Abstract
Aims
There are no data comparing the relative efficacy of hydrofluoroalkane (HFA) formulations of ciclesonide (CIC) and fluticasone propionate (FP) on airway hyper-responsiveness, in mild-to-moderate persistent asthma. We therefore elected to evaluate the comparative efficacy of HFA pressurized metered-dose inhaler formulations of CIC and FP, assessing methacholine challenge, in addition to exhaled nitric oxide, lung function, diary cards and quality of life.
Methods
Nineteen mild-to-moderate asthmatic patients completed the study per protocol in randomized, double-blind, double-dummy, crossover fashion. Patients were required to stop their usual inhaled corticosteroid therapy for the duration of the study. Pa-tients were commenced instead on salmeterol (SM) 50 µg one puff twice daily + montelukast (ML) 10 mg once daily for 2-week washout periods prior to each randomized treatment, in order to prevent dropouts. Patients received 4 weeks of either CIC 200 µg two puffs once daily (08.00 h) + CIC-placebo (PL) two puffs once daily (20.00 h) + FP-PL two puffs twice daily (08.00 h and 20.00 h), or FP 125 µg two puffs twice daily (08.00 h and 20.00 h) + CIC-PL two puffs twice daily (08.00 h and 20.00 h). SM + ML were withheld for 72 h prior to post-washout visits and CIC or FP was withheld for 24 h prior to study visits.
Results
There was no significant difference between CIC vs. FP for the primary outcome of methacholine PC20 as doubling dilution (dd) shift from respective baseline; mean difference: 0.4 dd (95% CI −0.4, 1.2). Moreover, there was no difference between treatments for the sequence of CIC first vs FP second; mean difference: 0.2 dd (95% CI −1.3, 1.7) or FP first vs CIC second; mean difference: 0.9 dd (95% CI −0.1, 1.8). There were also no differences for other secondary outcomes between treatments, either respective or irrespective of sequence, as change from baseline.
Conclusions
There were no differences between 4 weeks of CIC 400 µg once daily and FP 250 µg twice daily on methacholine hyper-responsiveness in mild-to-moderate persistent asthma. Longer-term studies are indicated to evaluate their relative efficacy on asthma exacerbations.
Keywords: asthma, asthma diary, ciclesonide, exhaled nitric oxide, fluticasone, hydrofluoroalkane formulations, inhaled corticosteroid, lung function, methacholine, mini asthma quality of life questionnaire
Introduction
Inhaled corticosteroids (ICS) are considered as optimal first-line anti-inflammatory therapy for mild-to-moderate persistent asthma. Current guidelines suggest titrating the dose of ICS against symptoms, reliever use and lung function [1, 2]. However, these conventional parameters of asthma control may be more downstream from the underlying inflammatory process. The dose–response curve with ICS for these conventional outcomes is rather shallow, as shown in a meta-analysis with fluticasone propionate (FP) [3], where a plateau effect on lung function was achieved above 100 µg twice daily, suggesting that other surrogate markers of inflammation may be more sensitive. Airway hyper-responsiveness is a noninvasive surrogate marker of airway inflammation and is a sensitive method of assessing the short- and long-term response to ICS [4–6].
Ciclesonide (CIC) is a novel lipophilic corticosteroid, under late stage clinical development for once or twice daily use [7]. CIC itself is relatively inactive in terms of its glucocorticoid receptor binding affinity but is activated on site in the lung by esterase cleavage to form the active metabolite, des-CIC, which exhibits similar glucocorticoid receptor binding affinity to budesonide [8]. It has been formulated in solution with hydrofluoroalkane-134a (HFA) as the propellant, providing an extra-fine aerosol with a high degree of lung deposition. This has the potential for treating small airway inflammation, which is not achievable with coarser conventional suspension aerosols [9, 10].
FP has been reformulated as a HFA suspension metered-dose aerosol and is currently recommended for twice daily use. In vitro data using an Anderson cascade impactor show similar particle size profiles when comparing CFC and HFA pressurized metered-dose inhaler (pMDI) suspension formulations of FP [11], and are therapeutically equivalent in clinical trials based on lung function as the primary outcome [12]. Furthermore, a study in mild-to-moderate asthmatics also found therapeutic equivalence when comparing the HFA vs CFC 250 µg FP pMDI formulations on methacholine hyper-responsiveness [13].
