Abstract
Aim
To construct a population pharmacokinetic model for methadone enantiomers in the setting of methadone maintenance treatment for opioid dependence.
Methods
A population pharmacokinetic model was developed using P-Pharm software for rac-, (R)- and (S)-methadone using data (8–13 plasma samples per subject) obtained from 59 methadone maintenance patients during one interdosing interval at steady state. The patients were randomly assigned to either a development (n = 38) or a validation dataset (n = 21). The model was refined by inclusion of all subjects to construct a final basic model, which was used to construct a covariate model.
Results
A population-based two-compartment open model with first-order absorption and lag time was developed and validated for all analytes. The population geometric mean (coefficient of variation) of maximum a posteriori probability Bayesian estimated values for clearance, terminal half-life and volume of distribution at steady-state of the active (R)-enantiomer were 8.7 (42%) l h−1, 51 (45%) h and 597 (45%) l, respectively. For all analytes, the volume of the central compartment was decreased with increasing plasma α1-acid glycoprotein concentration and was lower in females, while the delay in absorption was longer at higher doses. No covariates were identified for apparent oral clearance. The apparent oral clearance of (R)-methadone (geometric mean ratio; 95% confidence interval) was 105% (99, 110), that of (S)-methadone (P = 0.19), while (R)-methadone Vc/F (154%; 151, 157), Vdss/F (173%; 164, 183), t1/2β (162%; 153, 172) and mean residence time (166%; 156, 176) were significantly greater (P < 0.0001) than for (S)-methadone. The population pharmacokinetic models were able to predict accurately oral clearance values from limited (one or two samples) blood sampling protocols.
Conclusions
The substantial stereoselectivity in methadone disposition reinforces the potential for misinterpretation of racemic methadone disposition data. The marked interindividual variability in (R)-methadone clearance, with no covariates identified, highlights the need for alternative methods to determine an individual's metabolic clearance. The ability to predict (R)-methadone clearance from one to two blood samples at steady state may prove clinically useful if a drug–drug interaction or poor adherence are suspected and guide the prescriber in deciding if a client's request for a dose increase is warranted or whether an alternative opioid would be more appropriate.
Keywords: drug dependence, metabolism, methadone, population pharmacokinetic modelling, P-Pharm, stereoselectivity
Introduction
Methadone (6 - dimethylamino - 4, 4 - diphenyl - 3 -heptanone) is the most widely used pharmacological agent for the management of opioid dependence [1] and is administered as the racemic mixture of the (R)- and (S)- enantiomeric forms. (R)-methadone has a 10-fold higher affinity than (S)-methadone at µ and δ opioid receptors [2], possesses up to 50 times the analgesic activity of (S)-methadone in human and animal models of antinociception [3], and prevents the occurrence of opioid withdrawal symptoms while (S)-methadone is ineffective [4].
Although the pharmacokinetics of rac-methadone have been reported extensively, detailed examination of the disposition of the individual enantiomers is limited (see Eap et al. [5] for commentary). The limited data available demonstrate higher clearance and volume of distribution for the (R)- enantiomer after single doses in pain patients [6], while in methadone maintenance patients no difference in apparent oral clearance was observed [7]. CYP3A is the major isoform group involved in the clearance of the methadone enantiomers by N-demethylation to EDDP, with possible minor involvement of CYP2C9, whereas CYP2D6 plays a minor, if any, role in this pathway [8–10]. However, the involvement of CYP2D6 in other oxidative pathways, primarily for the (R)-enantiomer, has been suggested [11]. Recent studies have attempted to relate methadone clearance and indices of CYP3A activity using probe substrates. However, only weak correlations were found [12–14].
Substantial interindividual variability in rac-methadone disposition poses difficulties in dosage regimen design in opioid dependence treatment [15]. In addition, since a relationship between trough plasma methadone concentrations and urinalysis negative for illicit drugs has been reported [16], some authors have advocated greater individualization of methadone dosing regimens (see Eap et al. [5] for commentary). This might be facilitated if there were a clearer understanding of the factors governing methadone disposition (particularly clearance) in an individual patient. This might be achieved with population pharmacokinetic analysis. Furthermore, using Bayesian forecasting techniques, pharmacokinetic parameters for a given individual can be estimated from very sparse concentration–time and dosage data, thus providing guided dose adjustments.
The aim of the present study was to model the steady-state population pharmacokinetics of rac-, (R)- and (S)-methadone in a large number of methadone maintenance patients, and to examine factors which might contribute to their variability. A secondary objective was to examine the use of limited plasma concentration–time data to estimate apparent oral clearance, using a maximum a posteriori probability (MAP) Bayesian fitting procedure, as a prelude to possibly enhancing individualized dosing regimens.
Methods
Subjects and protocols
Ethical approval was obtained from the Royal Adelaide Hospital Research Ethics Committee. All subjects gave written informed consent. Fifty-nine subjects were studied during a single 24-h interdosing interval (once daily dosing) at steady state. The subjects had been enrolled in the South Australian Public Methadone Maintenance Programme for at least 6 months (range 6 months to 10 years) and had not had a methadone dose change for at least 2 months. Details of dosing history including times of previous doses were recorded. Patients were excluded from the study if they were pregnant or had positive HIV serology. Demographic details are provided in Table 1. Each subject was admitted to the inpatient facility of the maintenance programme 1 h before their scheduled daily dose (nominally 23 h after their previous witnessed dose) and all but six subjects remained in the facility for the subsequent 24 h. Each patient's usual methadone dose was administered as a syrup (5 mg ml−1; Glaxo Welcome Australia Ltd, Boronia, Australia) under supervision of the study personnel. Blood samples (5 ml) were obtained via an 18 G indwelling venous catheter (Jelco™; Critikon Corp., Tampa, FL, USA) inserted into a forearm vein prior to the daily dose and kept patent with a Teflon stylet (Jelco™). Blood was placed into heparinized tubes and centrifuged to obtain plasma. Exact times of blood sampling and dose administration were recorded.
Table 1.
Demographic characteristics of 59 methadone maintenance subjects studied for population pharmacokinetic analysis
| Model Development dataset | Model Validation dataset | Full data set | Significance | |
|---|---|---|---|---|
| Number of subjects | 38 | 21 | 59 | |
| Protocol 1* | 33 | 20 | 53 | |
| Protocol 2† | 5 | 1 | 6 | 0.41‡ |
| Number of observations | 463 | 262 | 725 | |
| Protocol 1* | 432 | 246 | 678 | |
| Protocol 2† | 31 | 16 | 47 | |
| Number of observationsper subject | 11.8 ± 2.2 (7–13) | 12.1 ± 1.7 (8–13) | 11.9 ± 2.0 (7–13) | 0.77§ |
| Protocol 1* | 12.9 ± 0.3 (12–13) | 12.5 ± 1.1 (10–13) | 12.8 ± 0.7 (10–13) | |
| Protocol 2† | 7.8 ± 0.5 (7–8) | 8 | 7.8 ± 0.7 (7–8) | |
| Age (years) | 36 ± 8 (20–47) | 32 ± 7 (21–48) | 34 ± 8 (20–48) | 0.12§ |
| Weight (kg) | 74 ± 15 (47–110) | 69 ± 12 (44–87) | 72 ± 14 (45–110) | 0.32§ |
| Sex (% male/% female) | 71/39 | 48/52 | 63/37 | 0.09‡ |
| α1-acid glycoprotein (mg. dl−1) | 103 ± 23 (67–157) | 112 ± 24 (73–160) | 109 ± 23 (67–160) | 0.30§ |
| Dose/day (mg day−1) | 76 ± 35 (7.5–150) | 74 ± 44 (20–160) | 76 ± 38 (7.5–160) | 0.67§ |
| Dose/day (mg kg−1 day−1) | 1.1 ± 0.5 (0.1–2.2) | 1.1 ± 0.7 (0.3–2.5) | 1.1 ± 0.6 (0.1–2.5) | 0.88§ |
| Rac-methadone MR¶ | 1.7 ± 0.8 (0.5–4.1)** | |||
| (R)-methadone MR¶ | 1.2 ± 0.9 (0.3–5.3) | |||
| (S)-methadone MR¶ | 2.8 ± 1.4 (0.7–6.3)** | |||
| Subjects with urinalysis positive for the following (%) | ||||
| Benzodiazepines | 50 | 43 | 48 | 0.09‡ |
| Barbiturates | 0 | 1 | 1 | |
| Opiates | 40 | 20 | 32 | 0.15‡ |
| Sympathomimetic amines | 11 | 10 | 10 | 0.99‡ |
| Subjects with the following withdrawal status (%) | ||||
| ‘Dose not holding’ | 60 | 62 | 61 | 0.99‡ |
Data are presented as mean ± SD (range).
