Abstract
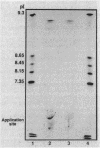
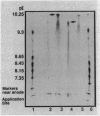
Staphylococcus aureus produces four types of beta-lactamase (A, B, C, and D). To investigate the effect of specific beta-lactamase type upon staphylococcal resistance, each beta-lactamase was purified to homogeneity, and the Michaelis constants (Km values) and turnover numbers (kcat values) for various penicillin and cephalosporin substrates were determined. Whereas Km values of the four beta-lactamases were comparable for penicillin G, cephalothin, and cefamandole, the type A and D enzymes exhibited greater affinity than the type B and C beta-lactamases for nitrocefin, cefazolin, and cephapirin. Conversely, the type B and C beta-lactamases exhibited greater kcat values than the type A and D enzymes against most of the cephalosporin agents, excluding nitrocefin. In contrast to earlier reports suggesting that the type B beta-lactamase is relatively inefficient in hydrolyzing penicillin G, we found only minor differences in the specific activities and kcat values of the type A, B, and C beta-lactamases. The type D beta-lactamase was distinctly less active against penicillin G, however, exhibiting only 15 to 25% of the kcat values of the other beta-lactamases. More than a 2,000-fold difference between the relative efficiencies of hydrolysis (kcat/Km) of cefazolin and cefuroxime by the type A beta-lactamase exists. This greatly exceeds the 60-fold difference in the stability of penicillin G and cefazolin with the same enzyme. Whereas the isoelectric points of the type A, B, and C beta-lactamases were similar, the value for the type D beta-lactamase was distinguishably lower (10.1 for types A, B, and C and 9.7 for type D).We conclude that marked differences in the stability of commonly used beta-lactams to hydrolysis by the staphylococcal beta-lactamases are present. This heterogeneity and the clinical implication thereof need to be considered in the antibiotic management of staphylococcal infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benner E. J., Bennett J. V., Brodie J. L., Kirby W. M. Inactivation of cephalothin and cephaloridine by Staphylococcus aureus. J Bacteriol. 1965 Dec;90(6):1599–1604. doi: 10.1128/jb.90.6.1599-1604.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bush K. Characterization of beta-lactamases. Antimicrob Agents Chemother. 1989 Mar;33(3):259–263. doi: 10.1128/aac.33.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Sykes R. B. Methodology for the study of beta-lactamases. Antimicrob Agents Chemother. 1986 Jul;30(1):6–10. doi: 10.1128/aac.30.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizosa J., Kobasa W. D., Snepar R., Kaye K. M., Kaye D. Cefazolin versus cephalothin in beta-lactamase-producing Staphylococcus aureus endocarditis in a rabbit experimental model. J Antimicrob Chemother. 1982 May;9(5):387–393. doi: 10.1093/jac/9.5.387. [DOI] [PubMed] [Google Scholar]
- Carrizosa J., Santoro J., Kaye D. Treatment of experimental Staphylococcus aureus endocarditis: comparison of cephalothin, cefazolin, and methicillin. Antimicrob Agents Chemother. 1978 Jan;13(1):74–77. doi: 10.1128/aac.13.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright S. J., Waley S. G. Purification of beta-lactamases by affinity chromatography on phenylboronic acid-agarose. Biochem J. 1984 Jul 15;221(2):505–512. doi: 10.1042/bj2210505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East A. K., Dyke K. G. Cloning and sequence determination of six Staphylococcus aureus beta-lactamases and their expression in Escherichia coli and Staphylococcus aureus. J Gen Microbiol. 1989 Apr;135(4):1001–1015. doi: 10.1099/00221287-135-4-1001. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Jr, Gramling P. K. Antistaphylococcal activity and beta-lactamase resistance of newer cephalosporins. J Infect Dis. 1976 Jun;133(6):691–695. doi: 10.1093/infdis/133.6.691. [DOI] [PubMed] [Google Scholar]
- Fong I. W., Engelking E. R., Kirby W. M. Relative inactivation by Staphylococcus aureus of eight cephalosporin antibiotics. Antimicrob Agents Chemother. 1976 Jun;9(6):939–944. doi: 10.1128/aac.9.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman P. L., Petersdorf R. G. Importance of beta-lactamase inactivation in treatment of experimental endocarditis caused by Staphylococcus aureus. J Infect Dis. 1980 Mar;141(3):331–337. doi: 10.1093/infdis/141.3.331. [DOI] [PubMed] [Google Scholar]
