Abstract
Aim
To determine the effect of food ingestion on the pharmacokinetic profile of solifenacin succinate (YM905; Vesicare®), a new bladder selective muscarinic receptor antagonist for the treatment of overactive bladder, a chronic disease usually caused by involuntary detrusor muscle contractions during bladder filling.
Methods
A randomized, two-period, crossover study in two groups of 12 healthy men (aged 18–45 years, body weight 60–100 kg, body mass index ≤30). A single 10-mg dose of solifenacin was administered to the first group in the fasting state during period 1 and in the fed state during period 2, and to the second group in the fed state during period 1 and in the fasting state during period 2 (10 mg is two times the suggested starting dose). There was a 14-day washout between treatment periods. Parameters obtained included Cmax, AUClast, and AUC0–inf, as well as t1/2, tmax, and tlag.
Results
One subject withdrew during the first period for personal reasons. No statistically or clinically significant pharmacokinetic differences occurred between subjects in the fed and fasting states. All geometric mean ratios were close to 1 (Cmax, 1.033; AUClast, 1.068; AUC0–inf, 1.040). The 90% confidence intervals (CIs) fell in the predefined no-food-effect boundaries of 0.8–1.25 (Cmax, 0.953–1.120; AUClast, 0.990–1.153; AUC0–inf, 0.976–1.109). The mean difference in t1/2 was −3.8 h (90% CI 7.6–0.0). There were no significant differences between the fed and fasting states with regard to tmax and tlag (P > 0.05).
Conclusions
The pharmacokinetics of oral solifenacin was not affected by food ingestion, suggesting that this drug may be administered with or without food. The results observed in this investigation are consistent with those of previous studies of solifenacin.
Keywords: food ingestion, muscarinic receptor antagonist, overactive bladder, pharmacokinetics, solifenacin succinate, YM905
Introduction
Overactive bladder (OAB) is a common chronic disease that is usually caused by involuntary contractions of the detrusor muscle during bladder filling. Symptoms include urinary urgency and frequency, with or without urinary incontinence. This disorder is further characterized by reductions in volume voided/micturition, which suggests a decreased bladder capacity. These bladder contractions are mediated by acetylcholine, which acts through muscarinic acetylcholine receptors found in the smooth muscle of the bladder [1].
Solifenacin succinate (YM905; Vesicare®; Yamanouchi Pharmaceutical Co., Ltd, Tokyo, Japan) is a new, once-daily, orally administered muscarinic receptor antagonist [1, 2]. In animal models, solifenacin, which shows promise for treating OAB at two dosage strengths, 5 mg and 10 mg, has been shown to be selective for the bladder over the salivary glands [1]. Pharmacokinetic (PK) studies have demonstrated that solifenacin has a long elimination half-life (t1/2) of 50 h and a time to maximal plasma concentration (tmax) of 4 h [3, 4]. The absolute bioavailability of solifenacin after a single oral dose of 10 mg was 90%[3].
For a drug such as solifenacin that is administered in tablet form, there is a potential for the pH of the stomach to affect both the degree and rate of breakdown of the tablet, leading to a change in the absorption of the drug [5]. Solifenacin succinate dissolves well in water, even in acidic conditions (K. Fujimoto et al., unpublished observations); therefore, gastric pH was not expected to have a significant effect on its absorption. Other effects of food intake that can affect drug absorption include delayed gastric emptying, stimulation of bile flow, increased splanchnic blood flow, altered lumenal drug metabolism, and chemical or physical drug interactions [6]. The present PK study was conducted to assess the effects of ingestion of food on solifenacin.
Methods
All subjects were screened before study entry and gave informed written consent. Screening included physical examination; medical and medication history; vital signs; 12-lead ECG; drug screening; and laboratory (haematology, biochemistry, and urinalysis), serology, and virology tests.
Healthy men (aged 18–45 years, body weight 60–100 kg, body mass index ≤30) were eligible for inclusion. Exclusion criteria were upper gastrointestinal symptoms within 4 weeks before enrolment; history of narrow-angle glaucoma, urine retention, or clinically significant constipation in the previous 6 months; and clinically significant abnormalities as revealed by physical examination, ECG, clinical laboratory tests, pulse rate, or blood pressure at the prestudy screening.
