Abstract
Aims
To assess the bioavailability of fluconazole (FLCZ) from phosphate pro-drug (fosfluconazole), to investigate the effect of loading doses on the time to achieve FLCZ steady state plasma concentrations and on safety, and to investigate the pharmacokinetics of fosfluconazole following once daily multiple bolus injection of fosfluconazole in healthy male volunteers.
Methods
The first study was a randomized, double-blind, double dummy, two-period crossover study. Subject received either 1000 mg fosfluconazole or 800 mg FLCZ once daily for 14 days in random order. The second study was an open label, randomized parallel group study. Subjects received one of three fosfluconazole once daily treatments: 500 mg for 10 days (no loading dose), a loading dose of 1000 mg on day 1 followed by 500 mg for 9 days (one loading dose), or loading doses of 1000 mg on days 1 and 2 followed by 500 mg for 8 days (two loading doses).
Results
The estimated mean (90% CI) bioavailability of FLCZ from fosfluconazole was 96.8% (94.5, 99.2), with a Cmax,ss ratio of 98.3% (93.3, 103.5) in the first study. Less than 1% of the administered dose of fosfluconazole was excreted unchanged in the urine and the majority (85.6%) was eliminated in the urine as FLCZ. In the second study two loading doses regimen led to earlier achievement of target steady state plasma concentrations (by day 3) compared with use of one or no loading dose (towards the end of the dosing period). Similar adverse event profiles were seen in all three treatment groups. Fosfluconazole did not accumulate after multiple dosing.
Conclusions
Multiple administration of 1000 mg fosfluconazole and 800 mg FLCZ produced equivalent systemic exposure to FLCZ. Steady state FLCZ plasma concentrations were achieved earliest when two loading doses were used.
Keywords: fosfluconazole, fluconazole, bioavailability, loading dose, pharmacokinetics
Introduction
Fluconazole (FLCZ) is an antifungal agent which is efficacious in the treatment of serious systemic fungal infections [1–3]. The current intravenous (IV) dosage form requires a high volume infusion which is undesirable in critically ill patients. There is, consequently, a need for a small volume formulation of FLCZ. Fosfluconazole is a phosphate pro-drug of FLCZ which is highly soluble (> 100 mg ml−1) compared with FLCZ (4 mg ml−1) [4].
In vitro, fosfluconazole is at least 25-fold less potent than FLCZ against single isolates of Candida species and Cryptococcus neoformans, and requires metabolic conversion to FLCZ for antifungal activity in vivo. The first study aimed to assess whether equivalent systemic exposure to FLCZ could be achieved following multiple bolus IV injections of fosfluconazole.
Prior in vivo studies have demonstrated that the PK/PD parameter predictive of FLCZ efficacy is the ratio of the area under the plasma concentration-time curve over the dosing interval (AUC(0,24 h)) to the minimum inhibitory concentration (MIC) (AUC(0,24 h)) : MIC ratio). An AUC(0,24 h) : MIC ratio in the range of 12–25 has been shown to be predictive of treatment success in clinical trials with FLCZ [5–8]. After FLCZ dosing, steady state plasma concentrations are reached within 5–10 days due to the long elimination half-life (approximately 35 h) [9, 10]. Rapid achievement of target steady state plasma concentrations is important in the treatment of severe, life-threatening, fungal infections. Pharmacokinetic simulations indicate that loading doses of fosfluconazole may help achieve FLCZ steady state earlier than 5–10 days. However it is important to confirm these simulations for determination of appropriate dosing regimens. The second study was conducted to investigate the effect of loading doses of fosfluconazole on the time to achieve FLCZ steady state and on the safety and tolerability during the loading dose period.
Methods
Subjects both studies
Healthy male subjects who gave their written informed consent underwent a medical examination. Volunteers were excluded if they had evidence of clinically significant disease, history of clinically significant allergies or abnormality in laboratory data and full physical examination; were taking any prescribed or over the counter drug (with the exception of paracetamol) in the 3 weeks; had received any experimental drug within the past 4 months; drank more than 28 units of alcohol per week; had evidence of drug abuse; had donated blood during the previous month.
Study design
The first study was a randomized, double-blind, double dummy, two-period crossover study. Subjects received either 1000 mg fosfluconazole and placebo for FLCZ or 800 mg FLCZ and placebo for fosfluconazole for 14 days in random order, with a washout period of at least 14 days. Placebo for fosfluconazole and FLCZ was presented as 0.9% sodium chloride which was virtually indistinguishable from both drugs.
