Abstract
Aims
Simvastatin, a substrate for CYP3A4, is extensively metabolized during the first pass. Our aim was to investigate the effect of regular consumption of grapefruit juice on the pharmacokinetics of simvastatin.
Methods
In a randomized cross-over study with two phases, 10 healthy volunteers ingested grapefruit juice 200 ml or water (control) for 3 days. On day 3, a single 40-mg dose of simvastatin was administered with grapefruit juice 200 ml or water. Plasma concentrations of simvastatin and simvastatin acid were determined up to 24 h.
Results
Grapefruit juice increased the area under the plasma concentration–time curves from 0 to 24 h [AUC(0–24)] of simvastatin 3.6-fold (range 1.8–6.0-fold; P < 0.01) and that of simvastatin acid 3.3-fold (range 2.1–5.6-fold; P < 0.01), respectively. The peak concentrations (Cmax) of simvastatin and simvastatin acid were increased 3.9-fold (range 2.3–9.3-fold; P < 0.01) and 4.3-fold (range 2.7–7.9-fold; P < 0.01) by grapefruit juice.
Conclusions
Even one glass of grapefruit juice, taken daily, considerably increases the plasma concentrations of simvastatin and simvastatin acid. Grapefruit juice may increase both the cholesterol-lowering effect and the risk of adverse effects of simvastatin.
Keywords: food–drug interaction, grapefruit juice, simvastatin
Introduction
Simvastatin, an HMG-CoA reductase inhibitor, is widely used in the treatment of hypercholesterolaemia. It has two separate metabolic pathways. The oxidative biotransformation of simvastatin is mediated primarily by CYP3A4 [1]. In the other primary route the inactive lactone prodrug is hydrolysed to the pharmacologically active simvastatin acid by carboxyl esterases and also non-enzymatically. Because of the extensive CYP3A4-mediated metabolism already during the first pass, simvastatin has a low mean oral bioavailability of about 5%[2, 3]. Some of the metabolites of simvastatin also show pharmacological activity. Simvastatin is usually well tolerated. However, high plasma concentrations increase the risk of musculoskeletal adverse effects. Thus, myopathies and even rhabdomyolysis with acute renal failure have been associated with simultaneous use of simvastatin with CYP3A4 inhibitors, e.g. erythromycin [4], diltiazem [5] and itraconazole [6], that increase plasma concentrations of simvastatin and simvastatin acid.
Grapefruit juice has increased plasma concentrations of dihydropyridine calcium channel blockers [7], cyclosporin [8], midazolam [9], and some HMG-CoA reductase inhibitors [10–12], probably by a selective downregulation of CYP3A4 at duodenal epithelium where CYP3A4 is localized [13]. Repeated consumption of high quantities of grapefruit juice has increased the AUC values of lovastatin and simvastatin over 10-fold [10, 11]. In a previous study a daily volume of 250 ml of grapefruit juice had a modest effect on lovastatin, but in that study grapefruit juice had been taken about 12 h apart from statin [14]. The present study was conducted to investigate the effect of a single daily glass of grapefruit juice on the pharmacokinetics of simvastatin and its active metabolite, simvastatin acid.
Methods
Subjects
Ten healthy volunteers (all men; age range 20–24 years; weight range 63–80 kg) participated in the study. Each subject was ascertained to be in good health by a medical history, clinical examination, and routine laboratory testing. The volunteers were not using any continuous medication, and all of them were nonsmokers. The consumption of grapefruit products was forbidden during the study (from 2 weeks before the first study day). The study protocol was approved by the Ethics Committee for studies in Healthy Subjects of the Hospital District Helsinki and Uusimaa, and by the Finnish National Agency for Medicines. The subjects gave written informed consent before entering the study.