Currently, there are no data comparing the relative efficacy of once daily administration of HFA CIC pMDI compared with twice daily HFA FP pMDI, in patients with mild-to-moderate persistent asthma. We therefore elected to evaluate the comparative efficacy of HFA pMDI formulations of CIC and FP, assessing methacholine hyper-responsiveness as the primary outcome, in addition to conventional measures of lung function, diary card evaluation, quality of life, and exhaled nitric oxide (NO), as secondary outcomes.
Methods
Patients
Eligible patients were non-smoking mild-to-moderate persistent asthmatics [1, 2] who were stable for at least 3 months prior to the study and none had received a course of oral corticosteroids or antibiotics during this period. Patients were required to be receiving either ICS alone in a daily dose of up to 2000 µg beclomethasone dipropionate/2000 µg budesonide/1000 µg of FP, or half the dose of the above ICS in combination with second-line controller therapy. Patients were required to exhibit airway hyper-responsiveness to methacholine on bronchial challenge testing with a provocative dose causing a 20% reduction from baseline FEV1 (PC20) of less than 4.0 mg ml−1[14]. Informed consent was obtained from all patients and the study was approved by the Tayside Committee on Medical Research Ethics.
Study design
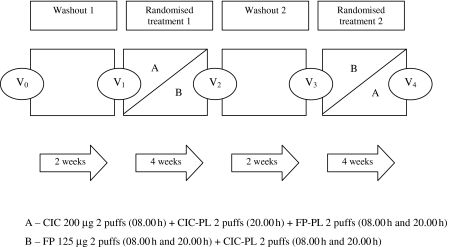
The study design schematic is shown in Figure 1. The study was conducted in a randomized, double-blind, double-dummy, crossover fashion. After an initial screening visit, patients were required to stop their usual ICS along with their second-line controller therapy for the duration of the study. Patients were commenced instead on salmeterol (SM) 50 µg (Serevent 50 Accuhaler®, Allen & Hanburys Ltd, Uxbridge, UK) one puff twice daily + montelukast (ML) 10 mg (Singulair®, Merck Sharp & Dohme Ltd, Hoddesdon, UK) once daily for 2-week washout periods prior to each randomized treatment, in order to prevent dropouts. Patients were randomized to receive for 4 weeks either CIC 200 µg (Altana Pharma AG, Konstanz, Germany) two puffs once daily (08.00 h) + CIC-placebo (PL) two puffs once daily (20.00 h) + FP-PL two puffs twice daily (08.00 h and 20.00 h), or FP 125 µg (Flixotide 125 Evohaler®, Allen & Hanburys Ltd, Uxbridge, UK) two puffs twice daily (08.00 h and 20.00 h) + CIC-PL two puffs twice daily (08.00 h and 20.00 h). All active and PL devices were masked to make them identical in external physical appearance. SM + ML were withheld for 72 h prior to post-washout visits and CIC or FP was withheld for 24 h prior to study visits.
Figure 1.
Study design schematic depicting washout and randomized treatment periods, with study visits V0 – V4
Measurements
Spirometry
Spirometry was performed according to the American Thoracic Society criteria [15] using a Micro Medical SuperSpiro® (Micro Medical Ltd, Rochester, UK).
Exhaled NO
Measurements were performed as previously described [16] using an integrated LR2000® clinical real-time NO gas analyser (Logan Research, Rochester, UK) with a flow rate of 250 ml min−1 and an accuracy of 2 parts per billion (p.p.b) NO with a response time of 2 s.
Methacholine bronchial challenge
Methacholine was administered using a standardized breath actuated Mefar® dosimeter (Markos-Mefar SpA, Bovezzo, Italy) at 5 min intervals in doubling cumulative concentrations from 0.03125 mg ml−1 to 64.0 mg ml−1 until a 20% reduction in FEV1 was recorded. Log linear interpolation was performed using a computer assisted programme (Micro Medical Ltd, Rochester, UK) to calculate the PC20 values.
Domiciliary peak expiratory flow (PEF), symptom score and rescue diary
Patients recorded morning and evening domiciliary PEF using a Mini-Wright® peak flow meter (Clement Clarke International Ltd, Harlow, UK) along with documentation of asthma symptom scores (4 point scale; 0 indicating no symptoms and 3 indicating severe symptoms) and rescue inhaler use for the duration of the study.
Mini Asthma Quality of Life Questionnaire (MiniAQLQ)
Patients completed the MiniAQLQ [17] on each study visit, which consisted of four domains; symptoms (5 items), activity limitation (4 items), emotional function (3 items), and environmental stimuli (3 items).