The second study was an open label, randomized parallel group study. Subjects received one of three treatments of fosfluconazole: 500 mg for 10 days (no loading dose), a loading dose of 1000 mg followed by 500 mg for 9 days (one loading dose), or loading doses of 1000 mg on days 1 and 2 followed by 500 mg for 8 days (two loading doses).
Fosfluconazole and FLCZ were administered once daily as a bolus IV injection over a maximum of 2 min and as an IV infusion over 4 h, respectively.
The first study was performed at the Pfizer Clinical Research Unit (Canterbury, UK) and the second study was at DDS Medicines Research Ltd. (Dundee, UK) in compliance with the guidelines and ethical principles originating from the revised Declaration of Helsinki (South Africa, 1996). The protocol was approved by Canterbury Research Ethics Committee (Canterbury, UK) and Tayside Committee on Medical Research Ethics (Dundee, UK), respectively.
Pharmacokinetic sampling
In the first study, blood samples were taken immediately before dosing on days 10–14, and at 5, 15, 30, 45 min and 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 16 and 24 h post final dose. In the second study, blood samples were collected at predose on days 1–10, at 1, 3, 6 and 12 h postdose on days 1–3, and at 1, 3, 6, 12 and 24 h post final dose. Within 1 h of collection, all samples were centrifuged (1500 g, 4 °C) for 10 min and the resulting plasma stored at −70 °C or below.
In the first study, urine samples for fosfluconazole and FLCZ analysis were collected from 0 to 24 h postdose on day 2 and 0 – 4 h, 4–8 h and 8–24 h postdose on day 14 and stored at −70 °C or below within 1 h of completion of the collection.
Drug assay
Plasma and urine concentrations of fosfluconazole and FLCZ were determined by solid phase extraction followed by liquid chromatography with atmospheric pressure ionization and tandem mass spectrometric detection (Sciex API III Plus) at Maxxam Analytics Incorporated (Mississauga, Canada). Urine samples were diluted with plasma (1 in 10). Briefly, samples with added internal standard (IS) solution were applied to the conditioned columns and washed with 10 mm phosphate buffer (pH 6.8). Analytes and IS were eluted with methanol, dried under a stream of nitrogen and then reconstituted in mobile phase (80% of acetonitrile and 20% of 15 mm ammonium acetate with 0.3% formic acid). The analytical column and guard column were a Zorbax RX-C8 (150 mm × 4.6 mm, 5 µm) and a Merck LiChroCART 4 – 4 LiChroSpher100 RP-8 (5 µm), respectively. The flow rate was 1.0 ml min−1.
The lower limits of quantification of both analytes were 0.2 µg ml−1 for plasma and 1.0 µg ml−1 for urine samples. The precision values for analysis (CV%) of plasma quality control samples were less than 13.7% for fosfluconazole and 12.4% for FLCZ in both studies. For urine quality control samples in the first study, those were less than 10.7% for fosfluconazole and 10.8% for FLCZ.
Pharmacokinetic analysis
The pharmacokinetic analysis of fosfluconazole and FLCZ plasma concentrations was performed by noncompartmental methods.
For fosfluconazole, the half-life of the terminal exponential phase (t1/2,z) was estimated as ln2/λz, where λz is the terminal rate constant which was estimated by linear regression of the log plasma concentration-time profile. Total area under the plasma concentration-time curve between time zero and time infinity (AUC) was calculated as AUCt + (Ct/λz), where AUCt was the area under the plasma concentration-time curve between time zero and the last measurable time, t, and Ct was the concentration at the last measurable time, t, estimated from the linear regression analysis. The mean residence time (MRT) was calculated as AUMC/AUC, where AUMC is the area under the first-moment of the concentration-time curve with extrapolation to infinity. The total clearance (CL) was calculated as Dose/AUC. The volume of distribution at steady state (Vss) was estimated as CL × MRT.
For FLCZ, AUC(0,24 h) was estimated by the linear trapezoidal method. The average plasma concentration at steady state over the 24 h sampling period (Cav,ss) was determined as AUC(0,24 h)/24. The maximum observed plasma concentration at steady state (Cmax,ss) and the first time to Cmax,ss (tmax,ss) of FLCZ after fosfluconazole dosing were taken directly from the recorded plasma concentration time data.
Safety assessments
Throughout the study, the investigator recorded all observed or volunteered adverse events. Classification by body system was according to the Coding Symbols for Thesaurus of Adverse Reaction Terms (COSTART) definitions (5th edn, Rockville, Md: US Food and Drug Administration; 1995).
Laboratory safety tests for blood and urine samples, supine blood pressure and pulse rate measurements, and a 12-lead ECG were performed at screening, predose, during and after dosing, and at follow-up.