This was a single-site, open-label, randomized, two-period crossover study with a 14-day washout between treatment periods. Twenty-four eligible men entered the study and were randomized to one of two groups (12 subjects each). Both groups received a single 10-mg oral dose of solifenacin in tablet form at 08.00 h, with 180 ml of water (10 mg is two times the suggested starting dose). Dose selection was based on both clinical relevance and safety as demonstrated by previous clinical data [4]. The first group received the drug in the fasting state during period 1 and in the fed state during period 2. The second group received the drug in the fed state during period 1 and in the fasting state during period 2. Fasting subjects began fasting (except for water) at 22.00 h before dosing and continued for 5 h after receiving the dose. They were given lunch at approximately 13.00 h; during the remainder of the admission period, normal meals with non-alcoholic drinks and decaffeinated beverages were given. Fed subjects followed the same schedule, except that they received the dose within 5 min after a standardized, high-fat (∼50% of total caloric meal content), high-calorie (∼1000 kcal) breakfast of two butter-fried eggs, two bacon strips, 113 g hash-brown potatoes, and 227 g whole milk (∼150 protein kcal, 250 carbohydrate kcal, and 500–600 fat kcal). The meal was selected based on US Food and Drug Administration guidelines [6]; contents were chosen based on their potential for the greatest effects on gastrointestinal physiology, and thus, on systemic drug availability.
Crossover occurred after a 2-week washout. Dosing and food schedules were equivalent to those of period 1. Approximately 2 weeks after the last dose, subjects returned for poststudy follow-up. This final evaluation consisted of physical examination, vital signs, 12-lead ECG, and laboratory tests.
The study protocol was approved by the Research Ethics Committee of the Queen's University of Belfast, Northern Ireland. The study was conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonization/World Health Organization Good Clinical Practice guidelines.
Venous blood samples (6 ml) were collected before dosing and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, 24, 36, 48, 96, and 144 h after the first and second dosing. Blood samples were collected in standard polyethylene tubes containing lithium heparin as an anticoagulant. Samples were kept chilled in ice and were centrifuged within 30 min of collection at ∼4 °C for 10 min at 1500 × g. Plasma (∼3 ml from a 6-ml blood sample) was harvested and stored at −70 °C at the study site in appropriately labelled tubes before being sent to the Bioanalysis and Drug Metabolism Section of Yamanouchi Europe BV (Leiderdorp, the Netherlands). Bioanalysis was performed using a validated tandem liquid chromatography–mass spectrometry method, based on liquid–liquid extraction followed by reversed-phase high-performance liquid chromatography. The limit of quantification was solifenacin 0.5 ng ml−1 in plasma and was linear for the concentration range from 0.5 to 1000 ng ml−1. Mean 3-day accuracy was assessed at low, medium, and high concentrations of solifenacin as −9.7%, −7.4%, and 6.0% (n = 6 at all levels), respectively; the within-day precision values were 4.4%, 4.4%, and 1.8%, whereas the between-day values were 6.8%, 6.6%, and 5.4%.
Noncompartmental PK analysis was performed using WinNonlin version 1.1 (Scientific Consulting, Inc., Apex, NC, USA). The following parameters were obtained: maximum plasma concentration (Cmax), area under the curve from time 0 to the last measurable plasma concentration (AUClast), area under the curve from time 0 to infinity (AUC0–inf), t1/2, tmax, and time to first measurable concentration (tlag). Cmax, tmax, and tlag values were determined from observed data. The linear logarithmic/trapezoidal rule was used to calculate AUClast. AUC0–inf values were calculated by dividing the predicted concentration of the last quantifiable sample by the terminal elimination rate constant and adding this value to AUClast. Values for t1/2 were calculated by linear regression of the log-transformed plasma concentration data without weighting.