Statistical analysis
The study was powered at 80% with the α-error set at 0.05 (two-tailed) in order to detect a 1 doubling dilution (dd) difference between treatments in methacholine PC20 (the primary outcome) calculated as the dd shift from respective pretreatment baseline (after each washout) with a sample size of 16 completed patients per protocol, in a crossover design. All other outcomes were considered as secondary. For all comparisons, an overall analysis of variance (anova) was performed, followed by multiple-range testing with Bonferroni correction set at 95% confidence interval (CI), in order to obviate multiple pairwise comparisons. The results of the multiple-range test were quoted as being either significant at P < 0.05 (two-tailed) or nonsignificant. In addition, anova was performed to assess sequence effects by comparing treatment responses for CIC first vs FP second, and vice versa. To normalize distribution, data for methacholine PC20 were logarithmically transformed, and analyses were performed using Statgraphics® statistical software package (STSC Software Publishing Group, Rockville, USA).
Results
Patients
Twenty-eight patients were initially recruited from the database based on previous history of lung function and bronchial hyper-responsiveness in keeping with mild-to-moderate persistent asthma. Four patients were subsequently found to be unresponsive to methacholine on the screening bronchial challenge prior to randomization, while one patient had a FEV1 of below 60% predicted which precluded a methacholine challenge. Out of the remaining 23 patients who were randomized, three patients dropped out due to asthma exacerbation during the study (one patient at each of the FP treatment limb, given first and second in sequence, and one patient during the first washout period) and one patient dropped out due to personal reasons. Nineteen patients (nine men and 10 women) with mean (SEM) age of 45 (3) years, FEV1 of 90 (4) % predicted and FEF25–75 of 66 (5) % predicted completed the study per protocol. The mean dose of ICS was 482 (75) µg daily, comprising beclomethasone dipropionate (n = 10), budesonide (n = 1) and FP (n = 8). Two patients were receiving SM as second-line controller therapy.
Methacholine challenge
Comparison of baseline values for methacholine PC20 according to treatment or sequence showed no significant difference (Tables 1 and 2). There were no significant differences in absolute PC20 values for either CIC or FP when compared with their respective baseline values (Table 1). There was no significant difference between CIC vs FP for the primary outcome of methacholine PC20, expressed as dd shift from respective baselines, amounting to a mean difference of 0.4 dd (95% CI −0.4, 1.2) (Table 3). There was also no significant difference between treatments, for the sequence of CIC first vs FP second; mean difference: 0.2 dd (95% CI −1.3, 1.7), or FP first vs CIC second; mean difference: 0.9 dd (95% CI −0.1, 1.8) (Table 3).
Table 1. Absolute mean values for randomized treatments and respective baselines with 95% CI for mean.
| CIC (baseline) | 95% CI | CIC | 95% CI | FP (baseline) | 95% CI | FP | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| NO (p.p.b) | 9.9 | 5.8, 14.1 | 8.1 | 4.0, 12.3 | 9.6 | 5.4, 13.8 | 5.1 | 1.0, 9.3 |