Statistical analysis
For the first study, natural log transformed AUC(0,24 h) and Cmax,ss of FLCZ were subjected to an analysis of variance (anova). The ratio between the antilogged treatment means and the corresponding antilogged 90% confidence interval (CI) was determined. As the 1000 mg dose of fosfluconazole was chemically equivalent to a 793 mg dose of FLCZ, FLCZ AUC following administration of FLCZ was dose adjusted by multiplying by 793/800 prior to analysis.
Using the normal bioequivalence criteria based on 90% CI of 80-125%, with 20 subjects, it was predicted that there was over 90% power of concluding bioequivalent FLCZ AUC if the real difference was less than 10%.
All statistical analyses were performed using SAS, version 6.09 [11].
Results
Subjects
In the first study, 24 healthy male subjects (age 18–43 years, weight 61–91 kg) entered and four subjects did not complete both study periods. One subject withdrew from the study having received five doses of fosfluconazole. Two subjects completed treatment with fosfluconazole in period 1 but discontinued after, respectively, withdrawing consent and experiencing a treatment related adverse event (thrombophlebitis). One subject withdrew in period 2 of FLCZ treatment due to an adverse event which was not considered treatment related (paronychia).
In the second study, 24 (eight per group) healthy male subjects (age 21–45 years, weight 63–100 kg) entered and one subject in the one loading dose group discontinued the study as a result of an adverse event (headache) which was not considered treatment related.
Pharmacokinetics
First study
The fosfluconazole pharmacokinetic parameters on day 14 are summarized in Table 1. The plasma concentrations of fosfluconazole immediately before dosing on days 10–14 and at 24 h postdose on day 14 were below the limit of quantification of the assay for all except one subject suggesting that no accumulation of fosfluconazole occurs after multiple dosing at 24 h intervals. The maximum recorded concentrations of fosfluconazole on day 14 (mean ± SD 142.5 ± 17.19 µg ml−1) occurred at the first sampling time. The mean ± SD percentages of the dose of fosfluconazole excreted in the urine after dosing on days 2 and 14 were 0.5 ± 0.60% and 0.7 ± 0.41%, respectively. On day 14 the majority of that was detected in the first 4 h collection interval.
Table 1. Fosfluconazole pharmacokinetic parameters on day 14 after multiple intravenous injections of 1000 mg fosfluconazole.
| Parameter | Fosfluconazole dosing(n = 23) |
|---|---|
| AUC (µg ml−1 h) | 185 (181) ± 40.4 |
| CL (ml min−1 kg−1) | 1.29 ± 0.311 |
| t1/2,z (h) | 2.5 ± 0.35 |
| MRT (h) | 2.3 ± 0.44 |
| Vss (l kg−1) | 0.17 ± 0.027 |
The arithmetic mean (geometric mean) ± SD for AUC and the arithmetic means ± SD for all other parameters are presented. One subject who did not complete a study period was excluded.
The plasma concentration profiles and pharmacokinetic parameters on day 14 of FLCZ following administration of fosfluconazole or FLCZ are presented in Figure 1 and Table 2. The mean trough concentrations of FLCZ over days 10–14 were similar and indicate that steady state had been reached by day 10 for both treatments.
Figure 1.
Mean ± SD plasma concentration profiles of fluconazole on day 14 after multiple intravenous injections of fosfluconazole (•) and after multiple intravenous infusions of fluconazole (○) in healthy male volunteers (n = 18)
Table 2. Fluconazole pharmacokinetic parameters on day 14 after multiple intravenous administration of 1000 mg fosfluconazole and 800 mg fluconazole.
| Parameter | Fosfluconazole dosing(n = 23) | Fluconazole dosing(n = 18) |
|---|---|---|
| AUC(0,24 h) (µg ml−1 h) | 572 (563) ± 100.5 | 608 (598) ± 115.4 |
| Cmax,ss (µg ml−1) | 32.1 (31.8) ± 4.38 | 33.6 (33.0) ± 6.11 |
| tmax,ss (h) | 2.5 (0.25, 6.0) | 4.0 (3.0, 8.0) |
| Cav,ss (µg ml−1) | 23.8 (23.5) ± 4.19 | 25.4 (24.9) ± 4.79 |
The median (range) for tmax,ssand the arithmetic mean (geometric mean) ± SD for the other parameters are presented. One subject who did not complete a study period was excluded from the fosfluconazole dosing group. Four subjects who did not complete a study period, one subject who received placebo instead of FLCZ on day 14, and one subject with technical problems during FLCZ infusion on day 14 were excluded from the fluconazole dosing group.
