |
AUC, Area under the plasma concentration vs. time curve between time zero and infinity; CL, total plasma clearance; CLu, clearance of unbound drug; Vss, volume of distribution at steady state; t1/2, terminal elimination phase half-life; MRT, mean residence time; Ae, amount excreted in the urine. The arithmetic mean (geometric mean) for AUC and the arithmetic means for all other parameters are presented.
Table 3.
Ratio or difference between means (95% CIs) of fosfluconazole pharmacokinetic parameters after intravenous injection of fosfluconazole in subjects with normal, mild, moderate or severe renal impairment
| Parameter | AUC (µg-h ml−1) | CL (ml min−1 kg−1) | Vss (l kg−1) | t1/2 (h) | MRT (h) |
|---|---|---|---|---|---|
| Mild | 98.7% | 0.21 | 0.02 | −0.1 | 0.1 |
| (72.3, 134.8) | (−0.24, 0.66) | (−0.005, 0.05) | (−0.6, 0.5) | (−0.6, 0.7) | |
| Moderate | 102.8% | 0.14 | 0.003 | −0.1 | −0.1 |
| (74.4, 142.1) | (−0.33, 0.61) | (−0.03, 0.03) | (−0.7, 0.4) | (−0.8, 0.5) | |
| Severe | 103.2% | 0.08 | 0.01 | −0.1 | 0.3 |
| (74.7, 142.7) | (−0.39, 0.55) | (−0.02, 0.04) | (−0.6, 0.5) | (−0.4, 0.9) |
AUC, Area under the plasma concentration vs. time curve between time zero and infinity; CL, total plasma clearance; Vss, volume of distribution at steady state; t1/2, terminal elimination phase half-life; MRT, mean residence time. The ratio (Renal impairment group/Normal group) of the means for AUC and the difference (Renal impairment group –Normal group) between the means for all other parameters are presented.
Protein binding for fosfluconazole varied from 85.1 to 96.1% for samples taken at 0.25 h and from 93.1 to 97.6% for the samples at 1 h postdose. As the degree of protein binding for fosfluconazole was high, the clearance of unbound fosfluconazole (CLu) compared with the CL was consequently high. There was no apparent relationship between the fosfluconazole CLu and renal impairment.
The results of the regression analyses did not indicate a linear relationship between CLcr and any of the fosfluconazole pharmacokinetic parameters CL, CLu, Vss, MRT; R2 was ≤0.04 for all regression analyses (R2 is the regression coefficient).
The majority detected in the urine was excreted in the first 0–4 h urine collection period, although 13 subjects (two subjects in the Normal group, two in the Mild group, five in the Moderate group and four subjects in the Severe group) still had quantifiable levels of fosfluconazole in the second (4–8 h) collection period. One subject in the Severe group had no quantifiable level of fosfluconazole over the whole collection period. The amount of fosfluconazole excreted (as a percentage of dose) was lowest in the Severe group (Table 2).
Fluconazole
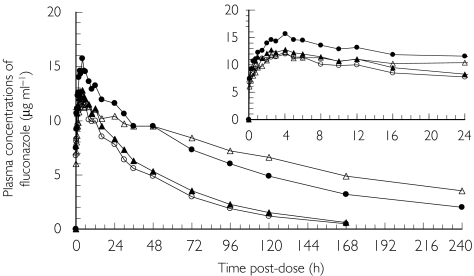
The mean plasma concentration profiles of FLCZ are presented by group in Figure 3. FLCZ was detected in the plasma of all subjects up to and including 120 h postdose. FLCZ was detected in 24 subjects (six subjects in each group) at 168 h postdose and in 16 subjects (two in the Normal group, three in the Mild group, five in the Moderate group and six in the Severe group) at 240 h postdose. Thus plasma concentrations of FLCZ were seen to stay higher at the later sampling times in subjects in the Moderate and Severe groups.
Figure 3.