| FEV1 (l) | 2.58 | 2.50, 2.66 | 2.66 | 2.58, 2.74 | 2.59 | 2.51, 2.67 | 2.72 | 2.63, 2.80 |
| FEV1 (% predicted) | 84 | 82, 87 | 86 | 84, 89 | 84 | 82, 87 | 88 | 86, 91 |
| FEF25–75 (l s−1) | 2.16 | 2.01, 2.32 | 2.26 | 2.11, 2.41 | 2.15 | 2.00, 2.31 | 2.42 | 2.27, 2.58 |
| FEF25–75 (% predicted) | 59 | 55, 63 | 61 | 57, 65 | 58 | 54, 62 | 65 | 61, 69 |
| Methacholine PC20 (mg ml−1) | 0.6 | 0.4, 1.0 | 0.8 | 0.4, 1.3 | 0.7 | 0.4, 1.3 | 1.3 | 0.7, 2.2 |
| PEF (am) (l min−1) | 471 | 461, 480 | 465 | 456, 475 | 469 | 460, 479 | 463 | 453, 472 |
| PEF (pm) (l min−1) | 472 | 463, 482 | 467 | 458, 476 | 473 | 464, 482 | 465 | 455, 474 |
| Asthma symptom score (am) | 0.2 | 0.1, 0.3 | 0.2 | 0.1, 0.3 | 0.1 | 0.1, 0.2 | 0.3 | 0.2, 0.4 |
| Asthma symptom score (pm) | 0.3 | 0.2, 0.4 | 0.2 | 0.1, 0.3 | 0.2 | 0.1, 0.3 | 0.2 | 0.1, 0.3 |
| Rescue (am) (puffs/day) | 0.2 | 0.1, 0.4 | 0.3 | 0.1, 0.4 | 0.1 | 0.1, 0.3 | 0.3 | 0.2, 0.5 |
| Rescue (pm) (puffs/day) | 0.4 | 0.3, 0.5 | 0.3 | 0.1, 0.4 | 0.3 | 0.2, 0.4 | 0.3 | 0.2, 0.4 |
| Activities (MiniAQLQ) | 6.46 | 6.21, 6.71 | 6.43 | 6.18, 6.69 | 6.68 | 6.43, 6.94 | 6.33 | 6.08, 6.58 |
| Symptoms (MiniAQLQ) | 5.81 | 5.50, 6.12 | 5.52 | 5.21, 5.82 | 6.02 | 5.71, 6.33 | 5.67 | 5.37, 5.98 |
| Emotions (MiniAQLQ) | 6.23 | 5.92, 6.54 | 5.95 | 5.63, 6.26 | 6.32 | 6.00, 6.63 | 6.23 | 5.92, 6.54 |
| Environment (MiniAQLQ) | 6.05 | 5.76, 6.35 | 5.60 | 5.30, 5.89 | 5.84 | 5.54, 6.14 | 5.91 | 5.61, 6.21 |
| Overall (MiniAQLQ) | 6.12 | 5.89, 6.35 | 5.87 | 5.64, 6.10 | 6.22 | 5.99, 6.45 | 6.01 | 5.78, 6.24 |
There were no significant differences for comparisons between baselines, between randomized treatments, and between randomized treatments and respective baselines. Data for methacholine PC20are given as geometric means.
Table 2. Absolute mean values for baselines by sequence with 95% CI for mean.
| Baseline (first) | 95% CI | Baseline (second) | 95% CI | |
|---|---|---|---|---|
| NO (p.p.b) | 10.5 | 5.3, 15.6 | 9.0 | 3.9, 14.2 |
| FEV1 (l) | 2.58 | 2.19, 2.97 | 2.59 | 2.20, 2.99 |
| FEV1 (% predicted) | 84 | 77, 92 | 84 | 77, 92 |
| FEF25–75 (l s−1) | 2.14 | 1.75, 2.54 | 2.18 | 1.78, 2.57 |
| FEF25–75 (% predicted) | 58 | 49, 67 | 59 | 50, 68 |
| Methacholine PC20 (mg ml−1) | 0.7 | 0.4, 1.2 | 0.6 | 0.4, 1.1 |
| PEF (am) (l min−1) | 474 | 429, 518 | 466 | 421, 510 |
| PEF (pm) (l min−1) | 475 | 433, 518 | 470 | 428, 513 |
| Asthma symptom score (am) | 0.1 | −0.1, 0.3 | 0.2 | 0.1, 0.3 |
| Asthma symptom score (pm) | 0.3 | 0.1, 0.5 | 0.3 | 0.1, 0.5 |
| Rescue (am) (puffs/day) | 0.1 | −0.1, 0.3 | 0.2 | 0.1, 0.5 |
| Rescue (pm) (puffs/day) | 0.4 | 0.1, 0.7 | 0.4 | 0.1, 0.7 |
| Activities (MiniAQLQ) | 6.51 | 6.27, 6.75 | 6.63 | 6.39, 6.87 |
| Symptoms (MiniAQLQ) | 5.93 | 5.50, 6.35 | 5.91 | 5.48, 6.33 |
| Emotions (MiniAQLQ) | 6.30 | 5.83, 6.77 | 6.25 | 5.78, 6.71 |
| Environment (MiniAQLQ) | 5.84 | 5.35, 6.33 | 6.05 | 5.56, 6.54 |
| Overall (MiniAQLQ) | 6.14 | 5.81, 6.47 | 6.20 | 5.87, 6.52 |
There were no significant differences for comparisons between baselines in sequence irrespective of randomized treatments. Data for methacholine PC20are given in geometric means.