Six subjects were excluded from an anova: four who discontinued the study, one who received placebo instead of FLCZ on day 14, and one with technical problems during FLCZ infusion on day 14. With adjustment for dose, the bioavailability of FLCZ from fosfluconazole was estimated to be 96.8% (90% CI 94.5, 99.2). The mean Cmax,ss ratio (fosfluconazole dosing/FLCZ dosing) was 98.3% (90% CI 93.3, 103.5). On the basis of these 90% CIs, a 1000 mg dose of fosfluconazole may be concluded to be bioequivalent to a dose of 793 mg FLCZ.
The mean ± SD percentages of the dose of FLCZ excreted in the urine on day 2 were 50.5 ± 7.50% and 48.1 ± 6.74% after administration of fosfluconazole and FLCZ, respectively. On day 14 these values were increased to 85.6 ± 8.33% and 80.9 ± 20.16%, respectively, reflecting the accumulation of FLCZ to steady state.
Second study
In any treatment group, plasma concentrations of fosfluconazole on day 10 (administered 500 mg) were similar to those on the days administered 500 mg fosfluconazole during days 1–3 and almost half of those on the days administered 1000 mg during days 1–3. This also suggests that there was no indication of accumulation of fosfluconazole in any treatment group.
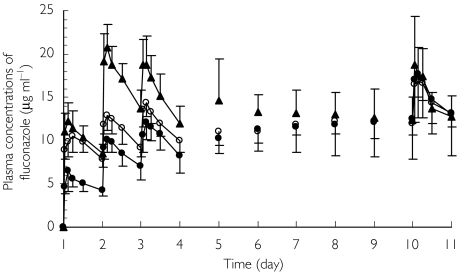
From mean FLCZ concentration profiles (Figure 2), the effect of the loading dose on plasma concentrations over the first 4 days can be seen. Between days 5 and 9, mean concentrations are similar in the no loading dose and one loading dose groups, but those in the two loading doses group remained slightly higher than the other two groups through to day 8. As assessed by trough plasma concentrations on each day, steady state FLCZ concentrations were reached towards the end of the dosing period for the no loading dose and one loading dose groups. In contrast, for the two loading doses group, the steady state was reached by day 3. The three treatment groups had similar concentration profiles on day 10. The AUC(0,24 h) (mean ± SD) were 364 ± 105.6, 349 ± 46.6 and 357 ± 56.2 µg ml−1 h in no, one and two loading doses groups.
Figure 2.
Mean ± SD plasma concentration profiles of fluconazole after multiple intravenous injections of fosfluconazole using no loading dose (•), one loading dose (○) and two loading doses (▴) in healthy male volunteers (n = 7–8)
Safety
In both studies, there were no serious adverse events, no discontinuations due to laboratory test abnormalities and no clinically significant change in vital signs.
In the first study, similar adverse event profiles were seen for the two treatments. There were no apparent differences in urine volume and in plasma phosphate and calcium concentrations between treatments.
In the second study, treatment emergent adverse events were reported by six out of eight subjects in all treatment groups, and only one was severe (tooth disorder). Adverse events thought to be treatment related were reported by three (two subjects with headache, one subject with somnolence, one subject with rash), four (one subject with headache, one subject with bradycardia, four subjects with paraesthesia) and two (nausea, paraesthesia each one subject) of the eight subjects in the no, one and two loading doses groups, respectively. Similar adverse event profiles were seen in all treatment groups.
Discussion
Based on the 90% CIs of bioavailability from fosfluconazole and Cmax,ss ratio of FLCZ, a 1000 mg dose of fosfluconazole may be concluded to be bioequivalent to a dose of 793 mg FLCZ. Less than 1% of the administered dose of fosfluconazole was excreted unchanged in the urine, and 85.6% and 80.9% of the dose were excreted in the urine as FLCZ at steady state after administration of fosfluconazole and FLCZ. These figures suggest that fosfluconazole was almost completely converted to FLCZ in the body and the majority of the administered fosfluconazole was eliminated in the urine as FLCZ.
Two loading doses regimen led to earlier achievement of steady state plasma concentrations (by day 3) compared with use of one or no loading dose (towards the end of the dosing period).