Study design
A randomized crossover study design with two phases was used at interval of 2 weeks. The volunteers ingested 200 ml normal-strength grapefruit juice (Valio Ltd, Helsinki, Finland) or water at 08.00 h for 2 days (with breakfast). On the third day the subjects received 40 mg simvastatin (Zocor, MSD, Haarlem, the Netherlands) at 09.00 h with 200 ml grapefruit juice or water. The volunteers fasted overnight before administration of simvastatin, and a standardized warm meal was served 3 h and a standardized warm light meal 7 h after simvastatin intake. The subjects were not allowed to drink coffee, tea, or cola during the study days.
Blood sampling
On the third study day, a plastic cannula was inserted into a forearm vein of each participant and was kept patent with an obturator. Timed blood samples were drawn into ethylenediaminetetraacetic acid (EDTA)-containing tubes before the administration of simvastatin and 0.5, 1, 2, 3, 4, 6, 8, 10, 12, and 24 h later. Plasma was separated within 30 min and stored at −70 °C until analysis.
Determination of plasma simvastatin and simvastatin acid concentrations
Plasma simvastatin and simvastatin acid concentrations were measured by liquid chromatography–ion spray tandem mass spectrometry [15]. The limit of quantification was 0.10 ng ml−1 for simvastatin and simvastatin acid. The between-day coefficient of variation (CV) was 14.8% at 0.10 ng ml−1, 6.2% at 5 ng ml−1, and 5.2% at 50 ng ml−1 (n = 3) for simvastatin and 15.3% at 0.10 ng ml−1, 8.5%at 5 ng ml−1, and 11.5%at 50 ng ml−1 (n = 3) for simvastatin acid.
Pharmacokinetic analysis
The Cmax and tmax of simvastatin and simvastatin acid were obtained directly from the original data. The terminal log-linear phase of the plasma concentration–time curve was visually identified for each curve. The elimination rate constant (ke) was determined by a linear regression analysis of the log-linear part of the plasma concentration–time curve. The area under the plasma concentration–time curve up to the last quantified data point [AUC(0–24)] was calculated by the linear trapezoidal rule. The elimination half-life (t1/2) was determined by the equation:
Statistical analysis
The data are expressed as mean values ± SD, except for tmax, which is presented as median with range. For all variables, except tmax, 95% confidence intervals (CI) were calculated on the mean difference between water and grapefruit juice phases. Data were analysed by Student t-test (two-tailed) for paired values or, in case of tmax, by the Wilcoxon test. The statistical program Systat for Windows, version 6.0.1 (Systat, Evanston, IL, USA) was used for the analysis. Differences were considered statistically significant at P < 0.05.
Results
Simvastatin
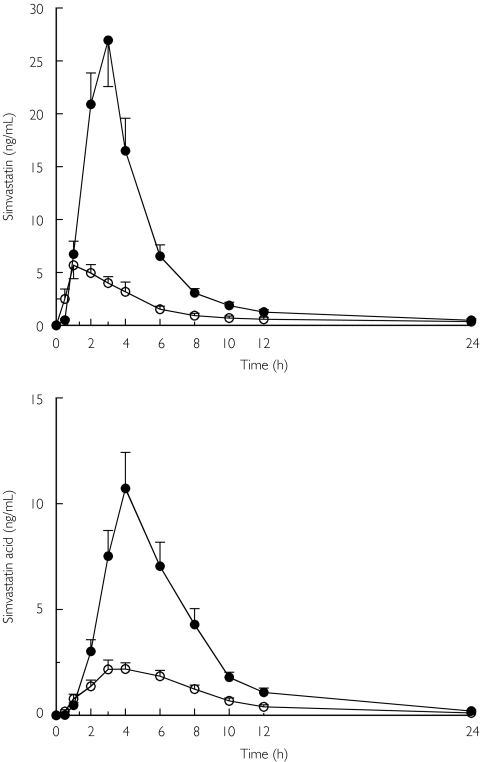
Grapefruit juice considerably increased plasma concentrations of simvastatin in each subject (Figure 1 and Table 1). The mean AUC(0–24) of simvastatin was increased 3.6-fold (range 1.8–6.0-fold; P < 0.001) and the mean Cmax was increased 3.9-fold (range 2.3–9.3-fold; P < 0.001) by ingestion of grapefruit juice. There were no statistically significant changes between the grapefruit juice and water phases in the tmax and t1/2 of simvastatin.