Mean plasma concentration profiles of fluconazole after intravenous injection of fosfluconazole in subjects with normal renal function (○), n = 6; mild renal impairment (▴), n = 7; moderate renal impairment (•), n = 6; and severe renal impairment (Δ), n = 6
The pharmacokinetic parameters AUC, Cmax, Tmax, t1/2, MRT, CL/F, Ae48 and CLR for FLCZ are shown in Table 4, and the results of the analysis comparing the three impairment groups with the normal group are summarized in Table 5. Mean AUC, t1/2 and MRT all increased with increasing renal impairment. This is consistent with Figure 3, which shows that mean concentrations of FLCZ stayed higher for longer in the groups with greater renal impairment. The ratios (95% CI) of AUC (Renal impairment group/Normal group) were 112.8% (89.5, 142.1), 240.6% (128.2, 451.4) and 355.1% (259.3, 486.3) for the Mild, Moderate and Severe groups, respectively. Comparing t1/2 in the Normal and Mild groups there was only a slight increase observed, but much larger increases were noted in the Moderate (two-fold) and Severe (3.5-fold) groups. Similar fold increases were seen with MRT across the renal impairment groups. The variability in t1/2 and MRT observed in the Moderate and Severe groups was greater than that seen in the Normal and Mild groups. There was no consistent change in FLCZ Cmax with increasing renal impairment. Similar mean Cmax results were seen for the Normal (12.0 µg ml−1), Mild (12.9 µg ml−1) and Severe (12.4 µg ml−1) groups, while Cmax in the Moderate group was higher (15.6 µg ml−1). The estimated ratio (Moderate group/Normal group) was 130.2% (95% CI 102.3, 165.8). There was no change in FLCZ Tmax with increasing renal impairment. The mean CL/F were 0.35, 0.36, 0.21 and 0.11 ml min−1 kg−1 for the Normal, Mild, Moderate and Severe groups, respectively.
Table 4.
Mean ± SD fluconazole pharmacokinetic parameters after intravenous injection of fosfluconazole in subjects with normal, mild, moderate or severe renal impairment
| Parameter | AUC (µg-h ml−1) | Cmax (µg ml−1) | Tmax (h) | t1/2 (h) | MRT (h) | CL/F (ml min−1 kg−1) | Ae48 (%) | CLR (ml min−1 kg−1) |
|---|---|---|---|---|---|---|---|---|
| Normal | 631.0 (628.5) | 12.3 (12.0) | 3.1 ± 1.07 | 36.5 ± 6.06 | 52.8 ± 9.79 | 0.35 ± 0.034 | 42.3 ± 16.65 | 0.21 ± 0.096 |
| (n = 6) | ± 60.31 | ± 2.96 | ||||||
| Mild | 727.2 (709.0) | 13.3 (12.9) | 3.9 ± 1.57 | 38.7 ± 11.95 | 56.4 ± 17.45 | 0.36 ± 0.092 | 44.4 ± 13.17 | 0.22 ± 0.070 |
| (n = 7) | ± 176.24 | ± 3.67 | ||||||
| Moderate | 1748.7 (1512.2) | 15.7 (15.6) | 3.4 ± 0.92 | 79.8 ± 46.46 | 115.7 ± 66.38 | 0.21 ± 0.133 | 25.1 ± 12.53 | 0.11 ± 0.082 |
| (n = 6) | ± 998.95 | ± 2.00 | ||||||
| Severe | 2317.2 (2232.0) | 12.5 (12.4) | 3.4 ± 0.66 | 129.8 ± 34.24 | 189.7 ± 49.03 | 0.11 ± 0.027 | 13.1 ± 5.93 | 0.05 ± 0.028 |
| (n = 6) | ± 692.19 | ± 1.24 |
AUC, Area under the plasma concentration vs. time curve between time zero and infinity; Cmax, maximum observed plasma concentration; Tmax, first time to Cmax; t1/2, terminal elimination phase half-life; MRT, mean residence time; CL/F, total plasma clearance; Ae48, amount excreted in the urine; CLR, renal clearance. The arithmetic mean (geometric mean) for AUC and Cmax, and the arithmetic means for all other parameters are presented.
Table 5.