Blood sampling protocol 1 (13 samples taken over the interdosing interval).
Blood sampling protocol 2 (eight samples taken over the interdosing interval).
Fisher's exact test for comparison of Model Development and Validation datasets.
Mann–Whitney U-test for comparison of Model Development and Validation datasets.
Metabolic ratio: ratio of EDDP/methadone concentration in pooled 24 h urine sample (n = 37).
Significantly different from (R)-methadone metabolic ratio (MR) (P < 0.0001).
The subjects were enrolled in one of four clinical trials, all aimed at examining the pharmacokinetics and pharmacodynamics of methadone, and which employed two different blood sampling protocols. In protocol 1 (53 subjects), a blood sample was collected 0–1 h before the dose and at the following nominal times after dosing: 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 9 and 12 h, and at the end of the interdosing interval (23–24 h). In protocol 2 (six subjects), a blood sample was collected 0–1 h before the dose and at the following nominal times after dosing: 0.5, 1, 2, 3, 4, 5, 6 and 8 h, and at the end of the interdosing interval (23–24 h). Data from 18 subjects conforming to blood sampling protocol 1 have been reported previously [7, 17, 18] in a study of the individual pharmacokinetics and pharmacodynamics of methadone. In 37 subjects (26 male, 11 female) of protocol 1, a 24 h pooled urine sample was also obtained, volume and pH measured and an aliquot stored at −20 °C until analysis.
Sample analysis
Quantification of the enantiomers of methadone in plasma [7], and methadone and its major N-demethylated metabolite (EDDP) in urine [19] were achieved using previously validated stereoselective high-performance liquid chromatography assays. For the methadone enantiomers in plasma, calibration curves ranged from 15 to 600 ng ml−1. For the methadone and EDDP enantiomers in urine, calibration curves ranged from 39 to 3900 ng ml−1 and from 35 to 3500 ng ml−1, respectively. For both assays, both intra- and interassay inaccuracy and precision were <12%, for all compounds, including at the limit of quantification [7, 19]. Concentrations of rac-methadone and rac-EDDP were calculated as the sum of the enantiomers. Plasma concentrations of α1-acid glycoprotein in prestudy dose plasma samples were determined using radial immunoassay plates (Behring Diagnostics, Marburg, Germany) [7]. Prestudy dose urine samples were screened for common drugs of abuse including nonmethadone opiates, barbiturates, benzodiazepines, cannabinoids and sympathomimetic amines, using an EMIT assay.
Demographic and pharmacokinetic data
The data collected for pharmacokinetic interpretation included rac-methadone dose, time of dose, blood collection time, and plasma (R)- and (S)-methadone concentrations. Dosage for the enantiomers was calculated as rac-methadone dose divided by 2. Clinical data included the continuous covariables of subject age, weight, weight0.75, and plasma α1-acid glycoprotein concentration in the predose plasma sample. Categorical binary covariables included: sex, urinalysis results (positive or negative) for nonmethadone opioids, barbiturates, benzodiazepines, cannabinoids, sympathomimetic amines, and withdrawal status (‘dose holding’ or ‘dose not holding’) [17]. In the 37 subjects from whom a 24 h pooled urine sample was obtained, the metabolic ratio (MR) of urinary EDDP/methadone concentration (corrected for molecular weight differences) for the individual enantiomers and the racemic compounds was calculated. These data were used in a subgroup analysis as potential covariates for apparent oral clearance.
Datasets
The Full dataset comprised 725 plasma concentration–time observations for rac-, (R)- and (S)-methadone, and covariable data from 59 subjects. In compliance with the Food and Drug Administration guidelines [20] on population pharmacokinetics, the data were randomly split in a ratio of 2 : 1 for the Model Development and Model Validation of the Basic Population Pharmacokinetic Model, using Microsoft Excel 2000 (Microsoft Corp., Seattle, WA, USA, version 9.0.4402). The Model Development dataset comprised 38 subjects with 463 plasma concentration–time observations, whilst the Model Validation dataset comprised 21 subjects with 262 plasma concentration–time observations.
Strategy for the population pharmacokinetic analysis
The population pharmacokinetic analysis was performed with the population pharmacokinetic modelling software P-Pharm (version 1.51; Innaphase, Philadelphia, PA, USA) running under Microsoft Windows Millennium on a PC with a Pentium III 1-GHz processor. P-Pharm implements a two-step expectation–minimization type algorithm for estimating the population parameters of a nonlinear mixed effects model [21].
The analysis was performed in two steps, similar to that described using P-Pharm software [22]: (i) development and validation of a Basic model without considering potentially important covariables; (ii) evaluation of the influence of subject characteristics on MAP Bayesian estimates of individual pharmacokinetic parameters, and optimization of the final model by including covariables found to be influential.
Development and validation of the Basic population pharmacokinetic model (Step 1)
The Model Development dataset was used to build a Basic population pharmacokinetic model. Various population pharmacokinetic and pharmacostatistical models were fitted to the data, which included variations of: (i) steady-state one- vs. two-compartment linear models with and without a lag time, (ii) a normal vs. log-normal pharmacokinetic parameter distribution, (iii) residual error variance. Standard evaluation criteria for model selection were employed, and included: (i) low estimates for the Akaike Information Criterion (AIC), the residual error term (σ), and intersubject variability in pharmacokinetic parameters; (ii) a high estimate for the Maximum Likelihood (ML) which is related to the negative of the Log-Likelihood (LL) of the data; (iii) a low range of residuals, and weighted residuals that were normally distributed about zero.
The primary structural pharmacokinetic parameters were apparent oral clearance (CL/F), apparent volume of distribution of the central compartment (Vc/F), transfer rate constants (k12, k21), absorption rate constant (ka), and lag time (tlag). Pharmacokinetic parameter estimates for individual subjects were obtained by the MAP Bayesian fitting procedure within P-Pharm, and used to calculate additional pharmacokinetic parameters (see Table 2) [23, 24]. Time (Tmax) to reach maximum plasma concentration (Cmax) was obtained by iteration. Briefly, the MAP Bayesian predicted pharmacokinetic parameters for each patient were used to iteratively solve for Tmax employing the Solver feature within Microsoft Excel. Cmax was taken as the concentration at which Tmax was observed. Cmax and measured trough (end of study, Clast) plasma methadone concentrations were corrected to a 70 mg dose of rac-methadone and 35 mg for each enantiomer.
Table 2.