Table 3. 95% CI for mean difference between CIC and FP, irrespective and respective of sequence.
| Irrespective of sequence | CIC (first) and FP (second) | FP (first) and CIC (second) | |
|---|---|---|---|
| NO (p.p.b) | −2.1, 7.3 | −9.0, 15.6 | −4.9, 0.6 |
| FEV1 (l) | −0.15, 0.06 | −0.17, 0.17 | −0.08, 0.23 |
| FEV1 (% predicted) | −5, 2 | −6, 5 | −2, 8 |
| FEF25–75 (l s−1) | −0.41, 0.06 | −0.32, 0.46 | −0.89, 0.18 |
| FEF25–75 (% predicted) | −12, 1 | −11, 13 | −24, 4 |
| Methacholine PC20 (doubling dilution shift) | −1.2, 0.4 | −1.3, 1.7 | −0.1, 1.8 |
| PEF (am) (l min−1) | −12, 14 | −13, 24 | −19, 24 |
| PEF (pm) (l min−1) | −11, 17 | −13, 29 | −22, 22 |
| Asthma symptom score (am) | −0.3, 0.1 | −0.2, 0.1 | −0.2, 0.5 |
| Asthma symptom score (pm) | −0.3, 0.1 | −0.5, 0.1 | −0.3, 0.3 |
| Rescue (am) (puffs/day) | −0.4, 0.2 | −0.3, 0.3 | −0.4, 0.7 |
| Rescue (pm) (puffs/day) | −0.3, 0.1 | −0.5, 0.2 | −0.3, 0.4 |
| Activities (MiniAQLQ) | −0.26, 0.92 | −0.08, 1.02 | −1.27, 0.81 |
| Symptoms (MiniAQLQ) | −0.45, 0.56 | −0.97, 1.22 | −0.60, 0.60 |
| Emotions (MiniAQLQ) | −0.76, 0.37 | −1.39, 0.47 | −0.83, 0.83 |
| Environment (MiniAQLQ) | −1.08, 0.02 | −0.97, 0.55 | −0.10, 1.61 |
| Overall (MiniAQLQ) | −0.43, 0.37 | −0.60, 0.70 | −0.52, 0.70 |
There were no significant differences for comparisons between CIC vs. FP, either irrespective or respective of sequence.
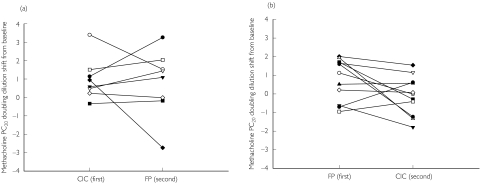
Mean values for PC20 as change from baseline, irrespective of sequence, showed a significant within treatment response for FP but not CIC, as indicated by the within treatment CI which excluded the zero value (Table 4). For mean PC20 as change from baseline according to sequence, CIC given first showed a significant within treatment response, as indicated by the CI which excluded the zero value, while for CIC given second, or FP given first or second, the CIs included the zero value, indicating a nonsignificant within treatment response (Table 5). Inspection of individual data for PC20 as change from baseline, showed no clear pattern for comparison between treatments, for either sequence (Figure 2).
Table 4. Mean values as change from respective baselines with 95% CI for mean.
| CIC | 95% CI | FP | 95% CI | |
|---|---|---|---|---|
| NO (p.p.b) | −1.8 | −5.1, 1.5 | −4.4 | −7.7, 1.1 |
| FEV1 (l) | 0.08 | 0.01, 0.15 | 0.12 | 0.05, 0.19 |
| FEV1 (% predicted) | 2 | −1, 4 | 4 | 1, 6 |
| FEF25–75 (l s−1) | 0.10 | −0.07, 0.26 | 0.27 | 0.11, 0.43 |
| FEF25–75 (% predicted) | 2 | −3, 6 | 7 | 3, 12 |
| Methacholine PC20 (doubling dilution shift) | 0.4 | −0.2, 0.9 | 0.8 | 0.2, 1.3 |
| PEF (am) (l min−1) | −5 | −15, 4 | −6 | −16, 3 |
| PEF (pm) (l min−1) | −5 | −15, 9 | −8 | −18, 2 |
| Asthma symptom score (am) | 0.0 | −0.1, 0.2 | 0.1 | −0.1, 0.3 |
| Asthma symptom score (pm) | −0.1 | −0.2, 0.1 | 0.0 | −0.1, 0.1 |
| Rescue (am) (puffs/day) | 0.1 | −0.1, 0.3 | 0.2 | −0.5, 0.4 |
| Rescue (pm) (puffs/day) | −0.1 | −0.3, 0.1 | 0.0 | −0.2, 0.1 |
| Activities (MiniAQLQ) | −0.03 | −0.45, 0.39 | −0.36 | −0.77, 0.06 |
| Symptoms (MiniAQLQ) | −0.29 | −0.65, 0.06 | −0.35 | −0.71, 0.01 |
| Emotions (MiniAQLQ) | −0.28 | −0.68, 0.12 | −0.09 | −0.49, 0.31 |
| Environment (MiniAQLQ) | −0.46 | −0.84, 0.07 | 0.07 | −0.32, 0.46 |
| Overall (MiniAQLQ) | −0.25 | −0.53, 0.04 | −0.21 | −0.50, 0.07 |
There were no significant differences for comparisons between randomized treatments.