Ratio or difference between means (95% CIs) of fluconazole pharmacokinetic parameters after intravenous injection of fosfluconazole in subjects with normal, mild, moderate or severe renal impairment
| Parameter | AUC (µg-h ml−1) | Cmax (µg ml−1) | Tmax (h) | t1/2 (h) | MRT (h) |
|---|---|---|---|---|---|
| Mild | 112.8% | 107.7% | 0.8 | 2.2 | 3.6 |
| (89.5, 142.1) | (80.0, 145.0) | (−0.8, 2.5) | (−9.4, 13.8) | (−13.7, 20.9) | |
| Moderate | 240.6% | 130.2% | 0.3 | 43.3 | 62.9 |
| (128.2, 451.4) | (102.3, 165.8) | (−1.0, 1.6) | (−5.4, 92.0) | (−6.6, 132.4) | |
| Severe | 355.1% | 103.4% | 0.3 | 93.3 | 136.9 |
| (259.3, 486.3) | (81.8, 130.6) | (−0.8, 1.5) | (57.4, 129.1) | (85.6, 188.2) |
AUC, Area under the plasma concentration vs. time curve between time zero and infinity; Cmax, maximum observed plasma concentration; Tmax, first time to Cmax; t1/2, terminal elimination phase half-life; MRT, mean residence time. The ratio (Renal impairment group/Normal group) of the means for AUC and Cmax, and the difference (Renal impairment group − Normal group) between the means for all other parameters are presented.
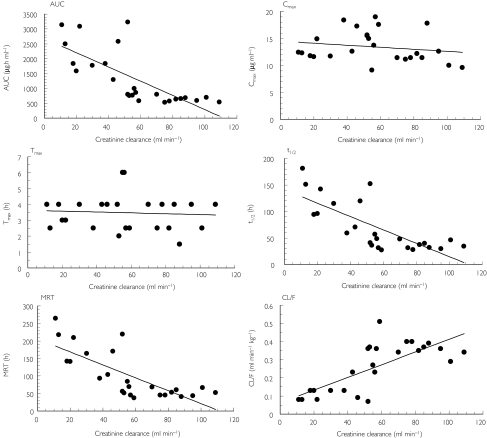
Results from the regression analysis of FLCZ pharmacokinetic parameters with CLcr suggest that there was a significant linear association (P < 0.01) between AUC, CL/F, t1/2, and MRT with Cockcroft and Gault CLcr (R2 ≥ 0.54, Figure 4) and measured CLcr (R2 ≥ 0.35). R2 for all regression analyses for Cmax or Tmax were ≤0.034. The models obtained from the regression analysis for CL/F were as follows:
Figure 4.
Results from the regression analysis of fluconazole pharmacokinetic parameters with Cockcroft and Gault creatinine clearance
FLCZ was detected in all urine collection periods (last collection period 24–48 h postdose). Both the amount excreted over 48 h in the urine and CLR decreased with an increase in renal impairment.
Safety
There were no serious adverse events, and no discontinuations due to adverse events. There was no observed relationship across the groups with few treatment emergent adverse events (Table 6). Treatment-related adverse events were mild to moderate in intensity and all resolved within 14 h without further treatment.
Table 6.
Incidence of treatment-emergent adverse events and clinically significant laboratory test abnormalities after intravenous injection of fosfluconazole in subjects with normal, mild, moderate or severe renal impairment
| Number of subjects with | Normal (n = 6) | Mild (n = 7) | Moderate (n = 6) | Severe (n = 6) |
|---|---|---|---|---|
| Adverse events | ||||
| All causality | 2 | 1 | 1 | 2 |
| Treatment related | 2 | 1 | 1 | 1 |
| Headache | 1 | 1 | 1 | 0 |
| Diarrhoea | 0 | 1 | 0 | 1 |
| Paresthsia | 1 | 0 | 0 | 0 |
| Clinically significant laboratory abnormalities | ||||
| With regard to normal baseline | 0 | 0 | 2 | 3 |
| Potassium (> 1.1 × ULN) | 0 | 0 | 0 | 2 |
| Phosphate (> 1.2 × ULN) | 0 | 0 | 0 | 1 |
| Urine blood (≥ 1 +) | 0 | 0 | 2 | 0 |
| Urea (> 1.3 × ULN) | 0 | 0 | 1 | 0 |
| With regard to abnormal baseline | 0 | 0 | 2 | 4 |
| Creatinine (> 1.3 × Baseline) | 0 | 0 | 0 | 1 |
| Sodium (< 0.95 × Baseline) | 0 | 0 | 0 | 1 |
| Potassium (> 1.1 × Baseline) | 0 | 0 | 0 | 2 |
| Urea (> 1.3 × Baseline) | 0 | 0 | 2 | 1 |
ULN, Upper limit of normal range; Baseline, predose level.