Comparison of (R)- and (S)-methadone pharmacokinetic parameters obtained from maximum a posteriori probability Bayesian predictions using the Full Basic population pharmacokinetics model in 59 methadone maintenance patients
| Parameter* | Rac-methadone | (R)-methadone | (S)-methadone | Significance (P-value)‡ |
|---|---|---|---|---|
| CL/F (l h−1) | 8.5 (7.6, 9.5) | 8.7 (7.9, 9.6) | 8.3 (7.3, 9.5) | 0.19 |
| Vc/F (l) | 112 (107, 118) | 145 (138, 152) | 94 (89, 99) | <0.0001 |
| k12 (h−1) | 0.57 (0.52, 0.61) | 0.54 (0.50, 0.58) | 0.55 (0.51, 0.59) | 0.16 |
| k21 (h−1) | 0.20 (0.19, 0.21) | 0.18 (0.16, 0.19) | 0.21 (0.20, 0.23) | <0.0001 |
| ka (h−1) | 0.59 (0.54, 0.63) | 0.55 (0.51, 0.60) | 0.62 (0.57, 0.67) | <0.0001 |
| tlag (h) | 0.45 (0.40, 0.49) | 0.53 (0.49, 0.58) | 0.39 (0.35, 0.43) | <0.0001 |
| t1/2α (h) | 0.83 (0.78, 0.88) | 0.90 (0.85, 0.95) | 0.82 (0.78, 0.87) | <0.0001 |
| t1/2β (h) | 39 (35, 43) | 51 (45, 57) | 31 (28, 35) | <0.0001 |
| Vdss/F (l) | 440 (398, 487) | 597 (538, 663) | 345 (312, 382) | <0.0001 |
| Vp/F (l) | 321 (283, 364) | 444 (390, 504) | 246 (217, 279) | <0.0001 |
| Vdβ/F (l) | 474 (428, 525) | 637 (573, 707) | 376 (339, 417) | <0.0001 |
| AUCτ (µg.hr. ml−1)† | 8.27 (7.39, 9.26) | 4.02 (3.64, 4.44) | 4.20 (3.69, 4.79) | 0.026 |
| MRT (h) | 52 (46, 58) | 69 (61, 77) | 41 (37, 47) | <0.0001 |
| Cmax (ng.ml−1)† | 494 (448, 544) | 225 (206, 246) | 268 (241, 298) | <0.0001 |
| Clast (ng.ml−1)† | 269 (236, 308) | 139 (124, 156) | 128 (109, 150) | 0.24 |
| Tmax (h) | 2.3 (2.2, 2.5) | 2.5 (2.3, 2.7) | 2.2 (2.1, 2.4) | <0.0001 |
Apparent oral clearance (CL/F), apparent volume of distribution of the central compartment (Vc/F), rate contrant for the transfer to (k12) and from (k21) the peripheral compartment, absorption rate constant (ka), absorption lag time (tlag), distribution (t1/2α) and terminal half-life (t1/2β), apparent volume of distribution at steady state (Vdss/F), and apparent volume of the peripheral compartment (Vp/F), apparent volume of distribution during the elimination phase (Vdβ/F), area under the plasma concentration–time curve during a steady-state interdosing interval (AUCô), mean residence time (MRT), time (Tmax) to reach maximum plasma concentration (Cmax).
AUCτ, Cmax and measured trough (end of study, Clast) plasma methadone concentrations were corrected to a 70 mg dose of rac-methadone and 35 mg for each enantiomer. Values are reported as geometric mean (95% confidence intervals) of the MAP Bayesian estimates of individual subject parameters assuming a log-normal parameter distribution.
Wilcoxon-matched pairs test for the comparison of (R)-methadone versus (S)-methadone.
Initial estimates of the pharmacokinetic parameters were obtained from previous literature values for rac-methadone [25–29]. In order to prevent a stereoselective bias in the final parameter estimates, the same initial estimates as those for rac-methadone were used for structural pharmacokinetic parameters for the individual methadone enantiomers. However, the pharmacokinetic models were tested for robustness by sequential fitting using initial estimates for CL/F and Vc/F, which were two-fold greater and lower than the original parameter estimates. The Basic population pharmacokinetic model was validated by examining the bias (not significantly different from zero) and precision (<20%) of the model-predicted methadone concentrations using the Model Validation dataset. Once the model had been validated, the Model Development and Validation datasets were combined. Thus, the Full dataset was used to produce a Full Basic model.
Exploratory identification of influential covariables (Step 2)
Preliminary examination of the relationship between the individual subject's MAP Bayesian estimates of the pharmacokinetic parameters obtained from the Full Basic model and subject covariables was performed with the use of linear regression analysis or the Mann–Whitney U-test, provided covariates were reasonable for the pharmacology of methadone. Stepwise inclusion and deletion of identified potentially explanatory covariables were performed using multiple linear regression analysis (P < 0.005) embedded within P-Pharm, and the standard model evaluation techniques outlined in Step 1 were employed.
To examine the predictive value of the urinary metabolic ratio, a dataset was constructed using only the pharmacokinetic and covariable data obtained from the 37 subjects from whom 24 h pooled urine samples were available. Multiple linear regression analysis, as described above, was used to investigate the relationship of these covariable data and apparent oral clearance.
Examination of the predictive performance of MAP Bayesian apparent oral clearance estimates from limited sampling protocols
Datasets were constructed that comprised only the concentrations of methadone obtained at the end of the interdosing interval alone (trough), or combined with samples taken at 4 h. The ability of the Full Basic model to predict apparent oral clearance values from limited concentration–time data was examined using the median (bias) and median absolute (precision) parameter prediction error [30]. Briefly, the MAP Bayesian estimate of apparent oral clearance obtained from the complete plasma concentration–time data (the ‘full data analysis’ value) for each individual was compared with that predicted from the limited concentration–time dataset and expressed as a percentage error. Analogously, the naive assumption that all subjects had the population median apparent oral clearance was also tested.
Other statistical analyses
GraphPad Prism v3.03 for Windows (GraphPad Software, San Diego, CA, USA) was used for statistical analyses. Significant differences between paired data were assessed using the Wilcoxon signed rank test. Comparisons of subgroups, defined by binary categorical covariables, were performed using the Mann–Whitney U-test. A one-tailed Student's t-test was used to test whether the residuals and weighted residuals were significantly different from zero and a Kolmogorov–Smirnov test was used to test for normality. The residuals were used to calculate the bias (mean concentration prediction error) and precision (root mean square prediction error), expressed as a percent of the concentration, and their 95% confidence intervals (CI) as previously described [31]. Linear regression analysis was performed to yield Pearson's r-values. For descriptive purposes, comparison of the pharmacokinetic parameters of the methadone enantiomers was performed using the geometric mean (95% CI) of the ratio of (R)- to (S)-methadone, while the Wilcoxon signed rank test was used for statistical comparisons. All data are expressed as geometric mean ±SD (lower 95% CI, upper 95% CI).
Results
Comparison of Model Development and Model Validation datasets
There were no statistically significant differences between the Model Development and Model Validation datasets in terms of demographic variables, doses or sampling protocols (Table 1). In the Full dataset, there were no significant differences (P > 0.19) between males and females for plasma α1-acid glycoprotein concentration, age, dose, or dose per kg body weight. Females (65 ± 13 kg) weighed significantly less than males (76 ± 13 kg, P = 0.004). Urinary MR values for rac- and (S)-methadone were significantly (P < 0.0001) different from those for (R)-methadone (Table 1).
Selection of the pharmacokinetic and pharmacostatisical model
Using the Model Development dataset, for all three compounds a two-compartment linear pharmacokinetic model with an absorption lag time provided the best fit of the data as assessed by the LL ratio test (P < 0.0001), smaller AIC values, and smaller estimates of σ. For all three analytes, a heteroscedastic error model (1/y) and log-normal parameter distribution were appropriate. For all three analytes, final population estimates of CL/F and Vc/F were insensitive (range −8% to +5%, respectively) to variation in the initial estimates of CL/F and Vc/F.