- Kernodle D. S., Classen D. C., Burke J. P., Kaiser A. B. Failure of cephalosporins to prevent Staphylococcus aureus surgical wound infections. JAMA. 1990 Feb 16;263(7):961–966. [PubMed] [Google Scholar]
- Kernodle D. S., McGraw P. A., Stratton C. W., Kaiser A. B. Use of extracts versus whole-cell bacterial suspensions in the identification of Staphylococcus aureus beta-lactamase variants. Antimicrob Agents Chemother. 1990 Mar;34(3):420–425. doi: 10.1128/aac.34.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernodle D. S., Stratton C. W., McMurray L. W., Chipley J. R., McGraw P. A. Differentiation of beta-lactamase variants of Staphylococcus aureus by substrate hydrolysis profiles. J Infect Dis. 1989 Jan;159(1):103–108. doi: 10.1093/infdis/159.1.103. [DOI] [PubMed] [Google Scholar]
- Kernodle D. S., Zygmunt D. J., McGraw P. A., Chipley J. R. Purification of Staphylococcus aureus beta-lactamases by using sequential cation-exchange and affinity chromatography. Antimicrob Agents Chemother. 1990 Nov;34(11):2177–2183. doi: 10.1128/aac.34.11.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Rosdahl V. T. An unusual "penicillinase plasmid" in staphylococcus aureus; evidence for its transfer under natural conditions. J Med Microbiol. 1974 Feb;7(1):1–9. doi: 10.1099/00222615-7-1-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Matthew M., Harris A. M. Identification of beta-lactamases by analytical isoelectric focusing: correlation with bacterial taxonomy. J Gen Microbiol. 1976 May;94(1):55–67. doi: 10.1099/00221287-94-1-55. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. PURIFICATION AND PROPERTIES OF THE EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Sep;88:452–459. doi: 10.1042/bj0880452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. WILD-TYPE VARIANTS OF EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1965 Mar;94:584–593. doi: 10.1042/bj0940584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regamey C., Libke R. D., Engelking E. R., Clarke J. T., Kirby M. M. Inactivation of cefazolin, cephaloridine, and cephalothin by methicillin-sensitive and methicillin-resistant strains of Staphylococcus aureus. J Infect Dis. 1975 Mar;131(3):291–294. doi: 10.1093/infdis/131.3.291. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. Beta-lactamase (Staphylococcus aureus). Methods Enzymol. 1975;43:664–672. doi: 10.1016/0076-6879(75)43131-7. [DOI] [PubMed] [Google Scholar]
- Rosdahl V. T. Naturally occurring constitutive -lactamase of novel serotype in Staphylococcus aureus. J Gen Microbiol. 1973 Jul;77(1):229–231. doi: 10.1099/00221287-77-1-229. [DOI] [PubMed] [Google Scholar]
- Ross G. W., Chanter K. V., Harris A. M., Kirby S. M., Marshall M. J., O'Callaghan C. H. Comparison of assay techniques for beta-lactamase activity. Anal Biochem. 1973 Jul;54(1):9–16. doi: 10.1016/0003-2697(73)90241-8. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Garner C., Wilcox C., Finland M. Effect of inoculum and of beta-lactamase on the anti-staphylococcal activity of thirteen penicillins and cephalosporins. Antimicrob Agents Chemother. 1975 Sep;8(3):344–349. doi: 10.1128/aac.8.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D. Reappraisal of the antistaphylococcal activities of first-generation (narrow-spectrum) and second-generation (expanded-spectrum) cephalosporins. Antimicrob Agents Chemother. 1989 Apr;33(4):407–411. doi: 10.1128/aac.33.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeg W., Bingöl R., Blobel H. Purification of penicillinase ( -lactamase) and acid phosphatase from Staphylococcus aureus in one procedure. Biochim Biophys Acta. 1972 May 12;268(2):542–549. doi: 10.1016/0005-2744(72)90351-8. [DOI] [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. J., Wu P. J., Livermore D. M. Biochemical characterization of a beta-lactamase that hydrolyzes penems and carbapenems from two Serratia marcescens isolates. Antimicrob Agents Chemother. 1990 May;34(5):755–758. doi: 10.1128/aac.34.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]