Three and four subjects in the Moderate and Severe impairment groups had clinically significant abnormalities (according to the sponsor-defined criteria). These seven subjects had six and seven abnormalities which developed from a normal and abnormal baseline, respectively (Table 6). Most of these laboratory abnormalities had returned to normal or near predose levels at follow-up. These values affected are typical abnormalities in renal impairment and of course there is a certain variability in urea, urine blood, etc. Apart from that, even withdrawal technique may have an impact on potassium, and intake of diuretics may have an impact on sodium. No trends of clinical concern could be identified from these abnormalities. The investigator assessed all abnormalities as being clinically acceptable.
No pattern could be seen across the time-points or groups in blood pressure or pulse rate. There were no clinically significant abnormalities in ECGs. In general, fosfluconazole was well tolerated.
Discussion
This was an open, parallel-group, two-centre study to determine the effects of renal impairment on the pharmacokinetics, safety and toleration of fosfluconazole and FLCZ following a single 1000-mg bolus i.v. injection of fosfluconazole. Subjects were categorized as Normal (> 80 ml min−1), Mild (51–80 ml min−1), Moderate (30–50 ml min−1) or Severe (< 30 ml min−1) according to their Cockcroft and Gault CLcr values. For subjects with apparent muscle wasting due to poor nutrition or long-term bed rest and subjects with hepatic dysfunction, Cockcroft and Gault equation using lean body weights, instead of total body weights, is a better predictor of CLcr [31–34]. In the current study, however, this has been partly corrected for by the use of subjects with a limited weight range through application of the Quetelet's index. There was a significant correlation between the Cockcroft and Gault CLcr and measured CLcr values (R2 = 0.72).
Fosfluconazole plasma concentration profiles were very similar across the four groups. There was no apparent relationship between any of the fosfluconazole pharmacokinetic parameters with increasing renal impairment. The pharmacokinetics of fosfluconazole, including its efficient conversion to FLCZ, were unaffected by the degree of renal impairment. Only small amounts of fosfluconazole were excreted in the urine (consistent with the pharmacokinetics of fosfluconazole in nonrenally impaired subjects), suggesting almost complete conversion to FLCZ. Fosfluconazole was not detectable in the plasma at 24 h or more postdose in all renal impairment groups, as in the Normal group. In the previous studies in healthy volunteers, fosfluconazole did not accumulate and was almost completely converted to FLCZ after multiple dosing [35]. These results suggest that after multiple dosing fosfluconazole does not accumulate in subjects with renal impairment.
Aweeka et al. [36] have investigated the effect of renal disease on the rate and extent of conversion of fosphenytoin (phosphate ester prodrug of phenytoin) to phenytoin. Following a single i.v. dose of fosphenytoin (250 mg over a period of 30 min) to subjects with renal disease and healthy subjects, the t1/2 of fosphenytoin was similar and the conversion of fosphenytoin to phenytoin was equally efficient in subjects with renal disease and healthy subjects. However, there was a trend toward increased fosphenytoin CL and earlier peak phenytoin concentration in subjects with renal disease. This finding is consistent with decreased binding of fosphenytoin to plasma proteins and increased fraction of unbound fosphenytoin resulting from decreased plasma protein concentrations associated with impaired renal states. For fosfluconazole the degree of protein binding was also high in subjects with renal impairment and unaffected by renal impairment. There was no apparent relationship between the fosfluconazole CLu and renal impairment.