Validation of the Basic pharmacokinetic model
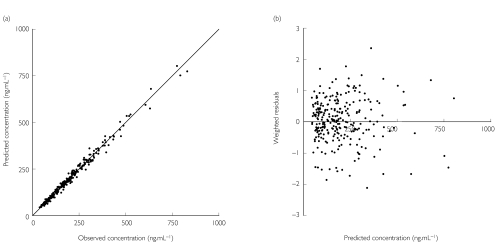
Validation of the Basic population pharmacokinetic model was performed using the bias and precision of the predicted concentrations for the Model Validation dataset. Figure 1 shows for (R)-methadone the close relationship of the model predicted and observed concentrations. For (R)-methadone, the mean concentration prediction error was without bias (P > 0.78) and was precise, with narrow 95% CIs (0.3 ng ml−1, −1.6 to 2.1; and 7.3%, 6.6 to 8.0, respectively). The weighted residuals were normally distributed (P > 0.1) and not significantly different from zero (P > 0.25). The Model Development dataset similarly internally predicted plasma methadone concentrations that were accurate, without bias (P > 0.4) and were precise for (R)-methadone. Similar validation data were obtained for rac- and (S)-methadone. Accordingly, the Basic population pharmacokinetic models passed the validation criteria.
Figure 1.
Scatter plot of model predicted and observed concentration (a) and the weighted residuals (b) in the Model Validation data set for (R)-methadone using the Basic population pharmacokinetic model. The solid line is the line of identity (y = x)
Refinement of the model using all subject data – the ‘Full Basic Model’
The Model Development and Validation datasets were combined to produce the Full dataset, which was then used to refine the final parameter estimates and evaluate the influence of covariables. The population estimates of the primary pharmacokinetic parameters were very close (<10%) to those obtained for the Model Development dataset, providing further support for the validity of the models. The performance of the Full Basic models for all three compounds was similar to that reported above for the Basic Models. The primary pharmacokinetic parameter CL/F was highly variable for all three analytes, with coefficients of variation (%CV) >40%, and values ranging seven-fold, from approximately 3 l h−1 to 25 l h−1(Figure 2). Similarly, volume of distribution at steady state was highly variable, with values ranging up to seven-fold, and estimates of terminal half-life were even more variable: (R)-methadone 15–140 h (45% CV); (S)-methadone 10–89 h (43% CV); rac-methadone 13–104 h (43% CV).
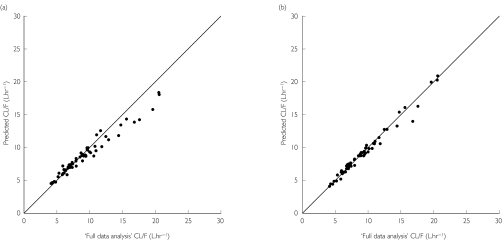
Figure 2.
Relationship of apparent oral clearance values determined from the full data analysis, and maximum a posteriori probability (MAP) Bayesian prediction for (R)-methadone using only a single trough plasma methadone concentration time-point (a) and combined trough and 4 h postdose plasma methadone concentration time-points (b). The solid line is the line of identity (y = x)
The Full Covariate population pharmacokinetic model
For rac-, (R)- and (S)-methadone, of the covariables identified in Step 2, multiple stepwise linear regression (P < 0.005) only identified sex and plasma α1-acid glycoprotein concentration as significant determinants of Vc/F, and dose as a significant determinant of tlag. The regression equations describing the relationship between the pharmacokinetic parameters and the covariables retained in the final model are shown in Table 3, and demonstrate that Vc/F is greater in males, and decreases with increasing plasma α1-acid glycoprotein concentration, while higher doses result in a longer tlag. Using the LL test, the combined addition of the covariables for Vc/F and tlag to the model resulted in a significantly improved fit for rac-methadone (P = 0.013) and (R)-methadone (P = 0.008), but not (S)-methadone (P = 0.11). However, for all three compounds, α1-acid glycoprotein and sex explained 52–53% of the variability in Vc/F, and dose explained 16–20% of the variability in tlag. Furthermore, the intersubject variability in Vc/F and tlag was reduced by approximately 19% and 5%, respectively, for all three compounds. Interestingly, females had significantly greater (R)-methadone Vc/F values when expressed per kg of body weight (2.1 ± 0.4 l kg−1vs. 1.7 ± 2.2 l kg−1, P < 0.0001), while other volumes of distribution were not significantly different between males and females whether or not they were corrected for body weight. Results were similar for rac- and (S)-methadone.
Table 3.
Regression equations describing the relationship between methadone pharmacokinetic parameters and covariates retained in the Full Covariate model
| Mean (SD) regression coefficient | |||
|---|---|---|---|
| Patient characteristic | Rac-methadone | (R)-methadone | (S)-methadone |
| Vc/F (l) = θ1 + θ2sex + θ3AAG | θ1: 146 | θ1: 182 | θ1: 128 |
| θ2: 25 (4.1) | θ2: 33 (5.1) | θ2: 21 (3.5) | |
| θ3: −0.455 (0.087) | θ3: −0.524 (0.108) | θ3: −0.434 (0.075) | |
| F-test (P-value) | 29.7 (< 0.0001) | 30.5 (< 0.0001) | 31.6 (< 0.0001) |
| tlag = θ4 + θ5dose | θ4: 0.295 | θ4: 0.456 | θ4: 0.249 |
| θ5: 0.0026 (0.0006) | θ5: 0.0043 (0.0013) | θ5: 0.0048 (0.0013) | |
| F-test (P-value) | 14.6 (0.0003) | 10.9 (0.002) | 14.1 (0.0004) |
AAG, Plasma α1-acid glycoprotein (mg dl−1); sex (female = 0, male = 1); dose, rac-methadone dose in mg day−1 (divided by 2 for the methadone enantiomers); θ, regression coefficient.
There were positive correlations between rac-, (R)- and (S)-methadone MAP Bayesian estimates of CL/F and dose. However, these correlations were highly dependent on values from only three subjects (out of a total of 59 subjects), who were receiving doses >130 mg and with CL/F estimates >16 l h−1. When the data from these subjects were removed, the regression relationship was markedly decreased in strength (P > 0.12, r2 < 0.05) for all three analytes. Furthermore, there were several subjects with similar CL/F estimates receiving doses approximately half those of these three subjects and vice versa. We did not further pursue a clearance covariate model which included dose.
As the volume of distribution and clearance of drugs are intuitively related to body weight on a physiological basis, we took the following approach to verify our decision to reject this covariate relationship. For each subject, the MAP Bayesian estimate of CL/F and Vc/F were divided by their body weight, and a correlation of the body weight-normalized parameter and body weight was examined. For (R)-methadone, there was a highly significant inverse correlation between body weight and body weight-normalized CL/F (P = 0.002, r = − 0.41) and body weight-normalized Vc/F (P < 0.0001, r =−0.70), indicting that body weight was not correlated with either parameter. Furthermore, the coefficients of variation of weight-normalized CL/F and Vc/F were 6% and 47% greater, respectively, than for nonbody weight-normalized CL/F and Vc/F. Similar results were obtained for rac- and (S)-methadone.
Inclusion of covariables had little effect on the magnitude of the population parameters (<10%) for all three analytes. The Full Covariate model gave predicted methadone concentrations that were without bias (P > 0.35, 0.5 ng ml−1; −0.7 to 1.7 ng ml−1) and precise (8.5%; 7.7 to 9.2%, respectively) for (R)-methadone. Similar results were obtained for rac- and (S)-methadone. Covariable analysis of the subset of subjects (n = 37) with urinary excretion data did not identify MR as a significant determinant of CL/F for any compound.