Figure 1.
Mean plasma concentrations of simvastatin (upper panel) and simvastatin acid (lower panel) after administration of a single 40-mg dose of simvastatin with 200 ml water (control) (○) or grapefruit juice (•) ingested once daily for 3 days
Table 1. The pharmacokinetic variables of simvastatin and simvastatin acid in 10 healthy subjects after the ingestion of a single dose of 40 mg simvastatin with water (control) or grapefruit juice 200 ml ingested once daily for 3 days.
| Variable | Water phase (control) | Grapefruit juice phase | Mean difference between water and grapefruit juice phase (95% CI) |
|---|---|---|---|
| Simvastatin | |||
| Cmax (ng ml−1) | 7.3 ± 3.4 | 28.5 ± 13.0* | 21.3 (12.6, 30.0) |
| Percentage of control (range) | 100% | 393% (232–932%) | |
| tmax (h) | 1.5 (1–4) | 3 (2–3) | |
| t1/2 (h) | 2.7 ± 1.9 | 2.0 ± 0.7 | −0.7 (−1.6, 0.2) |
| AUC(0–24) (ng ml−1 h−1) | 31.6 ± 17.2 | 112.5 ± 42.6* | 80.8 (54.6, 107.1) |
| Percentage of control (range) | 100% | 355% (178–604%) | |
| Simvastatin acid | |||
| Cmax (ng ml−1) | 2.6 ± 1.3 | 11.1 ± 5.1* | 8.5 (5.5, 11.5) |
| Percentage of control (range) | 100% | 432% (267–793%) | |
| tmax (h) | 4 (3–6) | 4 (3–4) | |
| t1/2 (h) | 2.9 ± 0.7 | 2.4 ± 0.5** | −0.5 (−0.9, −0.0) |
| AUC(0–24) (ng ml−1 h−1) | 18.5 ± 9.7 | 62.0 ± 29.9* | 43.5 (25.0, 62.0) |
| Percentage of control (range) | 100% | 334% (206–558%) | |
Data are mean values (± SD); tmax values are given as medians with ranges. Cmax, Peak plasma concentration; tmax, time to reach Cmax; t1/2, elimination half-life; AUC(0–24), area under the plasma concentration–time curve from time 0 to infinity.
P < 0.001 vs. control phase (water);
P < 0.05 vs. control phase (water).
Simvastatin acid
The mean AUC(0–24) and Cmax of simvastatin acid were increased about 3.3-fold and 4.3-fold [AUC(0–24): range 2.1–5.6-fold; P < 0.001; Cmax: range 2.7–7.7-fold; P < 0.001] by grapefruit juice compared with water (control). The tmax of simvastatin acid was unchanged, but the t1/2 averaged 2.9 h in the water phase and 2.4 h in the grapefruit juice phase (P < 0.05).
Discussion
The present study shows that even once daily consumption of a glass of grapefruit juice considerably increases plasma concentrations of simvastatin. There was a marked interindividual variability in the grapefruit juice–simvastatin interaction, e.g. the increase in the Cmax of simvastatin ranged from 2.3-fold to 9.3-fold and that in the AUC(0–24) from 1.8-fold to 6.0-fold. These findings show that the quantitative effects of grapefruit juice on the pharmacokinetics of simvastatin are hardly predictable on an individual level. For safety reasons, only a single dose of simvastatin was administered during both phases in the present study. However, previous studies suggest that the effect of grapefruit juice remains also in a steady-state administration of the study drug [16, 17].