FLCZ concentrations were still detected in plasma after 240 h postdose and remained higher at the later sampling times in subjects in the Moderate and Severe groups. AUC, t1/2 and MRT of FLCZ all increased with the degree of renal impairment and there was a linear relationship between the FLCZ CL/F value and renal impairment (as determined by Cockcroft and Gault CLcr or Measured CLcr), with FLCZ CL/F decreasing as renal impairment increased. Both the amount excreted in the urine and CLR decreased with an increase in renal impairment. These findings in FLCZ pharmacokinetics after fosfluconazole i.v. injection are consistent with the recommendations for dosage adjustments of FLCZ in renal failure. In the previous study Toon et al. [21] examined the pharmacokinetics of FLCZ in volunteers with various degrees of renal function after a single 50-mg oral dose of FLCZ. The FLCZ pharmacokinetics were markedly affected by impaired renal function. There is an inverse relationship between t1/2 and CLcr. Berl et al. [22] investigated the pharmacokinetics of FLCZ after multiple dose of FLCZ in the following groups: volunteers with CLcr >50 ml min−1, given a loading dose of 400 mg and a daily dose of 200 mg for 9 days (Group 1); subjects with CLcr between 21 and 50 ml min−1, given a loading dose of 200 mg and a maintenance dose of 100 mg for 9 days (Group 2); subjects with CLcr between 11 and 20 ml min−1, given a loading dose of 100 mg and a maintenance dose of 50 mg for 9 days (Group 3). At day 10 the mean CLR of FLCZ decreased as CLcr decreased, and the mean t1/2 was inversely related to mean CLcr (36.7 h in Group 1, 84.5 h in Group 2, and 101.9 h in Group 3). The mean AUC on day 10 was similar for Group 1 compared with Group 2, despite a reduction in the maintenance dose by 50%. The mean AUC for Group 3, for which the maintenance dose was 25% of that for Group 1, decreased by approximately 50% compared with Group 1. Based on these results, the daily dose of FLCZ in patients with renal impairment has been modified in accordance with CLcr (half normal dose for patients with CLcr at ≤50 ml min−1). Although renal impairment does not affect the conversion of fosfluconazole to FLCZ, CL/F of FLCZ decreased with CLcr and mean AUC, t1/2 and MRT all increased with the degree of renal impairment. Hence, the same dosage adjustments for FLCZ may be applied to fosfluconazole.
In conclusion, the pharmacokinetics of fosfluconazole, including its efficient conversion into FLCZ, were unaffected by renal impairment. For FLCZ, there was a significant linear relationship between CLcr and AUC, t1/2, MRT and CL/F, with AUC, t1/2 and MRT increasing and CL/F decreasing as renal impairment increased. The dose adjustment used for FLCZ (half normal dose for patients with CLcr at ≤50 ml min−1) can be applied to fosfluconazole as well. There were no safety concerns for any subject in this study, and fosfluconazole and FLCZ were well tolerated by all the treatment groups.
Acknowledgments
We thank Pr Françoise Mignon, Head of Department of Nephrology (Hôpital Bichat-Claude Bernard, France) for his advice in this study.