Isolates of Candida albicans, C. parapsilosis and C. tropicalis were highly susceptible to FLCZ (MIC90 values of 0.25–8 µg ml−1). However, MIC90 values for C. glabrata isolates and cryptococcal isolates were 16–32 µg ml−1[12–15]. By using the two loading doses regimen, the plasma FLCZ concentrations after administration on day 2 reached more than 16 µg ml−1, which are similar to MIC90 values for less susceptible species. In our second study, the mean AUC(0,24 h) on steady state were approximately 360 µg ml−1 h in all three groups. AUC(0,24 h) : MIC ratios were calculated as more than 45 for highly susceptible isolates and 11–23 for less susceptible species. These values are comparable with AUC(0,24 h) : MIC ratios of 12–25 which is associated with treatment efficacy [5–8]. These results suggest that the steady state plasma concentrations following 500 mg once daily fosfluconazole injection are effective against most systemic fungal infections. Rapid achievement of these target plasma concentrations is important in the treatment of severe, life-threatening, fungal infections.
In conclusion, multiple bolus administration of 1000 mg fosfluconazole and multiple infusion of 800 mg FLCZ produced equivalent systemic exposure to FLCZ. Target steady state FLCZ plasma concentrations were achieved earliest when two loading doses were used.
References
- 1.Rex JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:662–78. doi: 10.1086/313749. [DOI] [PubMed] [Google Scholar]
- 2.Goa KL, Barradell LB. Fluconazole. An update of its pharmacodynamic and pharmacokinetic properties and therapeutic use in major superficial and systemic mycoses in immunocompromised patients. Drugs. 1995;50:658–90. doi: 10.2165/00003495-199550040-00007. [DOI] [PubMed] [Google Scholar]
- 3.Troke PF. Large-scale multicentre study of fluconazole in the treatment of hospitalised patients with fungal infections. Multicentre European Study Group. Eur J Clin Microbiol Infect Dis. 1997;16:287–95. doi: 10.1007/BF01695633. [DOI] [PubMed] [Google Scholar]
- 4.Bentley A, Butters M, Green SP, et al. The discovery and process development of a commercial route to the water soluble prodrug, fosfluconazole. Org Process Res Dev. 2002;6:109–12. [Google Scholar]
- 5.Louie A, Drusano GL, Banerjee P, et al. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob Agents Chemother. 1998;42:1105–9. doi: 10.1128/aac.42.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andes D, van Ogtrop ML. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine model of disseminated candidiasis model. Antimicrob Agents Chemother. 1999;43:2116–20. doi: 10.1128/aac.43.9.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rex JH, Pfaller MA, Galgiani JN, et al. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro and in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–47. doi: 10.1093/clinids/24.2.235. for the NCCLS Subcommittee on Antifungal Susceptibility Testing. [DOI] [PubMed] [Google Scholar]
- 8.Lee SC, Fung CP, Huang JS, et al. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe Candida infections treated with fluconazole. Antimicrob Agents Chemother. 2000;44:2715–8. doi: 10.1128/aac.44.10.2715-2718.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brammer KW, Farrow PR, Faulkner JK. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev Infect Dis. 1990;12(Suppl 3):S318–S326. doi: 10.1093/clinids/12.supplement_3.s318. [DOI] [PubMed] [Google Scholar]
- 10.Debruyne D, Ryckelynck JP. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet. 1993;24:10–27. doi: 10.2165/00003088-199324010-00002. [DOI] [PubMed] [Google Scholar]
- 11.SAS Institute Inc. 4. Cary: NC, SAS Institute Inc; 1994. SAS/SAT Users Guide, Version 6.09. [Google Scholar]
- 12.Marco F, Danes C, Almela M, et al. Trends in frequency and in vitro susceptibilities to antifungal agents, including voriconazole and anidulafungin, of Candida bloodstream isolates. Results from a six-year study (1996–2001) Diagn Microbiol Infect Dis. 2003;46:259–64. doi: 10.1016/s0732-8893(03)00086-5. [DOI] [PubMed] [Google Scholar]
- 13.Pfaller MA, Diekema DJ, Jones RN, et al. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol. 2001;39:3254–9. doi: 10.1128/JCM.39.9.3254-3259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safdar A, Chaturvedi V, Koll BS, Larone DH, Perlin DS, Armstrong D. Prospective, multicenter surveillance study of Candida glabrata: fluconazole and itraconazole susceptibility profiles in bloodstream, invasive, and colonizing strains and differences between isolates from three urban teaching hospitals in New York City (Candida Susceptibility Trends Study, 1998–1999) Antimicrob Agents Chemother. 2002;46:3268–72. doi: 10.1128/AAC.46.10.3268-3272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta K, Jain N, Sethi S, Rattan A, Casadevall A, Banerjee U. Fluconazole and itraconazole susceptibility of clinical isolates of Cryptococcus neoformans at a tertiary care centre in India: a need for care. J Antimicrob Chemother. 2003;52:679–82. doi: 10.1093/jac/dkg399. [DOI] [PubMed] [Google Scholar]