Safety parameters assessed included incidence of adverse events (AEs), vital signs, ECGs, and laboratory tests (haematology, biochemistry, and urinalysis). Subjects were questioned about AEs at admission, immediately after dosing, and 1, 2, 5, and 7 days postdosing. The intensity of each AE was recorded as mild, moderate, or severe. The drug's relation to the AE was recorded as probable, possible, or unlikely. Vital signs and ECGs were recorded immediately before dosing and 4 and 24 h postdosing. Laboratory tests were conducted and recorded at baseline, at 24 h after dosing, and at poststudy follow-up.
After logarithmic transformation, data for AUC0–inf, AUClast, and Cmax were subjected to analysis of variance using SAS PROC GLM, option type SS3 (SAS Institute Inc., Cary, NC, USA). For AUC0–inf, AUClast, and Cmax, 90% confidence intervals (CIs) were determined for the ratios between fed and fasting states. Per US government guidelines, if the 90% CIs fell within the prespecified interval of 0.8–1.25, food intake would be determined to have no clinically relevant effect on solifenacin [6]. Effects of food on tmax, t1/2, and tlag were determined using Wilcoxon's matched-pairs signed ranks.
Results
Twenty-three of 24 subjects completed both periods of the study. One subject withdrew from the study (due to work commitments) during period 1 after taking the first dose of solifenacin and was therefore not included in the PK analyses. All 24 subjects received at least one dose of the study drug and were included in the safety analyses. Subject demographics are shown in Table 1. No clinically significant abnormalities were noted in physical examination, ECG, vital signs, serology, biochemistry, haematology, or urinalysis findings at pretreatment screening.
Table 1. Study data*.
| Mean ± SD | Cmax (ng ml−1) | AUClast (ng h−1 ml−1) | AUC0–inf (ng h−1 ml−1) | tlag (h)† | tmax (h)‡ | t1/2 (h) | |
|---|---|---|---|---|---|---|---|
| Subject demographics (n = 24) | |||||||
| Age, years (range) | 28.4 ± 7.0 | ||||||
| (19–41) | |||||||
| Height, cm | 176.7 ± 6.2 | ||||||
| (164–186) | ' | ||||||
| Weight, kg | 73.7 ± 10.1 | ||||||
| (60–99) | |||||||
| Body mass index | 23.6 ± 2.93 | ||||||
| (19.4–29.4) | |||||||
| Pharmacokinetic parameters§ | |||||||
| Fasting | 14.1 ± 4.3 | 691 ± 313 | 820 ± 423 | 0.46 ± 0.33 | 6.0 (3–12 [8]) | 50.8 ± 13.5 | |
| Fed | 14.7 ± 4.9 | 736 ± 290 | 842 ± 373 | 0.63 ± 0.48 | 5.8 (3–8 [6]) | 46.8 ± 10.7 | |
| Geometric mean | |||||||
| Fasting | 13.6 | 652 | 758 | ||||
| Fed | 14.0 | 697 | 789 | ||||
| Geometric mean ratio | 1.033 | 1.068 | 1.040 | ||||
| 90% CI | 0.953–1.120 | 0.990–1.153 | 0.976–1.109 | ||||
All values given as mean ± SD, unless otherwise noted.
Mean difference (fasting vs. fed); P > 0.05.
Reported as value (range [median]).
Following administration of a single oral dose of solifenacin 10 mg which is two times the suggested starting dose.
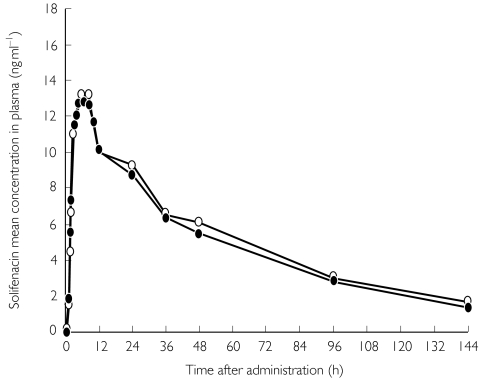
All PK parameters assessed in this study were similar between subjects in the fed and fasting states (Table 1). Mean plasma concentration–time profiles of solifenacin obtained in the fed and fasting states were also similar (Figure 1). The mean difference in t1/2 was −3.8 h; the 90% CI ranged from 7.6-0.0. There were no significant differences between the fed and fasting states with regard to tmax and tlag (P > 0.05) (Table 1).