Predictive performance of MAP Bayesian estimation of apparent oral clearance from limited sampling protocols
The naive predictor (i.e. the population median) provided a reasonably unbiased estimate of apparent oral clearance for all three compounds (+6 to +15%); however, it was imprecise (>25%). In contrast, the MAP Bayesian estimates for each individual obtained from the limited concentration–time data (trough sample only) for all three compounds were highly accurate (−1% to 0%) and precise (<9%). However, from inspection of Figure 2 for (R)-methadone, it can be seen that there was a systematic underprediction of apparent oral clearance at higher values. The same was true for rac- and (S)-methadone. Further examination revealed that for subjects with ‘full data analysis’ clearance values greater than the population median (approximately 8.5 l h−1 for all three analytes), the median prediction error values for all three compounds were between −12% and −9%, with precision values ranging from 9 to 12%. In contrast, for subjects with ‘full data analysis’ clearance values lower than the population median, clearance was accurately (+3 to +5%) and precisely (<6%) predicted for all three compounds. Addition of the 4 h sample to the trough plasma sample concentration provided highly accurate (−1%) and precise (<4%) predictions of ‘full data analysis’ clearance values for all three compounds, and did not show any bias over the entire range of clearance values, as shown in Figure 2 for (R)-methadone.
Comparison of the pharmacokinetics of the methadone enantiomers
Comparison of the MAP Bayesian estimates of the pharmacokinetic parameters of methadone from the Full Basic model revealed, with the exception of CL/F, marked stereoselectivity (P < 0.0001) in all primary and supplemental pharmacokinetic parameters (Table 2), while AUCτ was of borderline significance (P = 0.03). The apparent oral clearance of (R)-methadone [geometric mean ratio (95% CI)] was 105% (99, 110) that of (S)-methadone (P = 0.19), while (R)-methadone Vc/F (154%; 151, 157), Vdss (173%; 164, 183), t1/2α (109%; 106, 112), t1/2β (162%; 153, 172) and mean residence time (166%; 156, 176) were significantly greater (P < 0.0001) than for (S)-methadone. There was a significant correlation of AUCτ and dose for (R)-methadone (P < 0.0001, r2 = 0.46), (S)-methadone (P < 0.0001, r2 = 0.28) and rac-methadone (P < 0.0001, r2 = 0.36). There were no statistically significant (P > 0.24) differences between trough plasma (R)- and (S)-methadone concentrations, whether analysis was performed on the model-predicted trough concentrations or the observed pre- or poststudy samples (Clast, Table 2). In contrast, estimated Cmax for (R)-methadone was on average 84% (81, 87) that of (S)-methadone (P < 0.0001, Table 2). Dose-corrected Cmax values were highly variable for all three analytes: (R)-methadone 98–440 ng ml−1 (33% CV); (S)-methadone 93–708 ng ml−1 (41% CV); rac-methadone 191–1144 ng ml−1 (37% CV).
Discussion
The large amount of rich data from over 700 plasma concentration–time data points collected from 59 patients during an interdosing interval at steady-state allowed us to develop and validate a population pharmacokinetic model for rac-, (R)- and (S)-methadone in patients chronically maintained on methadone for opioid dependence, and to examine patient demographic characteristics that contribute to variability in methadone pharmacokinetics. A linear two-compartment model with first-order absorption and a lag phase provided the best description of the plasma concentration–time profiles for all three compounds. The pharmacokinetic parameters for rac-methadone and its enantiomers were influenced in a similar fashion by the same demographic variables. Although clearance and volume of distribution are related to body weight for many drugs, the apparent oral clearance and volume of distribution of methadone, and its individual enantiomers, were not influenced by body weight over the 44–110 kg range in our population, even when allometrically scaled. This finding is consistent with a previous report in cancer pain patients [32]. Therefore, we consider that expressing plasma methadone concentrations corrected for body weight may introduce errors in the interpretation of plasma concentration data in this subject population. Despite no relationship between body weight and apparent volumes of distribution, males had significantly greater volumes than females. However, when apparent volume of distribution of the central compartment was expressed on a per kg basis, women had significantly greater values than men. A possible explanation for these findings is that, although the men had a greater mean body weight (resulting in a higher volume of distribution), fat represents a greater proportion of body weight in female subjects, which would result in a greater mean volume of distribution per kg body weight. However, data on the body composition of our subjects are not available, preventing us from testing this hypothesis. Future studies should aim at investigating the role of body composition, such as body mass index or body surface area, on the volume of distribution of methadone, as this may have some utility if methadone is administered intravenously, especially in pain patients.
Plasma α1-acid glycoprotein concentration was a significant covariate for apparent volume of distribution for all three analytes, consistent with its known role in determining the plasma unbound fraction of methadone [7, 33]. The binding of methadone is selective for the ORM2A variant [33, 34]. Therefore, it is likely that interindividual variation in the relative expression of the ORM2A variant of α1-acid glycoprotein could have decreased the strength of the relationship observed. Despite this, sex and α1-acid glycoprotein concentration combined explained over 50% of the variability in apparent volume of distribution, and decreased the interindividual variability by approximately 20% for all three analytes. When the ORM2A variant of α1-acid glycoprotein was selectively quantified, Boulton and coworkers [35] observed no relationship between this covariate and apparent oral clearance or volume of distribution. However, they did report that plasma concentrations of the ORM2A variant of α1-acid glycoprotein was a significant predictor of the intercompartmental transfer rate constants (k12 and k21) and the absorption rate constant for (R)-methadone [35]. We did not include plasma α1-acid glycoprotein concentration as a covariate for apparent oral clearance for any analyte, as it did not provide further improvement in the Full Covariate model. For a hepatically cleared low extraction ratio drug, apparent oral clearance is a function of plasma unbound fraction and intrinsic metabolic clearance. These two parameters have been demonstrated to be important determinants of methadone systemic clearance in rats [36]. It is likely that the large interindividual variability in intrinsic metabolic clearance in our subjects [7] may have masked the expected effect of α1-acid glycoprotein, which is only a surrogate measure of plasma unbound fraction, on clearance.
CYP3A4 is the major CYP isoform involved in the N-demethylation of methadone and its individual enantiomers, whereas CYP2D6 does not appear to be involved in this metabolic pathway [8–10]. However, the role of genetic variability in CYP2D6-mediated metabolism of methadone has been suggested, based upon in vivo drug–drug interaction studies [11, 37]. A more recent study identified a minor CYP2D6 gene–dose relationship in methadone maintenance patients [38]. However, there was no difference in dose- and weight-corrected trough plasma methadone concentrations between poor metabolizers (no functional expression of CYP2D6) and subjects with a single copy of the CYP2D6 gene (extensive metabolizers of CYP2D6), or between extensive and ultrarapid metabolizers (multiple copies of the CYP2D6 gene), due to considerable overlap between groups [38]. Hence, assessment of CYP3A4 function rather than CYP2D6 function might prove to be more useful in predicting methadone pharmacokinetics. Recent studies have attempted to relate methadone clearance to indices of CYP3A. Variations in urinary cortisol excretion were not related to methadone clearance [12, 13], probably due to the limitations of this measure of CYP3A activity. More recently, the plasma 1′OH-midazolam/midazolam concentration ratio after oral administration of midazolam was not correlated with trough plasma concentrations of (R)-methadone, while only weak relationships were found for rac- and (S)-methadone [14]. Differences in the magnitude and relative extents of hepatic and intestinal first-pass metabolism may be one explanation, as has been observed with the erythromycin breath test and the 1′OH-midazolam/midazolam ratio after oral administration [39]. Additionally, it is presently unknown whether methadone is metabolized by the polymorphic CYP3A5 isoform. We were unable to investigate the influence of CYP3A phenotype or activity due to the retrospective nature of the present study.