Table 5. Mean values as change from respective baselines for CIC and FP, given first and second in sequence with 95% CI for mean.
| CIC (first) | 95% CI | CIC (second) | 95% CI | FP (first) | 95% CI | FP (second) | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| NO (p.p.b) | −3.0 | −9.1, 3.0 | −1.0 | −6.2, 4.2 | −3.1 | −8.3, 2.1 | −6.3 | −12.4, 0.2 |
| FEV1 (l) | 0.15 | −0.02, 0.31 | 0.03 | −0.11, 0.17 | 0.10 | −0.04, 0.24 | 0.15 | −0.02, 0.31 |
| FEV1 (% predicted) | 5 | −1, 10 | 0 | −4, 4 | 3 | −2, 7 | 5 | 1, 10 |
| FEF25–75 (l s−1) | 0.29 | −0.04, 0.61 | −0.04 | −0.32, 0.23 | 0.31 | 0.03, 0.59 | 0.22 | −0.11, 0.54 |
| FEF25–75 (% predicted) | 7 | −1, 15 | −2 | −9, 5 | 8 | 1, 15 | 6 | −2, 14 |
| Methacholine PC20 (doubling dilution shift) | 1.0 | 0.1, 1.9 | −0.1 | −0.9, 0.7 | 0.8 | −0.1, 1.6 | 0.8 | −0.1, 1.7 |
| PEF (am) (l min−1) | 11 | −6, 29 | −17 | −32, 2 | −15 | −30, 1 | 6 | −12, 23 |
| PEF (pm) (l min−1) | 10 | −9, 30 | −16 | −33, 1 | −16 | −33, 1 | 2 | −17, 22 |
| Asthma symptom score (am) | 0.0 | −0.3, 0.3 | 0.0 | −0.2, 0.2 | 0.2 | −0.1, 0.4 | 0.1 | −0.2, 0.3 |
| Asthma symptom score (pm) | −0.2 | −0.4, 0.1 | 0.0 | −0.3, 0.2 | 0.0 | −0.3, 0.2 | 0.1 | −0.2, 0.3 |
| Rescue (am) (puffs/day) | 0.1 | −0.3, 0.4 | 0.1 | −0.2, 0.4 | 0.3 | −0.1, 0.6 | 0.1 | −0.3, 0.4 |
| Rescue (pm) (puffs/day) | −0.2 | −0.5, 0.1 | −0.1 | −0.4, 0.2 | 0.0 | −0.3, 0.2 | −0.1 | −0.4, 0.3 |
| Activities (MiniAQLQ) | 0.34 | −0.25, 0.94 | −0.30 | −0.80, 0.21 | −0.52 | −1.03, 0.01 | −0.13 | −0.72, 0.47 |
| Symptoms (MiniAQLQ) | −0.15 | −0.78, 0.48 | −0.40 | −0.93, 0.13 | −0.40 | −0.93, 0.13 | −0.28 | −0.90, 0.35 |
| Emotions (MiniAQLQ) | −0.46 | −1.03, 0.11 | −0.15 | −0.64, 0.33 | −0.15 | −0.64, 0.33 | 0.00 | −0.57, 0.57 |
| Environment (MiniAQLQ) | −0.21 | −0.79, 0.37 | −0.64 | −1.13, 1.14 | 0.12 | −0.37, 0.62 | 0.00 | −0.58, 0.58 |
| Overall (MiniAQLQ) | −0.07 | −0.53, 0.38 | −0.37 | −0.76, 0.02 | −0.28 | −0.67, 0.11 | −0.13 | −0.58, 0.33 |
There were no significant differences for comparisons between randomized treatments according to sequence, i.e. CIC (first) vs FP (second), or FP (first) vs CIC (second).