References
- 1.Phillips P, De Beule K, Frechette G, et al. A double-blind comparison of itraconazole oral solution and fluconazole capsules for the treatment of oropharyngeal candidiasis in patients with AIDS. Clin Infect Dis. 1998;26:1368–73. doi: 10.1086/516342. [DOI] [PubMed] [Google Scholar]
- 2.Rex JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:662–78. doi: 10.1086/313749. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs LG, Skidmore EA, Freeman K, Lipschultz D, Fox N. Oral fluconazole compared with bladder irrigation with amphotericin B for treatment of fungal urinary tract infections in elderly patients. Clin Infect Dis. 1996;22:30–5. doi: 10.1093/clinids/22.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Levine J, Bernard DB, Idelson BA, Farnham H, Saunders C, Sugar AM. Fungal peritonitis complicating continuous ambulatory peritoneal dialysis: successful treatment with fluconazole, a new orally active antifungal agent. Am J Med. 1989;86:825–7. doi: 10.1016/0002-9343(89)90481-6. [DOI] [PubMed] [Google Scholar]
- 5.Anaissie EJ, Darouiche RO, Abi-Said D, et al. Management of invasive candidal infections: results of a prospective, randomized, multileft study of fluconazole versus amphotericin B and review of the literature. Clin Infect Dis. 1996;23:964–72. doi: 10.1093/clinids/23.5.964. [DOI] [PubMed] [Google Scholar]
- 6.Anaissie E, Bodey GP, Kantarjian H, et al. Fluconazole therapy for chronic disseminated candidiasis in patients with leukemia and prior amphotericin B therapy. Am J Med. 1991;91:142–50. doi: 10.1016/0002-9343(91)90006-j. [DOI] [PubMed] [Google Scholar]
- 7.Flanagan PG, Barnes RA. Hazards of inadequate fluconazole dosage to treat deep-seated or systemic Candida albicans infection. J Infect. 1997;35:295–7. doi: 10.1016/s0163-4453(97)93270-9. [DOI] [PubMed] [Google Scholar]
- 8.Ikemoto H. A clinical study of fluconazole for the treatment of deep mycoses. Diagn Microbiol Infect Dis. 1989;12(Suppl. 4):S239–S247. doi: 10.1016/0732-8893(89)90143-0. [DOI] [PubMed] [Google Scholar]
- 9.Van't Wout JW, Mattie H, van Furth R. A prospective study of the efficacy of fluconazole (UK-49,858) against deep-seated fungal infections. J Antimicrob Chemother. 1988;21:665–72. doi: 10.1093/jac/21.5.665. [DOI] [PubMed] [Google Scholar]
- 10.Goa KL, Barradell LB. Fluconazole. An update of its pharmacodynamic and pharmacokinetic properties and therapeutic use in major superficial and systemic mycoses in immunocompromised patients. Drugs. 1995;50:658–90. doi: 10.2165/00003495-199550040-00007. [DOI] [PubMed] [Google Scholar]
- 11.Troke PF. Large-scale multicentre study of fluconazole in the treatment of hospitalised patients with fungal infections. Multicentre European Study Group. Eur J Clin Microbiol Infect Dis. 1997;16:287–95. doi: 10.1007/BF01695633. [DOI] [PubMed] [Google Scholar]
- 12.Brammer KW. Management of fungal infection in neutropenic patients with fluconazole. Haematol Blood Transfus. 1990;33:546–50. doi: 10.1007/978-3-642-74643-7_97. [DOI] [PubMed] [Google Scholar]
- 13.Ikemoto H, Watanabe K, Mori T, et al. Clinical study of fluconazole on deep-seated fungal infections. Jpn J Antibiot. 1989;42:63–116. [PubMed] [Google Scholar]
- 14.Maruta A, Matsuzaki M, Fukawa H, Kodama F. Clinical evaluation of fluconazole in the case of deep mycosis associated with leukemia. Jpn J Antibiot. 1989;42:117–26. [PubMed] [Google Scholar]
- 15.Toyama K, Lin KY, Hojo H, Tsuda A, Torii Y, Yoshikawa O. A clinical evaluation of injectable fluconazole in the treatment of deep mycosis associated with hematological malignancy. Jpn J Antibiot. 1989;42:47–54. [PubMed] [Google Scholar]
- 16.Lee Y, Shiota T, Ikeda S, et al. Clinical efficacy of fluconazole in the patient with pulmonary mycosis. Jpn J Antibiot. 1989;42:138–43. [PubMed] [Google Scholar]