Time-dependent changes in rac-methadone apparent oral clearance, and to a lesser extent volume of distribution, have been reported using a population pharmacokinetic approach [25, 26]. The population mean apparent oral clearance value at steady state (171 ml min−1) was over three-fold greater than at the commencement of treatment (52 ml min−1). Whether this phenomenon is due to ‘autoinduction’ of methadone metabolism, or improving liver function due to the improved health status afforded by methadone maintenance therapy is not known. Prior to identifying time-related changes in apparent oral clearance and volume of distribution, these authors reported that the volume of distribution increased with body weight in methadone maintenance patients at the start of therapy and was higher in females for a given body weight [25]. However, once time-related changes in both apparent oral clearance and volume of distribution were included in the model, no covariates were identified. The lower number of subjects (21 males, 14 females), lack of sufficient data on α1-acid glycoprotein concentration, and the identified time-related changes in clearance and volume may explain the differences in covariable identification compared with the present study. Others have developed regression models for the prediction of total systemic clearance of rac-methadone in cancer pain patients [32]. Significant contributions to the predictiveness of the model included comedications that were found to increase (phenytoin, spironolactone, verapamil, diethylstilboestrol) or decrease (amitriptyline) clearance, while the presence of malignant disease and age over 65 years both decreased clearance, and haematocrit was related to increased clearance [32]. Our cohort of subjects was relatively younger and healthier, and we were not able to examine the potential influence of the comedications mentioned above. However, urinalysis results positive for several drug classes commonly abused in the methadone maintenance population were found to have no influence on apparent oral clearance.
Absorption lag-time for all three analytes was increased in subjects receiving higher doses, with dose explaining up to 20% of the variability in this parameter, and this supports previous observations in humans [28]. We hypothesize that this may be due to a local opioid receptor-mediated dose-dependent inhibitory effect of methadone on the gastrointestinal tract, to which tolerance does not fully develop [40]. Stereoselectivity in the absorption process was observed. The combined effect of longer lag-time and slower absorption rate resulted in the mean (R)-methadone Tmax value being approximately 20 min longer than for (S)-methadone, while (R)-methadone Cmax was on average 84% that of the (S)-enantiomer. Reasons for the stereoselective differences in tlag and ka are not readily apparent, but could relate to stereoselective active transport of methadone across the gastrointestinal lumen, given that methadone is known to be secreted in gastric fluid of humans after parenteral administration [41]. A role for p-glycoprotein in the gastrointestinal absorption of methadone has also been suggested, but remains controversial [42, 43]. Data during the lag phase were very sparse, which, in conjunction with model misspecification error, cannot be ruled out as a cause of the observed stereoselectivity.
We investigated the possible utility of the metabolic ratio of recovered EDDP/methadone as an index of total plasma clearance for methadone. However, in our analyses, this metabolic ratio was not related to apparent oral clearance, presumably due to interindividual variability in the extensive nonrenal (faecal) elimination of EDDP [44, 45]. Furthermore, the total combined urinary and faecal recovery of methadone and EDDP at steady state accounts for only 50–75% of the administered dose [44, 45], indicating that a significant proportion of total elimination is unexplained. In contrast to our results, Boulton and coworkers [35] demonstrated that the percent dose recovered as rac-EDDP negatively correlated with (R)-methadone apparent oral clearance. This is an unusual finding for a hepatically cleared drug, and may suggest that clearance by pathways other than urinary EDDP excretion becomes important at higher clearance values. Furthermore, our results in a large cohort of subjects confirm the urinary excretion of methadone and EDDP to be stereoselective. Given that the total apparent oral clearance of methadone was not stereoselective, then it is likely that the net nonrenal elimination (faecal elimination and/or further metabolism) of (R)-EDDP is greater than that of (S)-EDDP and/or methadone is eliminated by other pathways (faecal elimination and/or metabolism) which display net stereoselectivity.
Despite a weak positive correlation between prior MAP Bayesian estimates of apparent oral clearance and dose, these correlations were highly dependent on values from only three subjects receiving doses >130 mg and with CL/F estimates >16 l h−1. Furthermore, there were several subjects with similar CL/F estimates receiving doses approximately half those of these three subjects and vice versa. These results are consistent with the hypothesis that some subjects requiring high doses of methadone for control of withdrawal symptoms do so partly due to a high clearance of methadone [14, 46]. However, in the absence of these three subjects the regression relationship was not significant, and we did not further pursue a clearance covariate model which included dose. This was justified, as the programme from which these subjects were recruited allows dosage adjustment in consultation with the patient, dependent on the clinicians' evaluation of the control of withdrawal symptoms. Therefore, we consider not only that many subjects receiving higher doses of methadone for adequate control of withdrawal symptoms do so due to a high clearance, but that inadequate pharmacological control of withdrawal symptoms by the drug is also important. In such a scenario, inferring that dose influences clearance would result in a false post hoc ergo propter hoc assumption, as dose is not necessarily predictive of clearance in all cases.
The population mean value resulted in very poor naive predictions of CL/F when compared with the individual values obtained from the ‘full data analysis’. This was due to the large interindividual variability (up to seven-fold) in this parameter for all three analytes, consistent with the known variability in CYP3A4 expression and activity [47, 48]. However, when MAP Bayesian estimates were obtained using only trough samples, the predicted CL/F values were accurate and precise for individuals with clearance values <8.5 l h−1. This finding is not surprising, as the blood collection time-point was chosen deliberately to maximize predictive performance. This time-point is also of clinical utility, as patients generally visit the clinic at this time to ingest their daily methadone dose. However, MAP Bayesian prediction slightly underestimated clearance (∼10%) for subjects with high clearance values. When samples obtained at 4 h postdose were combined with a trough sample, MAP Bayesian-predicted clearance values were accurate and precise over the entire range of values. We have previously shown in methadone maintained patients that methadone pharmacological effects peak between 2 and 6 h postdose [17, 18], and that significant respiratory depression, which may be clinically relevant, occurs during this interval [17]. A 4 h sample to aid in the estimation of clearance was therefore chosen as a compromise between the availability of data at this time point in our dataset, its predictive performance, and clinical utility. A single trough blood sample taken at the clinic can then be used in conjunction with Bayesian forecasting to provide an accurate estimate of clearance (as would have been determined from a full data analysis requiring several plasma samples over an interdosing interval) for subjects with low to intermediate clearance, and only small underestimation for subjects with high clearance values. A supplementary 4 h sample can be taken to predict accurately clearance over the range of values seen in this large cohort (approximately 3–25 l h−1). Bayesian forecasting could thus be used by clinicians to improve dosing practice during dose modification in methadone maintained patients.
The estimates of the population pharmacokinetic parameters for rac-methadone were similar to those reported by other investigators (see Eap et al. [5] for commentary). Marked stereoselectivity was observed in all pharmacokinetic parameters, with the exception of apparent oral clearance. In contrast to the previous report of greater clearance of (R)-methadone (mean 158 ml min−1) compared with (S)-methadone (129 ml min−1) after acute administration in chronic pain patients [6], steady-state oral clearance was not stereoselective in our subjects (approximately 140 ml min−1). As commented earlier [7], the differences in the two study populations make comparison difficult. Additionally, comparison is further complicated by recently reported time-dependent changes in rac-methadone clearance in methadone maintenance patients while steady state is being achieved [25].
The 54% larger Vc/F for (R)-methadone is in close agreement with the known stereoselectivity of plasma protein binding of methadone [7, 33] and the reported Vdss/F values after acute administration in chronic pain patients [6]. The observed 70–80% greater values of Vp/F, Vdss/F and Vdβ/F for (R)-methadone compared with (S)-methadone demonstrate that there must be stereoselectivity in the tissue binding of methadone in addition to that known to occur in plasma [7, 33]. Indeed, (R)-methadone Vc/F (range 78–198 l) was greater than for (S)-methadone (range 54–137 l) in each subject (range of differences +24 to +77 l). In contrast, others [35] have reported much lower mean values for Vc/F for (R)-methadone compared with (S)-methadone. This could be due to three of the eight subjects with extremely high values for Vc/F (>300 l) for (S)-methadone [35]. At present, we cannot readily explain these contrasting observations.