Figure 2.
a) Individual methacholine PC20 values as doubling dilution shift from respective baselines for CIC and FP, given first and second, respectively, in sequence. A positive value indicates an improvement from baseline, and a negative value indicates a deterioration from baseline. b) Individual methacholine PC20 values as doubling dilution shift from respective baselines for CIC and FP, given second and first, respectively, in sequence. A positive value indicates an improvement from baseline, and a negative value indicates a deterioration from baseline
Secondary outcomes
Comparison of baseline values according to treatment or sequence showed no significant differences for all secondary outcomes (Tables 1 and 2). Absolute values for all secondary outcomes showed no difference for either CIC or FP compared with their respective baselines (Table 1). There were also no significant differences between randomized treatments, as change from baseline, for any of the secondary outcomes, either respective or irrespective of sequence (Table 3). Mean values for NO and FEF25–75, as change from baseline, irrespective of sequence, showed a significant within treatment response for effects of FP but not CIC, while for CIC there was a significant within treatment response for FEV1, as indicated by CIs which excluded the zero value (Table 4). Secondary outcomes, as change from baseline, according to sequence, which had CIs that excluded the zero value, included NO and FEF25–75, when FP was given first (Table 4).
Discussion
The present results showed no significant difference between once daily CIC and twice daily FP in mild-to-moderate persistent asthmatics for the primary outcome of methacholine hyper-responsiveness, amounting to a mean difference of 0.4 dd in PC20. This was also the case, when comparing the two treatments according to the sequence in which they were given. The lack of difference between treatments was also evident when inspecting the individual data for the primary outcome, as seen in Figure 2. Our study was powered to detect a 1 dd difference in methacholine PC20, as this is the magnitude of shift which was considered as being clinically relevant. There were also no significant differences between the two drugs for any of the secondary outcomes as change from baseline, irrespective or respective of sequence.
The baseline values for the primary outcome after each washout period showed similar values whether respective of sequence or treatment, which would suggest the lack of any carry-over effects between randomized treatments. We used the combination of SM + ML during each washout period, in order to prevent dropouts due to ICS withdrawal. This was based on data from a previous proof of concept study which showed that the combination of SM + ML prevented a decline in lung function and methacholine hyper-responsiveness after ICS withdrawal in moderate persistent asthma [18]. SM + ML were stopped for a 72-h period prior to each baseline measurement. We accept that there may have been a degree of carry-over for the effect on ML at each baseline measurement, although this would have been the same for each randomized treatment, as shown by the similar values for both baselines. Moreover, we were confident that any effect of ML would have disappeared by the end of each 4-week randomized treatment block.
We administered both drugs for a period of 4 weeks, as we considered this would achieve near maximal effects on methacholine PC20. This is supported by time profile sequential challenge studies, where effects of ICS on airway hyper-responsiveness show no difference in shift, when comparing values after 2, 4, and 6 weeks of treatment [19, 20].
We acknowledge that our study was inadequately powered to evaluate properly secondary outcomes such as lung function, symptoms and quality of life, which would require much larger and longer multicentre trials.
In comparing the two drugs, it should also be pointed out that the total daily dose of CIC was lower than FP, although the doses of both drugs were probably near the plateau of the dose–response curve for mild-to-moderate asthma [13]. We performed our challenges in the morning when the airway exhibits enhanced diurnal hyper-responsiveness, so as to coincide with trough protection at the end of the 24-h dosing interval with CIC. Previous data with twice daily CIC have shown a dose–response against adenosine monophosphate challenge with doses of 100, 400 and 1600 µg daily given each for 2 weeks [21].
In summary, our results showed no significant difference between HFA formulations of once daily CIC 400 µg and twice daily FP 250 µg over 4 weeks for effects on the primary outcome of methacholine hyper-responsiveness in mild-to-moderate asthma.
Acknowledgments
This study was supported by an unrestricted educational grant from Aventis Pharmaceuticals.