- 17.Nito H. Clinical efficacy of fluconazole in urinary tract fungal infections. Jpn J Antibiot. 1989;42:171–8. [PubMed] [Google Scholar]
- 18.Saag MS, Powderly WG, Cloud GA, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med. 1992;326:83–9. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi H, Ikemoto H, Watanabe K, Ito A, Hara K, Kohno S. Fluconazole monotherapy for cryptococcosis in non-AIDS patients. Eur J Clin Microbiol Infect Dis. 1996;15:787–92. doi: 10.1007/BF01701520. [DOI] [PubMed] [Google Scholar]
- 20.Brammer KW, Farrow PR, Faulkner JK. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev Infect Dis. 1990;12(Suppl. 3):S318–S326. doi: 10.1093/clinids/12.supplement_3.s318. [DOI] [PubMed] [Google Scholar]
- 21.Toon S, Ross CE, Gokal R, Rowland M. An assessment of the effects of impaired renal function and haemodialysis on the pharmacokinetics of fluconazole. Br J Clin Pharmacol. 1990;29:221–6. doi: 10.1111/j.1365-2125.1990.tb03623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berl T, Wilner KD, Gardner M, et al. Pharmacokinetics of fluconazole in renal failure. J Am Soc Nephrol. 1995;6:242–7. doi: 10.1681/ASN.V62242. [DOI] [PubMed] [Google Scholar]
- 23.Bentley A, Butters M, Green SP, et al. The discovery and process development of a commercial route to the water soluble prodrug, fosfluconazole. Org Process Res Dev. 2002;6:109–12. [Google Scholar]
- 24.Fernley HN. Mammalian alkaline phosphatases. In: Boyer PD, editor. The Enzymes. 3. New York and London: Academic Press; 1971. pp. 417–47. [Google Scholar]
- 25.Nouwen EJ, De Broe ME. Human intestinal versus tissue-nonspecific alkaline phosphatase as complementary urinary markers for the proximal tubule. Kidney Int. 1994;46(Suppl. 47):S43–S51. [PubMed] [Google Scholar]
- 26.Guder WG, Ross BD. Enzyme distribution along the nephron. Kidney Int. 1984;26:101–11. doi: 10.1038/ki.1984.143. [DOI] [PubMed] [Google Scholar]
- 27.Sobue S, Sekiguchi K, Shimatani K, Tan K. Pharmacokinetics and safety of fosfluconazole after single intravenous bolus injection in healthy male Japanese volunteers. J Clin Pharmacol. doi: 10.1177/0091270003262799. [DOI] [PubMed] [Google Scholar]
- 28.Garrow JS, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes. 1985;9:147–53. [PubMed] [Google Scholar]
- 29.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 30.SAS Institute Inc. SAS/SAT Users Guide. 4. Cary, NC: SAS Institute Inc; 1994. Version 6.09. [Google Scholar]
- 31.Pesola GR, Akhavan I, Madu A, Shah NK, Carlon GC. Prediction equation estimates of creatinine clearance in the intensive care unit. Intensive Care Med. 1993;19:39–43. doi: 10.1007/BF01709276. [DOI] [PubMed] [Google Scholar]
- 32.O'Connell MB, Dwinell AM, Bannick-Mohrland SD. Predictive performance of equations to estimate creatinine clearance in hospitalized elderly patients. Ann Pharmacother. 1992;26:627–35. doi: 10.1177/106002809202600503. [DOI] [PubMed] [Google Scholar]
- 33.Lam NP, Sperelakis R, Kuk J, Seeger JD, Lau AH. Rapid estimation of creatinine clearances in patients with liver dysfunction. Dig Dis Sci. 1999;44:1222–7. doi: 10.1023/a:1026600929277. [DOI] [PubMed] [Google Scholar]
- 34.Nawaratne S, Brien JE, Seeman E, et al. Relationships among liver and kidney volumes, lean body mass and drug clearance. Br J Clin Pharmacol. 1998;46:447–52. doi: 10.1046/j.1365-2125.1998.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobue S, Tan K, Layton G, Eve M, Sanderson JB. Pharmacokinetics of fosfluconazole and fluconazole following multiple intravenous administration of fosfluconazole in healthy male volunteers. Br J Clin Pharmacol. doi: 10.1111/j.1365-2125.2004.02107.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aweeka FT, Gottwald MD, Gambertoglio JG, et al. Pharmacokinetics of fosphenytoin in patients with hepatic or renal disease. Epilepsia. 1999;40:777–82. doi: 10.1111/j.1528-1157.1999.tb00778.x. [DOI] [PubMed] [Google Scholar]