The markedly longer terminal half-life of (R)-methadone (51 h) compared with (S)-methadone (31 h, P < 0.0001) is consistent with that observed in chronic pain patients [6] and was primarily due to stereoselective distribution. Stereoselectivity in the rate of accumulation of methadone is further reinforced by the 66% greater MRT for (R)-methadone (69 h) compared with (S)-methadone (41 h, P < 0.0001). Cmax was on average 85% that of the (S)-enantiomer (P < 0.0001), probably due to the stereoselectivity in volume of distribution, while trough plasma concentrations of the enantiomers were equivalent, due to a lack of stereoselectivity in clearance. The effect of stereoselectivity in distribution, but not clearance, manifests itself in marked changes in the ratio of plasma (R)- to (S)-methadone concentrations over the dosing interval, and highlights the importance of stereoselective quantification of methadone for examining pharmacokinetic–pharmacodynamic relationships.
There are several clinical implications emanating from our study. First, the time to reach steady-state concentrations of methadone is markedly longer for the active (R)-enantiomer than for the racemic compound, amounting to a difference of approximately 2.5 days when calculated over five half-lives. This has implications for the routine monitoring of changes in plasma concentration after dosage modification. Second, the apparent oral clearance of methadone displayed a seven-fold interindividual variability and was not significantly related to the demographic factors of weight, age and sex, or to complaints of withdrawal symptoms or plasma α1-acid glycoprotein concentration. Hence, dosage based on demographic characteristics (particularly body weight) is likely to be futile. While it is generally assumed that the trough plasma methadone concentration is inversely related to clearance, this is not necessarily the case, as for methadone with pronounced multicompartment kinetics the contribution of the distribution phase to the trough plasma concentration can be considerable and can mask the clearance component. Third, we have demonstrated that the clearance of methadone can be easily estimated from one or two conveniently timed plasma samples during a dosing interval. Thus, if a patient consistently experiences withdrawal symptoms during an interdosing interval and requests a dosage increase, then knowledge of that patient's clearance value could assist in making the decision for further dosage increase or use of an alternative opioid. Finally, if a clearance estimate is obtained during a period of known adherence, this value may be used as a reference for the future and may assist clinicians in differentiating between pharmacokinetic and pharmacodynamic factors if a drug–drug interaction is suspected, or identifying suspected poor adherence (diversion or supplementation).
Acknowledgments
The results were presented in part at the annual meeting of the Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists in December 2000. We thank Kyle Dyer, David Newcombe, Timothy Mitchell, Julia Hanna and Amanda Stegink for the recruiting of subjects and collection of blood samples, and Andrew Menelaou for performing plasma sample analysis. The authors thank the National Institute on Drug Abuse for supplying the drug compounds for analytical purposes and for financial support (Grant no. 1 R01 DA 13706-02), and also the Royal Adelaide Hospital, the Faculty of Health Sciences of the University of Adelaide, and the National Health and Medical Research Council of Australia (Grant no. 990586) for financial support. We thank the staff at the Warinilla Clinic of the South Australian Methadone Maintenance Programme for their invaluable assistance in this project. Dr Andrew McLachlan is gratefully acknowledged for his advice regarding the population pharmacokinetic modelling.
References
- 1.Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. Br Med J. 1994;309:997–1001. doi: 10.1136/bmj.309.6960.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kristensen K, Christensen CB, Christrup LL. The µ1, µ2, δ, κ opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:45–50. doi: 10.1016/0024-3205(94)00426-s. [DOI] [PubMed] [Google Scholar]
- 3.Scott CC, Robbins EB, Chen KK. Pharmacologic comparison of the optical isomers of methadon. J Pharmacol Exp Ther. 1948;93:282–6. [PubMed] [Google Scholar]
- 4.Isbell H, Eisenman AJ. The addiction liability of some of the drugs of the methadon series. J Pharmacol Exp Ther. 1948;93:305–13. [PubMed] [Google Scholar]
- 5.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–93. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- 6.Kristensen K, Blemmer T, Angelo HR, et al. Stereoselective pharmacokinetics of methadone in chronic pain patients. Ther Drug Monit. 1996;18:221–7. doi: 10.1097/00007691-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Foster DJR, Somogyi AA, Dyer KR, White JM, Bochner F. Steady-state pharmacokinetics of (R)- and (S)-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2000;50:427–40. doi: 10.1046/j.1365-2125.2000.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iribarne C, Berthou F, Baird S, et al. Involvement of cytochrome P450 3A4 enzyme in the N-demethylation of methadone in human liver microsomes. Chem Res Toxicol. 1996;9:365–73. doi: 10.1021/tx950116m. [DOI] [PubMed] [Google Scholar]
- 9.Moody DE, Alburges ME, Parker RJ, Collins JM, Strong JM. The involvement of cytochrome P450 3A4 in the N-demethylation of l-α-acetylmethadol (laam), norlaam, and methadone. Drug Metab Dispos. 1997;25:1347–53. [PubMed] [Google Scholar]
- 10.Foster DJR, Somogyi AA, Bochner F. Methadone N-demethylation in human liver microsomes: lack of stereoselectivity and involvement of CYP3A4. Br J Clin Pharmacol. 1999;47:403–12. doi: 10.1046/j.1365-2125.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begre S, von Bardeleben U, Ladewig D, et al. Paroxetine increases steady-state concentrations of (R)-methadone in CYP2D6 extensive but not poor metabolizers. J Clin Psychopharmacol. 2002;22:211–5. doi: 10.1097/00004714-200204000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Charlier C, Dessalles M-C, Plomteux G. Methadone maintenance treatment: is it possible to adapt the daily doses to the metabolic activity of the patient? Ther Drug Monit. 2001;23:1–3. doi: 10.1097/00007691-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Boulton DW, Arnaud P, DeVane CL. A single dose of methadone inhibits cytochrome P-4503A activity in healthy volunteers as assessed by the urinary cortisol ratio. Br J Clin Pharmacol. 2001;51:350–4. doi: 10.1046/j.1365-2125.2001.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinderman M, Maxwell S, Brawand-Amey M, Golay KP, Baumann P, Eap CB. Cytochrome P4503A4 metabolic activity, methadone blood concentrations, and methadone doses. Drug Alcohol Depend. 2003;69:205–11. doi: 10.1016/s0376-8716(02)00320-4. [DOI] [PubMed] [Google Scholar]
- 15.Somogyi AA. Proceedings of an Expert Workshop on the Induction and Stabilisation of Patients onto Methadone. Adelaide, South Australia: Commonwealth of Australia; 1999. Overview of methadone pharmacokinetics. [Google Scholar]
- 16.Eap CB, Bourquin M, Martin J, et al. Plasma concentrations of the enantiomers of methadone and therapeutic response in methadone maintenance treatment. Drug Alcohol Depend. 2000;61:47–54. doi: 10.1016/s0376-8716(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 17.Dyer KR, Foster DJR, White JM, Somogyi AA, Menelaou A, Bochner F. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: comparison of those who do and do not experience withdrawal and concentration–effect relationships. Clin Pharmacol Ther. 1999;65:685–94. doi: 10.1016/S0009-9236(99)90090-5. [DOI] [PubMed] [Google Scholar]