References
- 1.National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma Update on Selected Topics – 2002. J Allergy Clin Immunol. 2002;110:S141–S219. [PubMed] [Google Scholar]
- 2.British guidelines on the management of asthma. Thorax. 2003;58(Suppl 1):i1–94. doi: 10.1136/thorax.58.suppl_1.1i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt S, Suder A, Weatherall M, Cheng S, Shirtcliffe P, Beasley R. Dose–response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis. Br Med J. 2001;323:253–6. doi: 10.1136/bmj.323.7307.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sont JK, Han J, van Krieken JM, Evertse CE, Hooijer R, Willems LN, et al. Relationship between the inflammatory infiltrate in bronchial biopsy specimens and clinical severity of asthma in patients treated with inhaled steroids. Thorax. 1996;51:496–502. doi: 10.1136/thx.51.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson AM, Lipworth BJ. Dose–response evaluation of the therapeutic index for inhaled budesonide in patients with mild-to-moderate asthma. Am J Med. 2000;108:269–75. doi: 10.1016/s0002-9343(99)00435-0. [DOI] [PubMed] [Google Scholar]
- 6.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 7.Dent G. Ciclesonide (Byk Gulden) Curr Opin Invest Drugs. 2002;3:78–83. [PubMed] [Google Scholar]
- 8.Rohatagi S, Arya V, Zech K, Nave R, Hochhaus G, Jensen BK, et al. Population pharmacokinetics and pharmacodynamics of ciclesonide. J Clin Pharmacol. 2003;43:365–78. doi: 10.1177/0091270002250998. [DOI] [PubMed] [Google Scholar]
- 9.Goldin JG, Tashkin DP, Kleerup EC, Greaser LE, Haywood UM, Sayre JW, et al. Comparative effects of hydrofluoroalkane and chlorofluorocarbon beclomethasone dipropionate inhalation on small airways: assessment with functional helical thin-section computed tomography. J Allergy Clin Immunol. 1999;104:S258–S267. doi: 10.1016/s0091-6749(99)70043-6. [DOI] [PubMed] [Google Scholar]
- 10.Hauber HP, Gotfried M, Newman K, Danda R, Servi RJ, Christodoulopoulos P, et al. Effect of HFA-flunisolide on peripheral lung inflammation in asthma. J Allergy Clin Immunol. 2003;112:58–63. doi: 10.1067/mai.2003.1612. [DOI] [PubMed] [Google Scholar]
- 11.Cripps A, Riebe M, Schulze M, Woodhouse R. Pharmaceutical transition to non-CFC pressurized metered dose inhalers. Respir Med. 2000;94(Suppl B):S3–S9. [PubMed] [Google Scholar]
- 12.Tonnel AB, Bons J, Legendre M, Prud'Homme A, Bugnas B, Evano-Celli I, et al. Clinical efficacy and safety of fluticasone propionate 250 microg twice daily administered via a HFA 134a pressurized metered dose inhaler to patients with mild to moderate asthma. French Study Group. Respir Med. 2000;94(Suppl B):S29–S34. [PubMed] [Google Scholar]
- 13.Fowler SJ, Orr LC, Sims EJ, Wilson AM, Currie GP, McFarlane L, et al. Therapeutic ratio of hydrofluoroalkane and chlorofluorocarbon formulations of fluticasone propionate. Chest. 2002;122:618–23. doi: 10.1378/chest.122.2.618. [DOI] [PubMed] [Google Scholar]
- 14.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors July 1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 15.Standardization of Spirometry. 1994 Update, American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 16.Kharitonov S, Alving K, Barnes PJ. Exhaled and nasal nitric oxide measurements: recommendations. The European Respiratory Society Task Force. Eur Respir J. 1997;10:1683–93. doi: 10.1183/09031936.97.10071683. [DOI] [PubMed] [Google Scholar]
- 17.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32–8. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 18.Currie GP, Lee DK, Dempsey OJ, Fowler SJ, Cowan LM, Lipworth BJ. A proof of concept study to evaluate putative benefits of montelukast in moderate persistent asthmatics. Br J Clin Pharmacol. 2003;55:609–15. doi: 10.1046/j.1365-2125.2003.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dempsey OJ, Kennedy G, Lipworth BJ. Comparative efficacy and anti-inflammatory profile of once-daily therapy with leukotriene antagonist or low-dose inhaled corticosteroid in patients with mild persistent asthma. J Allergy Clin Immunol. 2002;109:68–74. doi: 10.1067/mai.2002.120559. [DOI] [PubMed] [Google Scholar]
- 20.Sovijarvi AR, Haahtela T, Ekroos HJ, Lindqvist A, Saarinen A, Poussa T, et al. Sustained reduction in bronchial hyperresponsiveness with inhaled fluticasone propionate within three days in mild asthma: time course after onset and cessation of treatment. Thorax. 2003;58:500–4. doi: 10.1136/thorax.58.6.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor DA, Jensen MW, Kanabar V, Engelstatter R, Steinijans VW, Barnes PJ, et al. A dose-dependent effect of the novel inhaled corticosteroid ciclesonide on airway responsiveness to adenosine-5′-monophosphate in asthmatic patients. Am J Respir Crit Care Med. 1999;160:237–43. doi: 10.1164/ajrccm.160.1.9809046. [DOI] [PubMed] [Google Scholar]