The increased Cmax and AUC of simvastatin and simvastatin acid during the grapefruit juice phase can be explained by inhibition of their CYP3A4-mediated first-pass metabolism in the intestinal wall. Because of its extensive CYP3A4-mediated first-pass metabolism, simvastatin is susceptible to metabolic pharmacokinetic interactions. For example, the potent CYP3A4 inhibitor itraconazole increased simvastatin AUC 19-fold [18] and double-strength grapefruit juice consumed t.i.d. for 3 days increased the AUC of simvastatin about 16-fold [11]. Two calcium channel blockers, verapamil and diltiazem, increased simvastatin AUC almost five-fold [19, 20]. Thus, the effect of one daily glass of grapefruit juice is comparable to these two calcium-channel blockers. Grapefruit juice increased simvastatin acid concentrations almost to the same degree as those of simvastatin. This can be explained by two mechanisms: first, due to the dynamic equilibrium between simvastatin lactone and simvastatin acid, and second, also the metabolism of simvastin acid by CYP3A4 can be inhibited by grapefruit juice.
This study suggests that even a small amount of grapefruit juice contains sufficiently active ingredients to inhibit considerably the first-pass metabolism of simvastatin in the intestinal wall. As a result of a considerable increase in simvastatin acid concentrations, an augmentation in both the cholesterol lowering effect and the risk of concentration-dependent adverse effects of simvastatin can be anticipated. In practice, it can be difficult to predict the increase in the therapeutic effect of simvastatin by grapefruit juice, because of the large interindividual variation in CYP3A4-mediated drug metabolism. For example, variation between juice batches and the potential of grapefruit juice to interact with other drugs are disadvantages of augmentation of simvastatin bioavailability by grapefruit juice. Common orange juice instead of grapefruit juice could be coadministered with simvastatin to avoid the potentially harmful interaction. Pravastatin, fluvastatin and rosuvastatin are HMG-CoA reductase inhibitors not metabolized to a relevant degree by CYP3A4, and hence their plasma concentrations are not likely to be markedly increased by concomitant ingestion of grapefruit juice [12].
In a previous study, a 250-ml volume of grapefruit juice ingested once daily for 3 days increased the AUC of lovastatin and lovastatin acid by 94% and 57%, respectively [14]. The authors of that study concluded that grapefruit juice administered once daily has only a minor effect on the pharmacokinetics of lovastatin. However, the range in the magnitude of the interaction (1.2–4.3-fold increase in the AUC of lovastatin) suggests that grapefruit juice–lovastatin interaction can be important in some subjects even if administered 12 h apart [14]. Results of that study and those of our study can be reconciled if different dosing schedules of grapefruit juice are taken into consideration, i.e. in the former study grapefruit juice was ingested 12 h before administration of lovastatin and in the present study grapefruit juice and simvastatin were ingested simultaneously. The effect of grapefruit juice on simvastatin pharmacokinetics dissipates considerably during the first 24 h after ingestion of grapefruit juice [15].
To conclude, once-daily consumption of normal-strength grapefruit juice considerably increases plasma concentrations of simvastatin and simvastatin acid, probably by inhibiting their CYP3A4-mediated first-pass metabolism in the intestinal wall. Because of the extent of this interaction and its unpredictability on an individual level, the concomitant intake of grapefruit juice and simvastatin is not recommended.
Acknowledgments
This study was supported by grants from the Helsinki University Central Hospital Research Fund and the Sigrid Juselius Foundation. We thank Mr Jouko Laitila, Mrs Eija Mäkinen-Pulli and Mrs Lisbet Partanen for skilful technical assistance.