- 18.Dyer KR, White JM, Foster DJR, Bochner F, Menelaou A, Somogyi AA. The relationship between mood state and plasma methadone concentration in methadone maintenance patients. J Clin Psychopharmacol. 2001;21:78–84. doi: 10.1097/00004714-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Foster DJR, Somogyi AA, Bochner F. Stereoselective quantification of methadone and its major oxidative metabolite, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, in human urine using high-performance liquid chromatography. J Chromatogr B. 2000;744:165–76. doi: 10.1016/s0378-4347(00)00246-2. [DOI] [PubMed] [Google Scholar]
- 20.FDA guidelines. Guidence for Industry: Population pharmacokinetics. Rockville, MD, USA: US Food and Drug Administration Centre for Drug Evaluation and Research; 1998. [Google Scholar]
- 21.P-Pharm Users Manual. Philadelphia: Innaphase Corporation; Version 1.51. [Google Scholar]
- 22.Nath CE, McLachlan AJ, Shaw PJ, Gunning R, Earl JW. Population pharmacokinetics of amphotericin B in children with malignant diseases. Br J Clin Pharmacol. 2001;52:671–80. doi: 10.1046/j.1365-2125.2001.01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowland M, Tozer TN. Clinical PharmacokineticsConcepts and Applications. 3. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- 24.Gibaldi M, Perrier D. Pharmacokinetics. In: Swarbrick J, editor. Drugs and the Pharmaceutical Sciences. Vol. 1. New York: Marcel Dekker; 1975. [Google Scholar]
- 25.Rostami-Hodjegan A, Wolff K, Hay AW, Raistrick D, Calvert R, Tucker GT. Population pharmacokinetics of methadone in opiate users: characterization of time-dependent changes. Br J Clin Pharmacol. 1999;48:43–52. doi: 10.1046/j.1365-2125.1999.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff K, Rostami-Hodjegan A, Hay AW, Raistrick D, Tucker G. Population-based pharmacokinetic approach for methadone monitoring of opiate addicts: potential clinical utility. Addiction. 2000;95:1771–83. doi: 10.1046/j.1360-0443.2000.951217717.x. [DOI] [PubMed] [Google Scholar]
- 27.Wolff K, Hay AW, Raistrick D, Calvert R. Steady-state pharmacokinetics of methadone in opioid addicts. Eur J Clin Pharmacol. 1993;44:189–94. doi: 10.1007/BF00315479. [DOI] [PubMed] [Google Scholar]
- 28.Wolff K, Rostami-Hodjegan A, Shires S, et al. The pharmacokinetics of methadone in healthy subjects and opiate users. Br J Clin Pharmacol. 1997;44:325–34. doi: 10.1046/j.1365-2125.1997.t01-1-00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Vos JW, Geerlings PJ, van den Brink W, Ufkes JG, van Wilgenburg H. Pharmacokinetics of methadone and its primary metabolite in 20 opiate addicts. Eur J Clin Pharmacol. 1995;48:361–6. doi: 10.1007/BF00194951. [DOI] [PubMed] [Google Scholar]
- 30.Bruno R, Vivler N, Vergniol JC, De Phillips SL, Montay G, Sheiner LB. A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J Pharmacokinet Biopharm. 1996;24:153–72. doi: 10.1007/BF02353487. [DOI] [PubMed] [Google Scholar]
- 31.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–12. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 32.Plummer JL, Gourlay GK, Cherry DA, Cousins MJ. Estimation of methadone clearance: application in the management of cancer pain. Pain. 1988;33:313–22. doi: 10.1016/0304-3959(88)90290-4. [DOI] [PubMed] [Google Scholar]
- 33.Eap CB, Cuendet C, Baumann P. Binding of d-methadone, l-methadone, and dl-methadone to proteins in plasma of healthy volunteers: role of the variants of α1-acid glycoprotein. Clin Pharmacol Ther. 1990;47:338–46. doi: 10.1038/clpt.1990.37. [DOI] [PubMed] [Google Scholar]
- 34.Hervé F, Duché JC, d'Athis P, Marché C, Barré J, Tillement JP. Binding of disopyramide, methadone, dipyridamole, chlorpromazine, lignocaine and progesterone to the two main genetic variants of human α1-acid glycoprotein: evidence for drug-binding differences between the variants and for the presence of two separate drug-binding sites on α1-acid glycoprotein. Pharmacogenetics. 1996;6:403–15. doi: 10.1097/00008571-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Boulton DW, Arnaud P, DeVane CL. Pharmacokinetics and pharmacodynamics of methadone enantiomers after a single oral dose of racemate. Clin Pharmacol Ther. 2001;70:48–57. doi: 10.1067/mcp.2001.116793. [DOI] [PubMed] [Google Scholar]
- 36.Garrido MJ, Valle M, Calvo R, Trocóniz IF. Altered plasma and brain disposition and pharmacodynamics of methadone in abstinent rats. J Pharmacol Exp Ther. 1999;288:179–87. [PubMed] [Google Scholar]
- 37.Eap CB, Bertschy G, Powell K, Baumann P. Fluvoxamine and fluoxetine do not interact in the same way with the metabolism of the enantiomers of methadone. J Clin Psychopharmacol. 1997;17:113–7. doi: 10.1097/00004714-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Eap CB, Broly F, Mino A, et al. Cytochrome P450 2D6 genotype and methadone steady-state concentrations. J Clin Psychopharmacol. 2001;21:229–34. doi: 10.1097/00004714-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Paine MF, Davis CL, Shen DD, Marsh CL, Raisys VA, Thummel KE. Can oral midazolam predict oral cyclosporine disposition? Eur J Pharm Sci. 2000;12:51–62. doi: 10.1016/s0928-0987(00)00139-1. [DOI] [PubMed] [Google Scholar]
- 40.Yuan CS, Foss JF, O'Connor M, et al. Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial. JAMA. 2000;283:367–72. doi: 10.1001/jama.283.3.367. [DOI] [PubMed] [Google Scholar]
- 41.Lynn RK, Olsen GD, Leger RM, Gordon WP, Smith RG, Gerber N. The secretion of methadone and its major metabolite in the gastric juice of humans: comparison with blood and salivary concentrations. Drug Metab Dispos. 1976;4:504–9. [PubMed] [Google Scholar]
- 42.Bouër R, Barthe L, Philibert C, Tournaire C, Woodley J, Houin G. The roles of P-glycoprotein and intracellular metabolism in the intestinal absorption of methadone: in vitro studies using the rat everted intestinal sac. Fundam Clin Pharmacol. 1999;13:494–500. doi: 10.1111/j.1472-8206.1999.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 43.Störmer E, Perloff MD, von Moltke LL, Greenblatt DJ. Methadone inhibits rhodamine123 transport in Caco-2 cells. Drug Metab Dispos. 2001;29:954–6. [PubMed] [Google Scholar]
- 44.Kreek MJ, Bencsath FA, Fanizza A, Field FH. Effects of liver disease on fecal excretion of methadone and its unconjugated metabolites in maintenance patients. Quantitation by direct probe chemical ionization mass spectrometry. Biomed Mass Spectrom. 1983;10:544–9. doi: 10.1002/bms.1200101003. [DOI] [PubMed] [Google Scholar]
- 45.Kreek MJ, Bencsath FA, Field FH. Effects of liver disease on urinary excretion of methadone and metabolites in maintenance patients: quantitation by direct probe chemical ionization mass spectrometry. Biomed Mass Spectrom. 1980;7:385–95. doi: 10.1002/bms.1200070906. [DOI] [PubMed] [Google Scholar]
- 46.Rostami-Hodjegan A, Foster DJR, Charlier C. A meta-analysis of the dose–concentration relationship for methadone and a nomogram to assess compliance and metabolic activity. J Psycopharmacol. 2001;15:A25. (Abstract) [Google Scholar]
- 47.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–23. [PubMed] [Google Scholar]
- 48.Watkins PB, Turgeon DK, Saenger P, et al. Comparison of urinary 6-β-cortisol and the erythromycin breath test as measures of hepatic P450IIIA (CYP3A) activity. Clin Pharmacol Ther. 1992;52:265–73. doi: 10.1038/clpt.1992.140. [DOI] [PubMed] [Google Scholar]