Figure 1.
Mean plasma concentrations of solifenacin after oral administration of a single 10-mg dose in healthy men (n = 23) under fed or fasting conditions. fed (○), fasted (•)
Geometric mean ratios and their 90% CIs for Cmax, AUClast, and AUC0–inf are shown in Table 1. These ratios were all very close to 1 and their 90% CI fell within the prespecified interval of 0.8–1.25.
Of the 24 subjects who received at least one dose of the study drug, 13 (54%) experienced AEs. Only four (17%) reported AEs probably or possibly related to the study drug. The most commonly reported AE with possible relation to treatment was headache (n = 3). Dry mouth was the only AE considered probably drug related (n = 1). All AEs considered possibly or probably treatment related (fasting, n = 5; fed, n = 3) were mild in intensity, and none led to discontinuation. There were no clinically significant changes in vital signs, physical examination, laboratory values, or ECG following dosing.
Discussion
In the present study, the pharmacokinetics of oral solifenacin was not clinically or statistically significantly affected by the ingestion of food. While tlag was slightly increased in fed subjects, tmax was actually slightly shorter, suggesting that food causes no relevant delay in absorption. The absence of a significant effect on the pharmacokinetics of solifenacin under the ‘extreme’ conditions represented by this high-fat, high-calorie meal provides additional support for the unlikelihood of a significant PK effect on solifenacin occurring with food intake in general. The PK results observed in this study are consistent with those of previous studies of solifenacin [3, 4].
In conclusion, solifenacin was well tolerated in this study, as in previous investigations [3, 4]. There was no significant difference in the incidence of AEs between subjects in the fed and fasting states. Our results indicate that oral solifenacin may be administered to patients without regard to food intake.
Acknowledgments
T.U. is an employee of Yamanouchi Pharmaceutical Co., Ltd, Tokyo, Japan. W.J.K., H.M., and R.A.S. are employees of Yamanouchi Europe B.V., Leiderdorp, the Netherlands. This study was supported by Yamanouchi Pharmaceutical Co.
References
- 1.Ikeda K, Kobayashi S, Suzuki M, et al. M(3) receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:97–103. doi: 10.1007/s00210-002-0554-x. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi S, Ikeda K, Suzuki M, Yamada T, Miyata K. Effects of YM905, a novel muscarinic M3-receptor antagonist, on experimental models of bowel dysfunction in vivo. Jpn J Pharmacol. 2001;86:281–8. doi: 10.1254/jjp.86.281. [DOI] [PubMed] [Google Scholar]
- 3.Kuipers M, Tran D, Krauwinkel W, Abila B, Mulder H. Heidelberg, Germany: 2002. Absolute Bioavailability of YM905 in Healthy Male Volunteers: a Single-Dose, Randomized, Two-Period Crossover Study. Presented at the 32nd International Continence Society Annual Meeting. [Google Scholar]
- 4.Smulders R, Tan H, Krauwinkel W, Abila B, van Zitjveld J. Heidelberg, Germany: 2002. A Placebo-Controlled, Dose-Rising Study in Healthy Male Volunteers to Evaluate Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Single Oral Doses of YM905. Presented at the 32nd International Continence Society Annual Meeting, August. [Google Scholar]
- 5.Williams L, Hill D, Davis J, Lowenthal D. The influence of food on the absorption and metabolism of drugs: an update. Eur J Drug Metab Pharmacokinet. 1996;21:201–11. doi: 10.1007/BF03189714. [DOI] [PubMed] [Google Scholar]
- 6.Guidance for Industry: Food-Effect Bioavailability and Bioequivalence Studies. Rockville, MD: Center for Drug Evaluation and Research, Food and Drug Administration, US Department of Health and Human Services; 2002. [Google Scholar]