References
- 1.Prueksaritanont T, Gorham LM, Ma B, et al. In vitro metabolism of simvastatin in humans: identification of metabolizing enzymes and effect of the drug on hepatic P450s. Drug Metab Dispos. 1997;25:1191–9. [PubMed] [Google Scholar]
- 2.Vickers S, Duncan CA, Vyas KP, et al. In vitro and in vivo biotransformation of simvastatin, an inhibitor of HMG CoA reductase. Drug Metab Dispos. 1990;18:476–83. [PubMed] [Google Scholar]
- 3.Vickers S, Duncan CA, Chen IW, Rosegay A, Duggan DE. Metabolic interaction studies on simvastatin, a cholesterol-lowering prodrug. Drug Metab Dispos. 1990;18:135–45. [PubMed] [Google Scholar]
- 4.Segaert MF, De Soete C, Vandewiele I, Verbanc J. Drug interaction-induced rhabdomyolysis. Nephrol Dial Transplant. 1996;11:1846–7. [PubMed] [Google Scholar]
- 5.Kanathur N, Mathai MG, Byrd RP, Jr, Fields CL, Roy TM. Simvastatin–diltiazem drug interaction resulting in rhabdomyolysis and hepatitis. Tenn Med. 2001;94:339–41. [PubMed] [Google Scholar]
- 6.Horn M. Coadministration of itraconazole with hypolipidemic agents may induce rhabdomyolysis in healthy individuals. Arch Dermatol. 1996;132:1254. doi: 10.1001/archderm.1996.03890340120028. [letter] [DOI] [PubMed] [Google Scholar]
- 7.Bailey DG, Spence JD, Munoz C, Arnold JMO. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991;337:268–9. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- 8.Ducharme MP, Warbasse LH, Edwards DJ. Disposition of intravenous and oral cyclosporine after administration with grapefruit juice. Clin Pharmacol Ther. 1995;57:485–91. doi: 10.1016/0009-9236(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 9.Kupferschmidt HHT, Ha HR, Ziegler WH, Meier PJ, Krähenbühl S. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58:20–8. doi: 10.1016/0009-9236(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 10.Kantola T, Kivistö KT, Neuvonen PJ. Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther. 1998;63:397–402. doi: 10.1016/S0009-9236(98)90034-0. [DOI] [PubMed] [Google Scholar]
- 11.Lilja JJ, Kivistö KT, Neuvonen PJ. Grapefruit juice–simvastatin interaction: effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin Pharmacol Ther. 1998;64:477–83. doi: 10.1016/S0009-9236(98)90130-8. [DOI] [PubMed] [Google Scholar]
- 12.Lilja JJ, Kivistö KT, Neuvonen PJ. Grapefruit juice increases serum concentrations of atorvastatin and has no effect on pravastatin. Clin Pharmacol Ther. 1999;66:118–27. doi: 10.1053/cp.1999.v66.100453001. [DOI] [PubMed] [Google Scholar]
- 13.Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99:2545–53. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers JD, Zhao J, Liu L, et al. Grapefruit juice has minimal effects on the plasma concentrations of lovastatin-derived 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Clin Pharmacol Ther. 1999;66:358–66. doi: 10.1053/cp.1999.v66.a101208. [DOI] [PubMed] [Google Scholar]
- 15.Lilja JJ, Kivistö KT, Neuvonen PJ. Duration of effect of grapefruit juice on the CYP3A4 substrate simvastatin. Clin Pharmacol Ther. 2000;68:384–90. doi: 10.1067/mcp.2000.110216. [DOI] [PubMed] [Google Scholar]
- 16.Lundahl JU, Regårdh CG, Edgar B, Johnsson B. The interaction effect of grapefruit juice effect is maximal after the first glass. Eur J Clin Pharmacol. 1998;54:75–81. doi: 10.1007/s002280050424. [DOI] [PubMed] [Google Scholar]
- 17.Dresser GK, Bailey DG, Carruthers SG. Grapefruit juice–felodipine interaction in the elderly. Clin Pharmacol Ther. 2000;68:28–34. doi: 10.1067/mcp.2000.107524. [DOI] [PubMed] [Google Scholar]
- 18.Neuvonen PJ, Kantola T, Kivistö KT. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin Pharmacol Ther. 1998;63:332–41. doi: 10.1016/S0009-9236(98)90165-5. [DOI] [PubMed] [Google Scholar]
- 19.Kantola T, Kivistö KT, Neuvonen PJ. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther. 1998;64:177–82. doi: 10.1016/S0009-9236(98)90151-5. [DOI] [PubMed] [Google Scholar]
- 20.Mousa O, Brates C, Sundblad KJ, Hall SD. The interaction of diltiazem with simvastatin. Clin Pharmacol Ther. 2000;67:267–74. doi: 10.1067/mcp.2000.104609. [DOI] [PubMed] [Google Scholar]